Abstract
Background: The minimum clinically important difference (MCID) for diffusing capacity of the lungs for carbon monoxide (DLCO) has not yet been solidly established. Methods: We used the dataset of surgical cohort of National Emphysema Treatment Trial. Briefly, severe and very severe chronic obstructive pulmonary disease (COPD) patients who were candidate for volume reduction surgery and who could provide sufficient data at 12-month follow-up were included. We used two anchor methods using 6-minute walk distance (6MWD. MCID = 40 m) and forced expiratory volume in 1 sec (FEV1. MCID = 100 ml) as anchors, and two distribution methods. We proposed MCID with a median of estimated values. We estimated MCID for DLCO in raw value and % change from the baseline independently. Results: The surgical cohort included 356 patients, whose average age was 66.6 ± 5.5 years, and the average % predicted FEV1 was 27.8 ± 7.3%. The estimated MCID for DLCO in raw value and % change from the baseline were as follows: anchor method (average, 6MWD) 1.2 ml/min/mmHg, 17%; anchor method (average, FEV1) 0.7 ml/min/mmHg, 11%; anchor method (receiver operating characteristic, 6MWD) 1.1 ml/min/mmHg, 10%; anchor method (receiver operating characteristic, FEV1) 1.2 ml/min/mmHg, 3%; distribution method (0.3 units of standard deviation), 0.9 ml/min/mmHg, 11%; distribution method (standard error of measurement), 1.1 ml/min/mmHg. The median of these values was 1.1 ml/min/mmHg and 11%. Conclusion: We estimated the group-level MCID for DLCO for patients with severe and very severe COPD patients as 1.1 ml/min/mmHg and 11% of baseline DLCO.
Introduction
A test of the diffusing capacity of the lungs for carbon monoxide (DLCO) is one of the most clinically important tests for monitoring patients with respiratory diseases (Citation1). Standard methods for using DLCO, performance of the test, and calculation of the results were initially described by the American Thoracic Society in 1987 (Citation1), and updated by a joint committee of the American Thoracic Society and European Respiratory Society in 2005 (Citation2). Even though the test is highly valuable for evaluating the lung function, it has two major limitations to interpret the small change of DLCO.
One is low reliability (or reproducibility) due to a large random error, i.e. the same results are not found even if the test is repeated after a short period. The reproducibility requirement by the standard methods is an intra-session error within 10% of the highest value (Citation1,2). That is, error < 10% is currently regarded as acceptable for DLCO. The other limitation is that the minimum clinically important difference (MCID) has not yet been established. MCID is a threshold to distinguish between a small meaningless change and a small but meaningful change (Citation3). Without knowing MCID, we cannot judge whether the small change is meaningful. The aim of the current study was to decide a group-level MCID for DLCO using a cohort of patients with severe and very severe chronic obstructive pulmonary disease (COPD).
Methods
Ethical statement
The National Heart, Lung, and Blood Institute (USA) provided the data set of the National Emphysema Treatment Trial (NETT) (Citation4). The Institutional Review Board of Yokohama City University approved this study (A110728010) and waived the requirement for informed consent for patients’ anonymity.
Patients and treatment
The major entry criteria for the NETT study were as follows: radiographic evidence of bilateral emphysema; participation in pulmonary rehabilitation; no high risk of perioperative morbidity or mortality; suitability for lung volume reduction surgery; % predicted forced expiratory volume in 1 second (FEV1) of 45% or less; a pressure of oxygen in artery of 45 mmHg or higher; a pressure of carbon dioxide in artery of 60 mmHg or less; 6-minute walking distance (6MWD) of 140 m or higher; and likelihood of completing the trial. During the screening process, 3777 patients in 17 clinical centers were evaluated from January 1998 to July 2002. Among them, 1218 patients met the entry criteria. From these, 608 and 610 were randomly allocated to the surgical and medical cohorts, respectively.
The original study demonstrated that surgically treated patients had large improvement in respiratory function, quality of life, and life prognosis (Citation4). We used the data of the surgical cohort for development and those of medical cohort for validation. A previous article described more detailed entry criteria (Citation4). We set the following additional exclusion criteria because we could not evaluate the longitudinal change for such patients: (i) death before 12-month follow-up; (ii) no record of DLCO, or any of anchor candidates, i.e., St. George Respiratory Questionnaire (SGRQ), 6MWD, and FEV1 at the baseline and/or 12-month follow-up.
Patients allocated to the surgical cohort were scheduled for bilateral lung volume reduction surgery within two weeks of randomization. Both median sternotomy and video-assisted thoracic surgery were conducted. Patients allocated to the medical cohort were treated by the primary care physicians using the method suggested in the guidelines: smoking cessation, regular inhalation of bronchodilators, oxygen therapy, immunization, pulmonary rehabilitation, and additional measures including oral corticosteroids (Citation4).
Measurements
The standard protocol for measuring DLCO was as follows (Citation1). A patient is asked to refrain from smoking for 24 hours preceding the test. The subject then sits throughout the test, and is encouraged to exhale to residual volume prior to inhaling the vital capacity of test gas. The breathhold time is 10 seconds. The initial 750 to 1000 ml of the exhaled gas is discarded before the 500 to 1000 ml of alveolar sample is collected. The average valued of at least two acceptable tests with at least a four-minute interval should be reported. DLCO is defined as “the fraction of inspired carbon monoxide × fraction of helium in the alveolar gas/fraction of inspired helium” (Citation1,2).
SGRQ, 6MWD, and FEV1 were considered as anchor candidates. The SGRQ is a measurement tool for evaluating quality of life mainly for patients with respiratory disease. The score was calculated from answering 76 questions. The score ranges was 0–100 where 0 indicated the best quality of life (Citation5). 6MWD expresses exercise capacity simply addressing the distance which a subject can walk in six minutes (Citation6). FEV1 is the maximal volume of air exhaled in the first second of a forced expiration from a position of full inspiration (Citation7). Established MCIDs for SGRQ (Citation8), 6MWD (Citation9–13), and FEV1 (Citation14–17) are four points, 40 m, and 100 ml, respectively; though some researchers propose different MCIDs for 6MWD and FEV1. ∆DLCO, ∆SGRQ, ∆6MWD, and ∆FEV1 in this study mean longitudinal change of values during the 12-month follow-up.
Estimating the cutoff values in the surgical cohort
We calculated MCID in the form of raw value of MCID and expressed as% change from the baseline. These two analysis were performed independently. For determination of the MCID, anchored-base and distribution-based methods were utilized. The anchored-based method compares changes in DLCO values with an “anchor” as reference with a well-established MCID. As an example, the present study, attempted to determine the DLCO value with similar impact to a change in 100 ml of FEV1. Before using the anchor methods, we checked the correlation between ∆DLCO and change in anchors.
Only anchors with a correlation coefficient > 0.3 were adopted as anchors in this study (Citation18,19). As a result, SGRQ was not adopted for an anchor. We used two anchor methods (Citation20–22) and two distribution methods (Citation19,20,Citation23). Then, we decided the final MCID by using the median of these values.
Anchor method (average): The cutoff values for the DLCO change in the form of raw value (∆DLCO) and% change were defined as the absolute values of the average longitudinal changes of anchors among patients whose improvement in anchors were in the range of the cutoff values ± 10%: 6MWD 36–44m, FEV1 90–110 (ml).
Anchor method (receiver operating characteristic (ROC)): We separated the patients into those with and without at least a minimal anchor improvement. We then, defined the MCID as the best cutoff values of the ∆DLCO and% change of DLCO with Youden's index, the cutoff value which gives the highest “sensitivity + specificity −1”.
Distribution standard deviation (SD) method: We defined the MCID for DLCO as 0.3 units of SD for DLCO at the baseline; and MCID for % change as 0.3 units of SD for % change from baseline to 12-month follow-up (Citation19,20).
Distribution standard error of measurement (SEM) method: We defined the MCID for DLCO as SD multiplied by the square root of 1 minus the reliability coefficient. This method was not adopted for % change of DLCO, because we did not have sufficient data for test-retest reliability. In total, we obtained six MCID for raw value of DLCO and five MCID for % change of DLCO from the baseline.
Application of the proposed cutoff values in the medical cohort
We applied estimated MCID for DLCO for patients in medical cohort. We compared the number of patients who had meaningless or meaningful change according to DLCO and the anchors.
Statistics
Data are presented as average ± SD (not standard error). Longitudinal changes of DLCO, 6MWD, and FEV1 values were evaluated using the Wilcoxon signed-rank test. Correlation was evaluated using Spearman's rank correlation test.
Results
Estimating the cutoff values in the surgical cohort
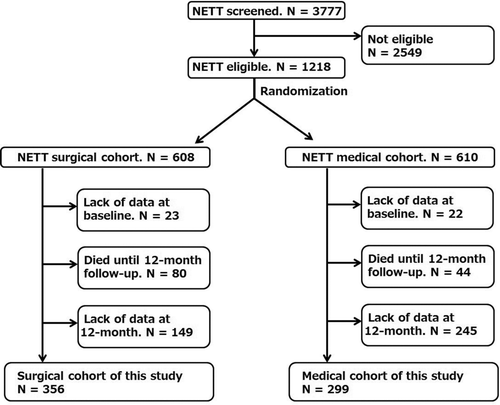
The NETT cohort consisted of 608 surgical patients and 610 medical patients, which was randomly divided. Of the 608 surgically treated patients who were originally included in the NETT dataset, 252 were excluded for the following reasons: 23 for lack of data at baseline, 80 due to death by the 12-month follow-up, and 149 for lack of data at the 12-month follow-up (Figure ). The final surgical cohort thus included 356 patients, whose average age was 66.6 ± 5.5 years and the average % predicted FEV1 was 27.8 ± 7.3%. Average DLCO of 8.1 ± 3.0 ml/min/mmHg (range: 1.5–17.7 ml/min/mmHg). It was much lower than the proposed predicted DLCO for elderly man of roughly 30 ml/min/mmHg and lower limit of that of roughly 20ml/min/mmHg, though there are many predictive formulas.
Figure 1. Flow chart for patient entry. N: number of patients; NETT: National Emphysema Treatment Trial.
From the baseline to the 12-months follow-up, DLCO, 6MWD, and FEV1(ml) on average improved 0.5 ml/min/mmHg, 42 m, and 154 ml respectively (P < 0.001 for all, Table ). These changes were explained by the therapeutic effect of the volume reduction surgery.
Table 1. Baseline characteristics and longitudinal changes
Anchor method (average, 6MWD): the average ∆DLCO and average % change from the baseline of the 19 patients whose ∆6MWD were in the range of MCID for 6MWD ± 10% (36 m ≤ ∆6MWD ≤ 44 m) were 1.2 ml/min/mmHg and 17%, respectively.
Anchor method (average, FEV1(ml)): the average ∆DLCO and average % change from the baseline of the 17 patients whose ∆FEV1(ml) were in the range of MCID for FEV1 (ml) ± 10% (90 ml ≤ ∆FEV1 ≤ 110 ml) were 0.7 ml/min/mmHg and 11%, respectively.
Anchor method (ROC, 6MWD): 184 patients showed improvement with ∆6MWD of 40 m or more. A cutoff value of 1.1 ml/min/mmHg and 10% change from the baseline yielded the best Youden's index of 0.22 and 0.24, respectively, to predict these 184 patients.
Anchor method (ROC, FEV1(ml)): 197 patients showed improvement with ∆FEV1 of 100 m or more. A cutoff value of 1.2 ml/min/mmHg and 3% change from the baseline yielded the best Youden's index of 0.23 and 0.25, respectively, to predict these 197 patients.
Distribution SD method: The SD for the DLCO at the baseline was 3.0 ml/min/mmHg. Therefore, 0.3 units of SD was equal to 0.9 ml/min/mmHg. The SD for% change from the baseline was 36%. Therefore, 0.3 units of SD was equal to 11%.
Distribution SEM method: SD (3.0) multiplied by the square root of 1 minus the reliability coefficient (0.85) was 1.1 ml/min/mmHg. The median of these estimated MCID for raw value of DLCO was 1.1 ml/min/mmHg and that for percentage change from the baseline was 11% (Table ).
Table 2. Summary of minimum clinically important difference (MCID) for diffusing capacity of the lungs for carbon monoxide (DLCO) evaluated in the surgical cohort
Application of the proposed cutoff values in the medical cohort
Of the 610 medically treated patients who were originally included in the NETT database, 311 patients were excluded for the following reasons: 22 for lack of data at baseline, 44 due to death by the 12-month follow-up, 245 for lack of data at the 12-month follow-up (Figure ). Thus, our final medical cohort included 299 patients (Figure ). The baseline characteristics of the medical cohort patients were similar to those of the surgical cohort patients because of randomization, but were not completely identical (Table ). The causes of difference between cohorts were that more patients died in the surgical cohort during the first year of randomization and there was greater unavailability of data at the 12-month follow-up in the medical cohort.
According to ∆DLCO, % change of DLCO from the baseline, ∆6MWD, and ∆FEV (ml), 134 (44.8%), 176 (58.9%), 121 (40.5%), and 144 (48.2%) experienced meaningful change, i.e. meaningful improvement of meaningful deterioration, at the 12-month follow-up (Table ).
Table 3. A comparison of the numbers of patients with meaningful and meaningless change in the medical cohort
Discussion
We estimated group-level MCID for DLCO for patients with severe and very severe COPD as 1.1 ml/min/mmHg in the form of raw value and 11% change from the baseline using both the anchor and the distribution methods. DLCO is an excellent index of the degree of anatomic emphysema in smokers with airflow limitation, especially when deciding diagnosis. For example, it is well known that DLCO is a useful indicator to distinguish COPD from bronchial asthma (Citation24). However, it was not sufficiently clear how to interpret the longitudinal change of DLCO measured for patients with COPD.
We cannot compare the results in this study with MCID previously estimated by the anchor or the distribution method, as there is no such a study. Therefore, we will try to evaluate the results using three methods. First, we adopted the results for a different cohort. In the medical cohort, a similar number of patients experienced meaningful or meaningless change according to ∆DLCO, % change of DLCO from the baseline, ∆6MWD, and ∆FEV. It reinforced the validity of the proposed value of MCID (Citation25).
Second, the American Thoracic Society/ European Respiratory Society Task Force indicated year-to-year MCID for DLCO for healthy adults as 10% (Citation14). This MCID set by the Task Force was not based on the current standard (Citation18–22) of the anchor method nor the distribution method, but was based on the observation of a cohort of eight healthy subjects; intra-patient DLCO measurements could vary as much as ± 10% over one year (Citation14,Citation26). The baseline DLCOs in our study in both surgical and medical cohorts were 8.1 ml/min/mmHg, and MCID for DLCO in the form of raw value in our study was 1.1 ml/min/mmHg. Therefore, 1.1 ml/min/mmHg was equivalent to 14%. MCID in the form of % change from the baseline in our study was 11%.
These results had a small discrepancy with the consensus in the Task Force of 10% but shows a resemblance to some extent. Third, we applied the distribution method (SD) for the previously published dataset. We found two published studies which could provide a DLCO value for more than 1000 subjects (Table ). Hadeli reported data of 4545 men and 3472 women who were referred to the pulmonary function laboratory (Citation27). Punjabi evaluated 6193 subjects to evaluate measurement variability in DLCO (Citation28). In both of these studies, 0.3 unit of SD is equivalent to 1.7–2.1 ml/min/mmHg, which are much larger than our estimation of 1.1 ml/min/mmHg. This difference is explained by the fact that inter-session intra-patient variability of DLCO increases with increasing baseline DLCO value (Citation29,30).
Table 4. Minimum clinically important difference (MCID) for diffusing capacity of the lungs for carbon monoxide (DLCO) estimated from previous studies using the distribution method
MCID is usually expressed as a fixed value rather than a proportion of the baseline value. However, we believe the proportion to the baseline value may be more suitable to address MCID for some measurements when some patients have very low values. For example, in the current study, the highest DLCO value of 17.7 ml/min/mmHg was almost 12 times higher than the lowest DLCO value of 1.5 ml/min/mmHg. The impact of ∆DLCO of 1.1 ml/min/mmHg is much stronger for a patient with baseline DLCO of 1.5 ml/min/mmHg than for a patient with baseline DLCO of 17.7 ml/min/mmHg. According to the consensus of the Task Force, data from Hadeli's and Punjabi's studies, and the current work, 10–14% of baseline DLCO may be used for a rough indication for MCID.
Pulmonary hypertension and DLCO may have interaction when dealing with the severe and very severe COPD patients. However, spearman's correlation coefficient between DLCO and peak pulmonary artery pressure was −0.13. The absolute value of a coefficient less than 0.2 is usually suggest a meaningless correlation. Therefore, the link between pulmonary hypertension and DLCO was not a serious concern for the current analysis.
The current study had some limitations. First, it was conducted with only severe and very severe COPD patients. Therefore, the external validity is not sufficiently confirmed. Second, because DLCO has low reproducibility, estimated MCID for DLCO in the current study were also affected by the low reproducibility. This error was not an error with a certain direction, which results in bias, but a random error without a certain direction. We tried to overcome this issue by using the maximal number of patients available in this study.
Third, the current study proposed MCID only for raw DLCO and its % change, though DLCO is usually evaluated in the form of DLCO (% predicted), DLCO adjusted for hemoglobin, volume of alveoli, and/or atmospheric pressure. It is difficult to decide the most valuable value among them, as the choice is depend on the situation. Therefore, we decided to propose MCID for the simple raw value and its % change of DLCO. Fourth, DLCO may be affected differently by medical and surgical interventions. This study used patients that underwent a surgical intervention as the primary study population, thus the results should be interpret with caution when applying it to cases with medical treatment.
Conclusions
We proposed 1.1 ml/min/mmHg in the form of raw value and 11% change from the baseline for the group-level MCID for DLCO among patients with severe and very severe COPD based on the anchor and distribution methods.
Acknowledgment
We thank the National Emphysema Treatment Trial and the National Heart, Lung, and Blood Institute for their help with providing data set, Mrs. Narisada for her help with data analysis, and Mr. Thomas Kiper for his proofreading.
Declaration of Interest Statement
All authors contributed conception, design, data acquisition, analysis, interpretation, drafting, revising, and final approval of the manuscript. Nobuyuki Horita served as a principal investigator and a guarantor and had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Naoki Miyazawa, Ryota Kojima and Miyo Inoue provided interpretation of data and drafting. Takeshi Kaneko and Yoshiaki Ishigatsubo provided study management.
None of the investigators declare any real or perceived conflicts of interest pertaining to the subject of this manuscript. No support in the form of grants, gifts, equipment, and/or drugs was provided.
The authors alone are responsible for the content and writing of the paper.
References
- American Thoracic Society. Single breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique. Statement of the American Thoracic Society. Am Rev Respir Dis 1987; 136:1299–1307.
- Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005; 26:720–735.
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Contr Clin Trials 1989; 10:407–415.
- Rationale and design of the National Emphysema Treatment Trial (NETT): A prospective randomized trial of lung volume reduction surgery. J Thorac Cardiovasc Surg 1999; 118:518–528.
- Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145:1321–1327.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166:111–117.
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
- Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005; 2:75–79.
- Wise RA, Brown CD. Minimal clinically important differences in the six-minute walk test and the incremental shuttle walking test. COPD 2005; 2:125–129.
- Holland AE, Hill CJ, Rasekaba T, et al. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2010; 91:221–225.
- Puhan MA, Chandra D, Mosenifar Z, et al. The minimal important difference of exercise tests in severe COPD. Eur Respir J 2011; 37:784–790.
- Puhan MA, Mador MJ, Held U, et al. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J 2008; 32:637–643.
- Redelmeier DA, Bayoumi AM, Goldstein RS, et al. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med 1997; 155:1278–1282.
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26:948–968.
- Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008; 31:416–469.
- Redelmeier DA, Goldstein RS, Min ST, et al. Spirometry and dyspnea in patients with COPD. When small differences mean little. Chest 1996; 109:1163–1168.
- Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005; 2:111–124.
- Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof 2005; 28:172–191.
- Revicki D, Hays RD, Cella D, et al. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 2008; 61:102–109.
- Guyatt GH, Osoba D, Wu AW, et al. Methods to explain the clinical significance of health status measures. Mayo Clin Proc 2002; 77:371–383.
- Hays RD, Farivar SS, Liu H. Approaches and recommendations for estimating minimally important differences for health-related quality of life measures. COPD 2005; 2:63–67.
- Coeytaux RR, Kaufman JS, Chao R, et al. Four methods of estimating the minimal important difference score were compared to establish a clinically significant change in Headache Impact Test. J Clin Epidemiol 2006; 59:374–380.
- Leidy NK, Wyrwich KW. Bridging the gap: using triangulation methodology to estimate minimal clinically important differences (MCIDs). COPD 2005; 2(1):157–165.
- Saydain G, Beck KC, Decker PA, Cowl CT, Scanlon PD. Clinical significance of elevated diffusing capacity. Chest 2004; 125:446–452.
- Horita N, Miyazawa N, Morita S, Kojima R, Kimura N, Kaneko T, et al. Small, Moderate, and Large Changes, and the Minimum Clinically Important Difference in the University of California, San Diego Shortness of Breath Questionnaire. COPD 2014; 11:26–32.
- Hathaway EH, Tashkin DP, Simmons MS. Intraindividual variability in serial measurements of DLCO and alveolar volume over one year in eight healthy subjects using three independent measuring systems. Am Rev Respir Dis 1989; 140:1818–1822.
- Hadeli KO, Siegel EM, Sherrill DL, et al. Predictors of oxygen desaturation during submaximal exercise in 8,000 patients. Chest 2001; 120:88–92.
- Punjabi NM, Shade D, Patel AM, et al. Measurement variability in single-breath diffusing capacity of the lung. Chest 2003; 123:1082-1089.
- Drummond MB, Schwartz PF, Duggan WT, et al. Intersession variability in single-breath diffusing capacity in diabetics without overt lung disease. Am J Respir Crit Care Med 2008; 178:22–232.
- Robson AG, Innes JA. Short term variability of single breath carbon monoxide transfer factor. Thorax 2001; 56:358–361.

