Abstract
Objective: To describe CT features associated with severe exacerbations of Chronic Obstructive Pulmonary Disease (COPD). Materials and Methods: In this prospective ethical-committee-approved study, 44 COPD patients (34 men, 10 women, age range 49–83 years) who provided written informed consent were included at the time of hospital admission for severe exacerbation. Pulmonary function tests (PFT) and chest CT scans were performed at admission and after resolution of the episode following a minimum of 4 weeks free of any acute symptom. For each CT scan, two radiologists independently scored 15 features in each lobe and side. CT features and PFT results were compared for exacerbation and control through Mac-Nemar tests and paired t-tests, respectively. Results: Forced expiratory volume in 1 second and vital capacity improved significantly after exacerbation (p = 0.023 and 0.012, respectively). Bronchial wall thickening and lymphadenopathy were graded significantly higher at exacerbation than at control by both readers (p ranging from < 0.001 to 0.028). Other CT features were not observed during exacerbation, or were so only by one reader (p ranging from < 0.001 to 0.928). Conclusion: Only lymphadenopathy and bronchial wall thickening are CT features associated with severe COPD exacerbation, respectively in 25% and 50% of patients. Our findings do not advocate a role for CT in the routine work-up of patients with severe COPD exacerbation.
Introduction
The natural course of Chronic Obstructive Pulmonary Disease (COPD) is associated with episodes of exacerbation which are clinically defined as acute events characterized by a worsening of the patient's respiratory symptoms that is beyond normal day-to-day variations and which leads to a change in medication (Citation1). Moreover, these episodes can be classified into mild, moderate, or severe exacerbations, following patient's need for medical environment (Citation2). Although the cause of about one-third of severe exacerbation episodes cannot be identified (Citation1), two-thirds are associated with certain conditions such as respiratory tract infections (viral or bacterial) (Citation3–9), air pollution (Citation10–12), as well as pulmonary embolism (PE), pulmonary edema, cardiac arrhythmia, pneumothorax, or pleural effusion (Citation13–15).
As COPD exacerbations are associated with increased morbidity and mortality, as well as with increased healthcare costs, their prevention and treatment are two major objectives in COPD management with subsequent requirement for appropriate assessment tools (Citation16). As imaging tool, chest radiography is limited to the detection of pneumonia and pleural abnormalities, and only leads to change in managements in a marginal proportion of patients (Citation17, 18). However, while CT scans allows detecting more chest abnormalities, the knowledge of CT features at the time of exacerbation is a pre-requisite for determining possible role of CT in routine work-up of exacerbation. Nevertheless these features remain widely unknown, previous studies having focused on the prevalence of PE (Citation19, 20). The aim of our study was therefore to describe these features by comparing CT scans performed at severe exacerbation with control scans.
Materials and Methods
Study population
This prospective study was approved by the institutional ethical committee and written informed consent was obtained from all patients. In addition, its registration identifier on ClinicalTrials.gov was NCT01922180. Between July 2007 and August 2009, 44 COPD patients followed in our hospital (mean age, 69 years ± 9 (standard error of mean); range 49–83 years), including 34 men and 10 women were prospectively included in this trial at the time of an exacerbation episode leading to admission in our hospital, which corresponds to a severe episode (Citation2). There were no exclusion criteria. COPD was graded as GOLD I in 2 patients, GOLD II in 13 patients, GOLD III in 18 patients, and GOLD IV in 11 patients (Citation1). At the time of admission, pulmonary function tests (PFT) and chest CT scans were performed. As there is no definition of the resolution of an exacerbation episode, we arbitrarily considered that a minimum of 4 weeks free of any acute symptom after discharge was reasonable before redoing PFT and chest CT scan. The mean interval between CT scans at exacerbation and at control was 76 days.
Pulmonary function tests
PFT were carried out with the patient in the seated position. Forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) were measured with a Lilly-type pneumotachograph, while functional residual capacity (FRC) was measured in a constant volume body plethysmograph (MasterScreen Body, Jaeger, Würzburg, Germany). Total lung capacity (TLC) and residual volume (RV) were calculated using the values of FRC and the subdivisions of FVC. The measured values were then compared to the predicted values established by the European Respiratory Society (Citation21).
CT examinations
CT examinations were performed with a commercially available 64-detector row scanner (Somatom Sensation, Siemens Healthcare, Forchheim, Germany). Images were acquired in supine position after full inspiration and full expiration, using the following parameters: slice thickness, 0.6 mm; pitch, 1.4; rotation time, 330 msec; tube voltage, 120 kV; and tube current-time product, 100 mAs, with automatic exposure control (CareDose 4D, Siemens Healthcare) switched on. From raw data, 1-mm-thick section images were reconstructed at 0.7-mm intervals by using a high spatial algorithm (B60S, Siemens Healthcare) and a soft-tissue algorithm (B30S). The inspiratory CT scan at the time of exacerbation was performed with intravenous iodinated contrast material (100 mL of iomeprol administrated at a rate of 3.5 mL/sec, Iomeron 400; Bracco Diagnostics, Milan, Italy), whereas the control scan was unenhanced.
Image analysis
Informations on CT images that could identify the patients were removed and examinations were randomly numbered by using tables from Fisher and Yates (Citation22). Thereafter, two independent radiologists with 21 (reader 1) and 23 (reader 2) years of experience in chest imaging analyzed the CT images utilizing a reading sheet adapted from that used for scoring exacerbation in cystic fibrosis (Citation23). A preliminary reading session of eight CT examinations obtained in patients not involved in the present study was performed in consensus to learn the grading system and improve inter-reader agreement.
For each lobe (with the lingula considered as a separate lobe), readers were asked to grade severity of the following seven features: bronchiectasis, mucous plugging, bronchial wall thickening, pulmonary consolidation, ground glass opacity, cysts or bullae, and air trapping. All features with the exception of air trapping (Citation24) and bronchial wall thickening (Citation25) were defined according to the Fleischner Society Glossary of Terms for Thoracic Imaging (Citation26). Air trapping was considered to be present on the expiratory CT images when lung regions failed to increase in attenuation and/or failed to decrease in volume, when compared with the corresponding inspiratory images (Citation24). Bronchial wall thickening was defined as thick and less well-defined bronchial walls (Citation25). Bronchial wall thickening was graded as mild (wall thickness equal to the diameter of the adjacent vessel), moderate (wall thickness greater than up to twice the diameter of the adjacent vessel), or severe (wall thickness >2 times the diameter of the adjacent vessel).
After the preliminary reading session, we decided to consider eight additional features possibly associated with COPD exacerbation: three were graded per lobe (centrilobular micronodules, platelike atelectasis, and emphysema) and five were graded per side (PE, pleural effusion, mediastinal or hilar lymphadenopathy, reticular pattern or honeycombing, and pulmonary mass or nodule). These eight additional features with the exception of PE (Citation25) and pleural effusion (Citation25) were also defined according to the Fleischner Society Glossary of Terms for Thoracic Imaging (Citation26). PE was defined as a filling defect within an opacified pulmonary artery (Citation25). Pleural effusion was defined as a homogeneous crescentic opacity in the most dependent part of the pleural cavity (Citation25). An extract for one lobe of the reading sheet is shown in Figure . In order to keep readers blinded to the correspondence between enhanced CT scans at the time of exacerbation and unenhanced control CT scans, the reading sessions were spread over 6 months and reading sheets could not be modified after each session.
Statistical analysis
We began the analysis of reading sheets by transforming our results in a way that would allow for further computation. This resulted in four steps:
reading sheets for exacerbation and control were compared, and changes in grades were computed for each feature in each lobe/side of each patient;
changes in grades were classified as “improvement” (grade of exacerbation > grade of control), “worsening” (grade of exacerbation < grade of control), and “no change” (grade of exacerbation = grade of control);
four features (i.e. bronchiectasis, mucous plugging, bronchial wall thickening, and air trapping) had related subfeatures as detailed in Figure , whereby the subfeatures were considered if the primary feature was present at exacerbation and control; and,
changes in feature/subfeature in various lobes/sides of a particular patient were classified as follows: if unchanged in each lobe/side, it was considered as “unchanged”, if unchanged in each lobe/side but improved in one, it was considered as “improved”, if unchanged in each lobe/side but worsened in one, it was considered as “worsened”, and if improved and simultaneously worsened in different lobes/sides, it was considered as “unchanged”.
For each feature/subfeature, a Mac-Nemar test assessed whether the number of patients who improved or worsened from exacerbation to control was significant.
Inter-reader agreement was evaluated through weighted Kappa statistics comparing the observed proportion of agreements with the proportion of agreements expected by chance. The observed worsening-unchanged, improvement-unchanged disagreements were weighted by a factor of 1, and the worsening-improvement disagreements were weighted by a factor of 2. All kappa values were interpreted as proposed in the literature: a kappa value of 0.20 or less indicated poor agreement, 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 good agreement, and 0.81–1.00 excellent agreement (Citation27).
Finally, paired t-tests were used to compare PFT results obtained at exacerbation and control. All analyses were performed by using Statistica 6.0 (StatSoft France, Maisons-Alfort, France) except for the Kappa statistics (Prism 6, Graphpad Software, La Jolla, USA). p < 0.05 was considered to indicate a statistically significant difference.
Results
Pulmonary function tests
Table shows comparisons between PFT at exacerbation and at control. There was a significant functional recovery of the airflow obstruction from exacerbation to control; shown through increases of FEV1 and FVC (p = 0.023 and 0.012, respectively).
Table 1. Comparisons of PFT at Exacerbation and at Control
CT examinations
One patient had a left upper lobectomy, one had a left lower lobectomy, and one had both middle and right lower lobectomy. Our readers thus reviewed 520 lobes and 88 sides. The frequencies of a CT feature graded by each reader at exacerbation and at control are listed in Table . Almost all CT features were detected with various grades at both exacerbation and control, indicating that they could remain present after an exacerbation episode, even without any acute symptom. Reader 1 and 2, respectively, detected 3/39 (7.7%) and 2/39 (5.1%) of patients with PE, with five CT examinations at exacerbation where enhancement was missing or inappropriate for evaluating PE for both readers. Nevertheless, as control scans were unenhanced, we did not investigate the evolution of PE at control. All patients showed at least one significant change at CT between exacerbation and control.
Table 2. Frequency of CT features detected by each reader at exacerbation and at control, and corresponding grades
A comparison of the numbers of patients with worsening, improvement, or no change from exacerbation to control for each CT feature by both readers are shown in Table . The severity of bronchial wall thickening (Figure ) and the presence of mediastinal or hilar lymphadenopathy (Figure ) were graded significantly higher by both readers (p ranging from < 0.001 to 0.028), during exacerbation than at control. The proportion of patients with mediastinal or hilar lymphadenopathy present at exacerbation were 13/44 (29.5%) for reader 1 and 8/44 (18.2%) for reader 2.
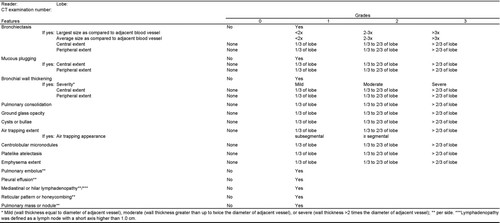
Figure 2. Axial enhanced CT scan through the right lower lobe of a 71-year-old man (GOLD IV) at exacerbation (a) with mild bronchial wall thickening (arrows) that disappeared at unenhanced control (b).
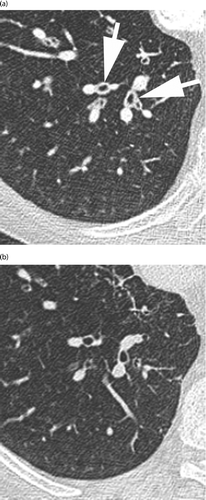
Figure 3. Axial enhanced CT scan through the right upper lobe of a 79-year-old man (GOLD II) at exacerbation (a) with mild mediastinal lymphadenopathy (arrow) that disappeared at unenhanced control (b).
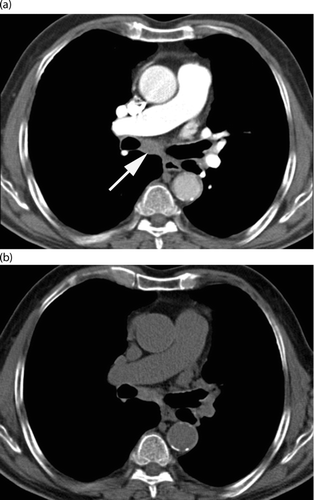
Table 3. Comparisons of numbers of patients with improvement, worsening or no change from exacerbation to control
In patients with bronchial wall thickening present at both exacerbation and control, the proportion with increased severity at exacerbation were 14/27 (51.8%) for reader 1 and 12/27 (44.4%) for reader 2. In addition, other items, such as the presence of bronchial wall thickening, the central extent of bronchial wall thickening, the peripheral extent of bronchial wall thickening, and the extent of centrilobular micronodules were graded significantly higher at exacerbation than at control by only one of our readers (p ranging from < 0.001 to 0.039).
Results of weighted kappa statistics are listed in Table . Agreement between readers was fair for the bronchial wall thickening severity, and moderate for the presence of mediastinal or hilar lymphadenopathy.
Table 4. Inter-reader agreements
Discussion
Our study showed that 1) severe exacerbation of COPD is associated with bronchial wall thickening and mediastinal or hilar lymphadenopathy, 2) inter-reader agreements were “fair” for the severity of bronchial wall thickening and “moderate” for the presence of mediastinal or hilar lymphadenopathy, and 3) the prevalence of PE is low. This study is important because it shows that only two chest CT features are observed during severe COPD exacerbations, with fair-to-moderate inter-reader agreement.
Previous studies on COPD exacerbations involving chest radiographs have been unable to determine which features were associated with an exacerbation episode (Citation18, Citation28, Citation29). Based on CT images, our study allowed us to subjectively quantify the features that we observed and therefore to determine those associated with severe exacerbation. Our comparisons revealed that the severity of bronchial wall thickening and the presence of mediastinal or hilar lymphadenopathy –two features commonly reported in various conditions, including pulmonary inflammatory disease and edema (Citation25) –are indeed associated with severe COPD exacerbation, in respectively 50% and 25% of patients.
On one hand, it is feasible that these proportions could have been underestimated as our analysis of reading sheets in four steps may have given more weighting to the unchanged status compared to an improved or worsened status. On the other hand, important features such as consolidation or pleural effusion –that could have a clinical impact –were not observed during severe COPD exacerbation, even though we considered all possible causes of exacerbation. Our study thus advocates that chest CT scans should not be performed in the routine work-up of patients with severe COPD exacerbation.
Previous studies of COPD exacerbation with CT have been focused on the prevalence of PE. Depending on the reader, we observed prevalence of 7.7% and 5.1%; figures that are very close to 3.3% reported by Rutschmann et al. (Citation19) and 5% reported by Choi et al. (Citation20), both studies having had recruitment processes very close to ours. Because our control scans were unenhanced, we were not able to detect any difference between exacerbation and control. Nevertheless, the prevalence of PE during severe COPD exacerbation is low but repeatedly reported in various studies (Citation19, 20).
Most inter-reader agreements were weak, and four features (i.e. the presence of bronchial wall thickening, the central extent of bronchial wall thickening, the peripheral extent of bronchial wall thickening, and the extent of centrilobular micronodules) were graded higher at exacerbation than at control by only one of our readers. Because it has been previously observed that discrepancy can occur between experienced readers, we tried to “calibrate” our readers at the start of the trial by having them perform a preliminary reading session in consensus in order to minimize discrepancies.
Moreover, CT scans obtained during exacerbations were compared to controls scored by the same reader in order to compute changes in grades with minimized systematic over- or underestimation by this reader. Despite this, however, there were still reader disagreements. Considering the severity of bronchial wall thickening, its assessment could have been visually subtle and could therefore also have contributed to the corresponding fair inter-reader agreement. In addition, our analysis of the reading sheets that considered “worsened” and “improved” status obtained in two different lobes as a resulting “no change” status, could also have contribute to weak inter-reader agreements.
Our study has several limitations. First, the number of patients in our study is relatively small (44 patients) and GOLD I patients are poorly represented (two patients). This can be explained by our recruitment process based on severe exacerbations. Nevertheless despite the size of our study group, we detected significant CT features, and GOLD II-III-IV patients are almost equally represented.
Second, the control scans were obtained after exacerbation recovery with a minimum of four weeks free of any acute symptom, which could have resulted in observing residual features that would not have been present before the exacerbation episode. On the one hand, this particular design could have lead us to observe more non-significant differences between exacerbation and control than we did, but on the other hand, this may have also reinforced the significance of the differences we observed. We used this particular design as it would have been impossible to predict the occurrence of an exacerbation episode and to perform baseline scans just before it occurred. In order to minimize possible residual features and as there is no definition of a recovery from COPD exacerbation, the control CT scan was arbitrarily obtained after at least a four-week interval free of any acute symptom after discharge.
Third, CT scans at exacerbation were enhanced whereas control scans were not as it would have been ethically unjustified to administrate intravenous iodinated contrast material at control CT scans on the basis of the low prevalence of PE at exacerbation (Citation19, 20). Moreover, the consistency of the visual assessment between enhanced CT scans at exacerbation and unenhanced control CT scans is unknown. As a result, readers were not blinded to the exacerbation or control status, but were blinded to the correspondence between control and exacerbation CT scans. We therefore spread the reading sessions over six months and reading sheets could not be modified after each session. Considering the low inter-reader agreements and the fact that some features even showed an increase in severity from exacerbation to control, we believe that the effect of this bias was as much as possible reduced.
Fourth, reading by our two readers was based on visual grading, as usually done in routine practice. Objective quantification would have been very appropriate, but as we obtained enhanced CT scans at exacerbation and unenhanced CT scans at control, comparisons based on attenuation measurements (for example, the extent of pulmonary emphysema) would have been biased. In addition, objective quantification techniques are still missing for almost all features considered in this study. Nevertheless, quantitative evaluation of airway wall thickening should be advocated in future studies to objectively assess CT changes between exacerbation and control. In this perspective, attention should be paid when objectively quantifying airways walls in a short period after an exacerbation episode.
In conclusion, lymphadenopathy and bronchial wall thickening are the only CT signs associated with severe COPD exacerbation. These signs occur in 25% and 50% of patients, respectively. Thus, our findings do not advocate a role for CT in the routine work-up of patients with severe COPD exacerbation.
Declaration of Interest Statement
We declare that there is no potential conflict of interest associated with this manuscript. ClinicalTrials.gov identifier of this protocol was NCT01922180. The authors are responsible for the writing and the content of this article.
References
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011. http://www.goldcopd.org/ December 2012.
- Rodriguez-Roisin R. Towards a consensus definition for COPD exacerbations. Chest 2000; 117:398S–401S.
- Monso E, Rosell A, Bonet G, et al. Risks factors for lower airway bacterial colonization in chronic bronchitis. Eur Respir J 1999; 13:338–342.
- Pela R, Marchesani F, Agostinelli C, et al. Airways microbial flora in COPD patients in stable clinical conditions and during exacerbations; a bronchoscopic investigation. Monaldi Arch Chest Dis 1998; 53:262–267.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008; 359:2355–2365.
- Fagon JY, Chastre J, Trouillet JL, et al. Characterization of distal bronchial microflora during acute exacerbation of chronic bronchitis. Use of the protected specimen brush technique in 54 mechanically ventilated patients. Am Rev Respir Dis 1990; 142:1004–1008.
- Monso E, Ruiz J, Rosell A, et al. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am J Respir Crit Care Med 1995; 152:1316–1320.
- Soler N, Torres A, Ewig S, et al. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med 1998; 157:1498–1505.
- Sethi S, Wrona C, Grant BJ, Murphy TF. Strain-specific immune response to Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 169:448–453.
- Ling SH, van Eeden SF. Particulate matter air pollution exposure: role in the development and exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2009; 4:233–243.
- Sint T, Donohue JF, Ghio AJ. Ambient air pollution particles and the acute exacerbation of chronic obstructive pulmonary disease. Inhal Toxicol 2008; 20:25–29.
- Peacock JL, Anderson HR, Bremner SA, et al. Outdoor air pollution and respiratory health in patients with COPD. Thorax 2011; 66:591–596.
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23:932–946.
- Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161:1608–1613.
- Adams SG, Melo J, Luther M, Anzueto A. Antibiotics are associated with lower relapse rates in outpatients with acute exacerbations of chronic obstructive pulmonary disease. Chest 2000; 117:1345–1352.
- Decramer M, Nici L, Nardini S, Reardon J, Rochester CL, Sanguinetti CM, Troosters T. Targeting the COPD Exacerbation. Respir Med 2008; 102 Suppl 1:S3–S15.
- Myint PK, Lowe D, Stone RA, Buckingham RJ, Roberts CM. U.K. National COPD Resources and Outcomes Project 2008: patients with chronic obstructive pulmonary disease exacerbations who present with radiological pneumonia have worse outcome compared to those with non-pneumonic chronic obstructive pulmonary disease exacerbations. Respiration 2011; 82:320–327.
- Sherman S, Skoney JA, Ravikrishnan KP. Routine chest radiographs in exacerbations of chronic obstructive pulmonary disease. Arch Intern Med 1989; 149:2493–2496.
- Rutschmann OT, Cornuz J, Poletti PA et al. Should pulmonary embolism be suspected in exacerbation of chronic obstructive pulmonary disease? Thorax 2007; 62:121–125.
- Choi KJ, Cha SI, Shin KM, et al. Prevalence and predictors of pulmonary embolism in Korean patients with exacerbation of chronic obstructive pulmonary disease. Respiration 2013; 85:203–209.
- Quanjer P, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Eur Respir J 1993; 6:5–40.
- Fisher RA, Yates F. Statistical Tables for Biological, Agricultural and Medical Research. London, England: Oliver and Boyd, 1963.
- Brody AS, Kosorok MR, Li A, et al. Reproducibility of a scoring system for computed tomography scanning in cystic fibrosis. J Thorac Imag 2006; 21:14–21.
- Arakawa H, Webb WR. Air trapping on expiratory high-resolution CT scans in the absence of inspiratory scan abnormalities: correlation with pulmonary function tests and differential diagnosis. AJR 1998; 170:1349–1353.
- Hansell DM, Lynch DA, McAdams HP, Bankier AA. Imaging Diseases of the Chest, 5th ed. Philadelphia, PA: Mosby Elsevier, 2010.
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246:697–722.
- Altman DG. Practical Statistics for Medical Research. London, England: Chapman & Hall, 1991.
- Jain P, Misra A. Routine chest x-ray in chronic obstructive pulmonary disease: a myth. N Z Med J 1990; 11:163.
- Emerman CL, Cydulka RK. Evaluation of high-yield criteria for chest radiography in acute exacerbation of chronic obstructive pulmonary disease. Ann Emerg Med 1993; 22:680–684.