Abstract
The objective of this study was to estimate the annual resource use and costs before and after COPD diagnosis and compare it across stages of airflow obstruction and levels of dyspnoea in the UK primary care setting. A retrospective cohort of newly diagnosed COPD patients (1/1/2008-31/12/2009) was identified in the UK Clinical Practice Research Datalink (CPRD). Resource use did not include medication costs and comprised of exacerbations, all cause GP interactions, and non-COPD hospitalisations, which were estimated for up to 12 months before and 24 months after COPD diagnosis. It was further stratified using baseline characteristics, Medical Research Council (MRC) dyspnoea score, and stages of airflow limitation. COPD costs were estimated using NHS reference costs. The analysis included 7881 newly diagnosed COPD patients (mean age, 67.2 years; 45% females). In the 2 years follow-up, the cohort experienced moderate and severe exacerbations, non-COPD hospitalisations, and GP surgery visits at an annual rate of 0.51, 0.13, 0.47, and 12.85, respectively. All resource components showed an upward trend with increase airflow limitation and dyspnoea. GP interactions accounted for 58.5% of annual per patient COPD management costs, estimated to be £2047 during the observation period. The annual costs doubled from patients with low levels of dyspnoea (MRC = 1; £1473) to those with high levels of dyspnoea (MRC = 5; £3243). COPD management costs in the primary care setting continued to remain high up to 2 years following initial diagnosis. The cost burden increased with high levels of dyspnoea and airflow obstruction, suggesting that both measures can identify patients requiring increased monitoring.
Introduction
There is an increasing interest in chronic obstructive pulmonary disease (COPD) worldwide because of its high prevalence and contribution to morbidity and mortality (Citation1). Furthermore, the high costs associated with COPD are a major concern for health care systems (Citation2). The burden of COPD is usually measured in terms of progressive decline in lung function, effect on patients’ symptoms, disability, and quality of life. In addition, the corresponding use of health care resources is a major focus point for this disease state (Citation1) and has been recognised by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) (Citation3).
The assessment of burden of COPD at the primary care level requires longitudinal estimation of patient characteristics, disease diagnosis, and changes in costs corresponding with disease progression. Such an assessment should help identify clinically relevant COPD subgroups affected by a higher burden of disease and consequently, provide insights for the holistic management of the disease. The UK Clinical Practice Research Datalink (CPRD) is an electronic medical record database used extensively for studying patients in primary care; it provides longitudinal details of patients linking primary, secondary, pharmacy records, and clinical parameters including symptoms and lung function as a part of the Quality Outcomes Framework. In addition, it classifies disease severity by stages of airflow obstruction and Medical Research Council (MRC) dyspnoea categories at incident diagnosis and follow-up time points, as per the GP's recording (Citation4).
This study examined COPD progression and the cost burden over 24 months following the first recorded diagnosis of COPD. The specific objectives of this study were to estimate the annual resource use and costs 12 months prior to and up to 24 months post-diagnosis, and to compare the disease burden among patients with various stages of airflow obstruction, based on 2006 GOLD classification (Citation3) and MRC dyspnoea scale categories (Citation5).
Methods
Study design
This retrospective cohort study evaluated patient and disease characteristics and the resource use among newly diagnosed patients identified from the primary care setting using CPRD (known as the General Practice Research Database (GPRD) during time of study). CPRD is an observational data and interventional research service jointly funded by the National Health Services (NHS), the National Institute for Health Research (NIHR), and the Medicines and Healthcare products Regulatory Agency (MHRA) of the UK (Citation4). For the present analysis, we sourced information from CPRD primary care electronic medical records (Citation4).
Patients aged ≥40 years with the first recorded diagnosis of COPD confirmed by spirometry (ie, forced expiratory volume at 1 second/forced vital capacity, FEV1/FVC < 0.7) between January 1, 2008 and December 31, 2009 were included in this study. The date of diagnosis was considered as the COPD index date/cohort entry date.
Patients were considered to be newly diagnosed only if they had no record of COPD in their medical history prior to cohort entry. Furthermore, these patients were required to have at least 24 months of follow-up history, unless death occurred during the follow-up period. Patients with a medical code of a condition incompatible with the diagnosis of COPD at any time in their history were excluded. These included lung or bronchial developmental anomalies, bronchiectasis, pulmonary resection, degenerative processes (cystic fibrosis or pulmonary fibrosis), or other significant respiratory disorders (excluding cancer) that could interfere with clinical diagnosis of COPD or substantially change the natural history of the disease. These patients were further stratified by severity of airflow limitation using the levels based on 2006 GOLD lung obstruction classification (Citation3) and MRC dyspnoea score (Citation5) collected at time closest to diagnosis. Patient characteristics such as age, gender, body mass index (BMI), smoking status, and prior diagnosed and/or treated co-morbid conditions (according to the Charlson index) (Citation6) were recorded at or a time nearest to the COPD cohort entry date.
The observation period comprised of a fixed period of 24 months of follow-up after cohort entry. The corresponding data collected 12-month prior to the diagnosis defines the pre-observation period. The health care resource use during the pre-observation and observation periods was captured through GP recordings as moderate and severe COPD exacerbations, all cause GP interactions, and non-COPD hospitalisations. Exacerbations captured during the pre-observation period were considered as suspected moderate exacerbations due to lack of formal COPD diagnosis in patient records. A moderate exacerbation was defined as an episode characterised by management with COPD-specific antibiotics and oral corticosteroids (within 5 days of initiation of antibiotics) and/or a medical diagnosis of COPD exacerbation.
A severe exacerbation was defined as an episode characterised by hospitalisation for COPD. During the 12-month pre-observation period, events indicative of possible moderate COPD exacerbations included medical diagnosis for acute bronchitis or concurrent treatment with oral corticosteroids and antibiotics. Recorded resource use also included GP interactions further classified as administrative contact, surgery correspondence, visit to a surgery nurse, in-person surgery visit, out-of-office GP visit, or a GP home visit. Non-COPD hospitalisations were defined as hospital admissions with specific non‑COPD causes or general hospital admissions (without a specific cause) with no record of COPD diagnosis or medication recorded 3 days before and up to 7 days after the index hospitalisation record. In addition, the total number of deaths reported during the 24-month observation period were recorded, and patients were censored at the earliest of death or end of study period.
The costs were expressed as mean annual costs per patient, and all costs were calculated using 2011 data (Table ). The cost of an exacerbation was estimated using relevant COPD-related health care resource group (HRG) codes (DZ21A-K for short stay and long stay) (Citation7). The cost of a moderate exacerbation was compiled based on resource use as stated in GOLD Strategy Group report (Citation3) and combined with costs data from the NHS reference costs (Citation7), the Personal Social Services Research Unit (PSSRU) 2011 (Citation8), and the British National Formulary 65 (BNF 65) (Citation9). This included a GP consultation lasting for 11.7 minutes, accident and emergency (A&E) visit with no admission (in 29% of the cases), and a prescription of prednisolone (30 mg) and co-amoxiclav (500 mg) (Citation10). The cost of a non-COPD hospitalisation was estimated as a weighted average of short- and long-stay hospital episodes from PSSRU costs (Citation8).
Table 1. Unit costs estimated for the resource use categories
Unit costs estimated for resource use categories were based on NHS reference costs (Citation7) and PSSRU 2011 costs (Table ) (Citation8). These unit costs were then applied to annual event rates for resource use components to calculate the annual costs per patient. Costs estimated during the observation period were further summarised based on the stages of airflow obstruction (Citation3) and the level of dyspnoea estimated using MRC dyspnoea scale (Citation5) captured nearest to the cohort entry. This study analysed anonymised electronic records, and the protocol WEUSKOP5904 was approved by CPRD Independent Scientific Advisory Committee (ISAC).
Results
Patient disposition and demographic characteristics
The cohort consisted of 7881 patients with a mean age of 67.2 years at baseline. These included 45.0% females, and 38.0% current smokers with a mean BMI (m/kgCitation2) of 26.9. A majority of patients (54.1%) had moderate airflow limitation (50% ≤ FEV1 <80% predicted or GOLD stage 2), and more than a third (37.5%) were suffering from clinically significant dyspnoea (MRC ≥ 3). Table shows patient disposition and demographic characteristics. Of these patients, 59.3% had at least one prescription of a short-acting bronchodilator (SABD) in 12 months prior to diagnosis. Proportions of patients having at least one prescription of long-acting beta-agonist (LABA), long-acting muscarinic agent (LAMA), or a combination of inhaled corticosteroid (ICS) and LABA with or without SABD prior to their COPD diagnosis were 4.7%, 8.4%, and 21.7%, respectively. In addition, 19.6% patients also received at least one prescription of ICS in 12 months prior to diagnosis. A total of 618 (7.8%) patients died during the 24-month observation period.
Table 2. Patient disposition and demographics
Resource use before and during the observation period
In the pre-observation period, the cohort experienced an annual per patient rate of 0.50 for suspected moderate exacerbations and 0.41 for non-COPD hospitalisations. After diagnosis, the cohort experienced an annual per patient rate of 0.51 for moderate exacerbations, 0.13 for severe exacerbations, and 0.47 for non-COPD hospitalisations in the observation period. In the 2 years after diagnosis, the annual per patient rates for GP surgery visit, GP out of office visits, GP home visits, GP administrative contacts, GP surgery correspondence, and GP practice nurse visits were 12.85, 0.15, 0.26, 27.99, 1.24, and 4.92, respectively.
During the observation period, the annual rates of moderate and severe exacerbations and non-COPD hospitalisations showed an upward trend with an increase in airflow obstruction level (Appendix Table ). The annual rates of moderate exacerbation recorded were 0.45, 0.47, 0.60, and 0.86 for patients with increasing airflow obstruction from mild to very severe. The corresponding rates for severe exacerbations were 0.11, 0.12, 0.14, and 0.19, and for non-COPD hospitalisations were 0.42, 0.48, 0.47, and 0.52. Similar trends of increased exacerbation rates were observed with an increase in the level of dyspnoea. The annual rates of moderate and severe exacerbations increased from 0.36 to 0.71 and from 0.08 to 0.25, respectively, with an increase in the level of dyspnoea from MRC grade 1 to MRC grade 5.
The annual rate of non-COPD hospitalisations also increased from 0.3 to 0.9 with an increase in MRC from grade 1 to 5. In addition, GP interactions and its components showed a similar trend in the observation period post-diagnosis (Appendix Table ). The resource use components also varied by BMI (Appendix Table ). Among BMI categories, moderate and severe exacerbations and non-COPD hospitalisations were the most common events for underweight patients (0.54, 0.18, and 0.49 events per person per year, respectively, for the underweight subgroup).
Logistic regression analysis (odds ratio (95% confidence interval)) showed that female gender (1.42 (1.21–1.66)), obesity (1.51 (1.23–1.85)), depression (1.75 (1.38–2.23)), anxiety (1.45 (1.17–1.81)), and presence of one (2.86 (2.44–3.35)) or more (5.32 (3.12–9.06)) co-morbidities were associated with more than one GP visit among patients with significant dyspnoea (MRC grade ≥ 3). All the above variables with the exception of anxiety were also associated with a single GP visit. Similarly, female gender (1.43 (1.22–1.69)), congestive heart disease (1.51 (1.05–2.15)), severe (1.51 (1.16–1.97)) or very severe (2.29 (1.51–3.48)) airflow obstruction, depression (1.37 (1.10–1.72)), anxiety (1.50 (1.21–1.84)), and presence of asthma (2.06 (1.75–2.42)) were associated with frequent (>1) exacerbations among patients with significant dyspnoea (MRC grade ≥ 3).
Annual costs of COPD management before and during the observation period
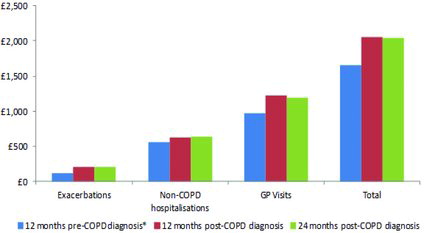
The annual estimated COPD management costs per patient excluding medication costs were £1654 in the pre-observation period and £2047 during the 24 months observation period (Figure ). GP interactions (68.3% and 58.5%) contributed more to these costs compared to exacerbations (5.4% and 10.2%) and non-COPD hospitalisations (26.2% and 31.4%) during the pre-observation and observation periods, respectively.
Among GP interactions, administrative contacts with GP surgery and GP surgery visits contributed the most with annual per patient costs of £496.4 and £401.3 in the pre-observation period and £615.8 and £462.6 during the observation period, respectively. Among exacerbations, severe exacerbations contributed more to the annual costs of exacerbations compared with moderate exacerbations. In the pre-observation period, the annual per patient cost of a suspected moderate exacerbation was £42.5. The costs in the observation period were £43.5 for moderate exacerbations and £164.3 for severe exacerbations. Mean annual costs with increase in airflow limitation and dyspnoea levels during the pre-observation period could not be determined, as these measures are not routinely evaluated in symptomatic patients who are not yet diagnosed with COPD.
In the entire cohort, the mean annual total COPD management costs showed an upward trend with an increase in airflow limitation and dyspnoea levels. This trend was particularly striking among patients with increasing level of dyspnoea, with the costs doubling from patient with low levels of dyspnoea (MRC = 1; £1473.4) to patients with high levels of dyspnoea (MRC = 5; £3243.3). Similar trends were observed in each cost component showing higher values for higher levels of dyspnoea (Table ). This was further validated by a cross-table presenting total annual COPD management costs by levels of airflow obstruction and MRC dyspnoea score for the 24-month periods post-diagnosis (Appendix Table ).
Table 3. Annual COPD management costs (excluding medication costs) by levels of airflow obstruction and dyspnoea for the 12- and 24-month periods post-diagnosis
Discussion
This study examined CPRD data to assess the costs of COPD over 24 months following the first recorded diagnosis. We estimated the resource use consisting of GP interactions, moderate to severe exacerbations, and non-COPD hospitalisations 12 month prior and up to 24 months post-diagnosis. We also recorded COPD-related medications post-diagnosis and analysed the treatment patterns during the observation period. The results published elsewhere indicated nearly half (48.7%) the patients changing their treatment at least once during the first year and more than 70% during the 2-year follow-up. Such high prevalence of treatment additions, switches, and step-downs, and in many cases more than once for a single patient, would have led to imprecise estimates of treatment durations. Therefore, considering the complexity of assessing individual medication costs in terms of symptom control and disease progression, we excluded medication costs from our analysis.
Our results showed that the annual costs of COPD management were significant before and immediately after diagnosis even after excluding medication costs (except those used to treat a moderate exacerbation). A study by Akazawa and colleagues estimated the annual treatment costs for a COPD patient to be £4052.5 (£1 = US$1.6) prior to diagnosis in a US managed care population in 2008 (Citation11). A similar study by Chittleborough and colleagues in Australia estimated the treatment costs to be £2222.9 (£1 = AU$1.7) (Citation12). The estimated annual COPD management costs in our study were £2047 for up to 2 years following diagnosis.
GP visits, in our study, constituted a higher proportion of COPD management costs compared to exacerbations and non-COPD hospitalisations. These visits averaged one per month even for those with mild to moderate airflow obstruction, indicating that attempts at reducing GP visits by better symptom control, patient education regarding disease progression, and telephonic consultation might significantly impact the COPD management cost. A study by Neilson and colleagues in 2005 showed similar results, wherein medication costs were the main cost driver followed by health care provider visits and exacerbations (Citation13). However, in general, our results are contrary to the published literature where frequency and severity of exacerbations are the driving factors that influence COPD costs (Citation14). Such results may be explained by our newly diagnosed cohort consisting of relatively milder COPD patients; a majority (71.3%) had mild to moderate airflow obstruction at baseline and thus, were expected to experience fewer and less severe exacerbations than other cohorts published in the literature (Citation15).
Furthermore, we observed that the estimated COPD management costs increased more sharply with an increase in level of dyspnoea compared with an increase in the level of airflow obstruction. Studies have already established the burden of dyspnoea on health-related quality of life and patient well-being (Citation16–Citation18). Our results showed that increasing levels of dyspnoea also exert substantial burden on health care resource use. This impact was observed through more frequent GP visits, exacerbations, and non-COPD hospitalisations. However, the entire dyspnoea burden and its effect on health resource use cannot be explained by COPD alone. Significant proportion of these patients suffered from co-morbidities including cardiovascular disease, which may also lead to dyspnoea. Interventions that help reduce dyspnoea through multiple clinical mechanisms and enable patients to lead a more active life may substantially improve disease outcomes.
Our study has several limitations. We excluded the cost of non-exacerbation‑related medications from our cost calculations. A previous study has shown medication cost to be a major contributor to overall healthcare costs (Citation19). This may significantly underestimate the annual COPD costs per patient in our analysis. In addition, we used tariff-based cost estimates applicable across England and Wales. Although this improves the generalisability of our findings, the actual costs in local settings may differ from national estimates, thereby limiting their applicability to local health economies. The CPRD only captures diagnosed diseases in the primary care setting.
After diagnosis, the study cohort was required to have at least 24 months of follow-up history, unless death occurred during the follow-up period. Therefore, contributions from undiagnosed/underdiagnosed patients, patients excluded due to shorter duration of follow-up or unavailability of spirometry data, and patients with more severe disease who died early may have influenced the generalisability of the results. It is possible that some outcomes recorded only in the hospital might not have been reported or experienced delay in reporting to the GP. Additionally, Strong et al. have reported that the quality of spirometry measurement is variable in the primary care population; however, this variability is not accounted for in the CPRD database (Citation20). Finally, the generalisability of the findings may be limited by differing diagnostic practices in the UK compared with the rest of the world.
Conclusions
In the primary care setting, COPD management costs of newly diagnosed patients are high and continue to be high for up to 2 years after diagnosis. GP interactions contributed to the majority of these costs followed by non-COPD hospitalisations and exacerbations. The cost burden of both non-COPD hospitalisations and GP visits is indicative of high co-morbidity and poor dyspnoea control. These findings suggest that measures taken to effectively reduce dyspnoea and better manage co-morbid conditions may help reduce the COPD cost burden.
Declaration of Interest Statement
KW, YSP, and AS are employees of GlaxoSmithKline.
Author contributorship: YSP has made substantial contribution towards conception and design, data analysis and interpretation, and manuscript preparation. KW has made substantial contributions towards all the aspects of the study. AS has contributed towards data acquisition, data analysis and interpretation, and manuscript preparation. KW and YSP are the guarantors for this study.
Acknowledgments
Funding for this study was provided by GlaxoSmithKline. All listed authors met the criteria for authorship set forth by the International Committee for Medical Journal Editors. Editorial support, in the form of development, assembling tables and figures, collating author comments, copyediting, fact checking, and referencing, was provided by Dr. Annirudha Chillar of Cactus Communications Inc., and funded by GlaxoSmithKline.
References
- Dal Negro R. Optimizing economic outcomes in the management of COPD. Int J Chron Obstruct Pulmon Dis 2008; 3(1):1–10.
- Borg S, Ericsson A, Wedzicha J, et al. A computer simulation model of the natural history and economic impact of chronic obstructive pulmonary disease. Value Health 2004; 7(2):153–67.
- Global Initiative for Chronic Obstructive Lung Disease. Available at http://www.goldcopd.org/uploads/users/files/GOLDReport2006_0122.pdf. Accessed on September 27, 2013.
- Welcome to The Clinical Practice Research Datalink. Available at http://www.cprd.com/home/. Accessed on September 27, 2013.
- MRC dyspnoea scale.. National Institute for Health and Care Excellence. http://www.nice.org.uk/usingguidance/commissioningguides/pulmonaryrehabilitationserviceforpatientswithcopd/mrc_dyspnoea_scale.jsp. Accessed on June 20, 2011.
- Khan NF, Perera R, Harper S, Rose PW. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam Pract 2010; 11:1.
- Department of Health, UK. NHS reference costs 2010–2011. Available at: https://wwwgovuk/government/publications/2010- 11-reference-costs-publication. Accessed on October 4, 2012.
- Personal Social Services Research Unit U. Unit Costs of Health and Social Care 2011. Available at: http://wwwpssruacuk/project-pages/unit-costs/2011/indexphp. Accessed on October 4, 2012.
- Society JFCBMAaRP. British National Formulary 65. 2012. Available at: http://bnforg/bnf/indexhtm. Accessed on October 4, 2012.
- Hertel N, Kotchie RW, Samyshkin Y, Radford M, Humphreys S, Jameson K. Cost-effectiveness of available treatment options for patients suffering from severe COPD in the UK: a fully incremental analysis. Int J Chron Obstruct Pulmon Dis 2012; 7:183–199.
- Akazawa M, Halpern R, Riedel AA, Stanford RH, Dalal A, Blanchette CM. Economic burden prior to COPD diagnosis: a matched case-control study in the United States. Respir Med 2008; 102(12):1744–1752.
- Chittleborough CR, Burke MJ, Taylor AW, et al. Medicare-related service use and costs among people with diagnosed and undiagnosed diabetes and respiratory conditions. Austral Health Rev 2009; 33(1):107–116.
- Nielsen R, Johannessen A, Schnelle HM et al. Repeatability of health economic data in COPD. Respir Med 2008; 102(11):1556–1562.
- Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD 2010; 7(3):214–228.
- Donaldson GC, Seemungal TA, Patel IS, Lloyd-Owen SJ, Wilkinson TM, Wedzicha JA. Longitudinal changes in the nature, severity and frequency of COPD exacerbations. Eur Respir J 2003; 22(6):931–936.
- Mahler DA, Ward J, Waterman LA, Baird JC. Longitudinal changes in patient-reported dyspnea in patients with COPD. COPD 2012; 9(5):522–527.
- Blinderman CD, Homel P, Billings JA, Tennstedt S, Portenoy RK. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Sympt Manage 2009; 38(1):115–123.
- Müllerova H LC, Tabberer M. Prevalence and burden of breathlessness in COPD patients. Manuscript under review.
- McGuire A, Irwin DE, Fenn P, et al. The excess cost of acute exacerbations of chronic bronchitis in patients aged 45 and older in England and Wales. Value Health 2001; 4(5):370–375.
- Strong M, South G, Carlisle R. The UK Quality and Outcomes Framework pay-for-performance scheme and spirometry: rewarding quality or just quantity? A cross-sectional study in Rotherham, UK. BMC Health Serv Res 2009; 9:108.
Appendix
Table A1. Annual rates of resource use among COPD patients in 24-months’ observation period
Table A2. Total annual COPD management costs (excluding medication costs) by levels of airflow obstruction and Medical Research Council (MRC) dyspnoea score for the 24-month periods post-diagnosis