Abstract
Background: The prevalence of obesity has increased during the last decades and varies from 10-20% in most European countries to approximately 32% in the United States. However, data on how obesity affects the presence of airflow limitation (AFL) defined as a reduced ratio between forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) are scarce. Methods: Data was derived from the third examination of the Copenhagen City Heart Study from 1991 until 1994 (n = 10,135). We examine the impact of different adiposity markers (weight, body mass index (BMI), waist circumference, waist-hip ratio, and abdominal height) on AFL. AFL was defined in four ways: FEV1/FVC ratio < 0.70, FEV1/FVC ratio < lower limit of normal (LLN), FEV1/FVC ratio <0.70 including at least one respiratory symptom, and FEV1/FVC ratio < LLN and FEV1% of predicted < LLN. Results: All adiposity markers were positively and significantly associated with FEV1/FVC independent of age, sex, height, smoking status, and cumulative tobacco consumption. Among all adiposity markers, BMI was the strongest predictor of FEV1/FVC. FEV1/FVC increased with 0.04 in men and 0.03 in women, as BMI increased with 10 units (kg · m-2). Consequently, diagnosis of AFL was significantly less likely in subjects with BMI ≥ 25 kg · m-2 with odds ratios 0.63 or less compared to subjects with BMI between 18.5–24.9 kg · m-2 when AFL was defined as FEV1/FVC < 0.70. Conclusion: High BMI reduces the probability of AFL. Ultimately, this may result in under-diagnosis and under-treatment of COPD among individuals with overweight and obesity.
Introduction
Chronic obstructive pulmonary disease (COPD) and obesity are responsible for significant morbidity and mortality worldwide (Citation1–3). Although COPD is estimated to become the fourth leading cause of mortality worldwide by the year 2020 (Citation4), the prevalence of obesity has increased almost exponentially during the last decades and varies from 10-20% in most European countries to approximately 32% in the United States (Citation5, 6).
In the clinical setting, the combination of COPD and obesity is increasing (Citation6). Obesity may cause dyspnea, which is also a major symptom of COPD (Citation7). Thus in obese individuals, dyspnea could be solely attributed to obesity and other possible causes such as COPD may not be pursued. Another problem is that increasing weight and body mass index (BMI) is associated with both decreasing forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) (Citation8, 9). However, FVC is more affected than FEV1 leading to an increase in FEV1/FVC (Citation10–12). As the presence of airflow limitation defined as a reduced FEV1/FVC ratio is a requisite finding in COPD, high BMI could ultimately result in under-diagnosis of COPD in individuals with obesity precluding them from adequate treatment.
In the present study, we investigate the association between various adiposity markers (weight, BMI, waist circumference, waist-hip ratio (WHR), abdominal height) with lung function, and how the presence of airflow limitation is affected by being overweight and obese. We use data from the Copenhagen City Heart Study (CCHS), which comprises approximately 10,000 individuals selected from the general population of Denmark. We have hypothesized that a high BMI would reduce the probability of having airflow limitation, and as a consequence lead to under-diagnosis of COPD among individuals with overweight and obesity conditions.
Methods
Subjects and design
Data comes from the CCHS, which is an ongoing epidemiological survey and has previously been described in detail (Citation13). In summary, the population was derived from a random and age-stratified sample of 19,698 individuals aged ≥ 20 years, who were recruited from among 90,000 people living in a predefined area of Copenhagen in 1976. In subsequent follow-up surveys, additional subjects have been invited. We have used data from the third examination of CCHS from 1991 to 1994 (CCHS III), as this examination includes various markers of obesity including abdominal height. We have in total 9,719 subjects with sufficient data for the analyses. We have decided not to exclude subjects with self-reported asthma to simulate a realistic setting of diagnosing airflow limitation in individuals with respiratory symptoms.
Variables of interest
We have used different measurements as adiposity markers: weight, BMI, waist circumference, WHR, and abdominal height. BMI was calculated as weight · height−2 (kg · m−2) and categorized according to World Health Organization (WHO) with a slight of modification: underweight (<18.5 kg · m−2), normal weight (18.5–24.9 kg · m−2), overweight (25–29.9 kg · m−2), obesity (30–34.9 kg · m−2), and severe obesity (≥35 kg · m−2) (Citation14). Waist circumference was measured between the lowest edge of the ribcage and the highest edge of the iliac crest, whereas hip circumference was measured at the widest point between the iliac crest and symphysis pubis. WHR was calculated as waist circumference · hip circumference−1. Abdominal height was defined as the sagittal diameter of the abdomen and measured from the iliac crest when the participants were placed in a supine position.
Spirometry was performed with a dry wedge spirometer (Vitalograph, Maidenhead, UK), which was calibrated daily with a 1-L syringe. FEV1 and FVC were obtained under a pre-bronchodilatory setting. We have obtained at least three sets of values, and had two measurements differing less than 5% as a criterion for reproducibility. Only the highest measurements of FEV1 and FVC were used in the analyses. FEV1 and FVC as percentages of predicted values were calculated using internally derived references based on a subsample of never-smokers with age and height as explanatory covariates separately for men and women.
We have defined airflow limitation in four different ways: (Citation1) according to FEV1/FVC <0.70, (Citation2) according to FEV1/FVC <lower limit of normal (LLN) calculated as the difference between the predicted value based on a subsample of never-smokers with age and height as explanatory covariates and 1.645 times the SE of the estimate separately for men and women, (Citation3) according to FEV1/FVC < 0.70 together with at least one of the following respiratory symptoms: dyspnea, sputum, cough, chest pain or tightness, and/or wheezing, and (Citation4) according to FEV1/FVC < LLN and FEV1% of predicted < LLN.
Dyspnea was considered when participants answered affirmative to at least one of the following questions: “Do you get breathless, when hurrying on level ground or walking up a slight hill?”, “Do you get breathless when walking on level ground with people of the same age?”, “Do you stop for a breath when walking on level ground in your own tempo?”, “Do you occasionally wake up at night because of breathlessness or troubled breathing?”, “Do you get breathless when taking a bath or getting dressed?”, and “Are you often bothered by breathlessness?”.
Sputum was considered when participants answered affirmative to at least one of the following questions: “Do you usually cough up phlegm from the lungs in the morning and/or during the day?” and “Do you usually cough up phlegm (in the morning or during the day) as long as three consecutive months each year?”. Cough was considered when participants answered affirmative to at least one of the following questions: “Do you occasionally cough when performing a strenuous activity?” and “Do you occasionally cough at nighttime?”. Chest pain or tightness was considered when participants answered affirmative to the question: “Do you get chest pain or tightness, when hurrying or taking the stairs?”. Wheezing was considered when participants answered affirmative to the question: “Do you occasionally have whistling or wheezing while breathing?”.
All participants were classified as never-, former-, or current-smokers based upon information on duration of tobacco smoking, current amount of tobacco consumed, and inhalation. We define cumulative tobacco consumption as tobacco consumed through smoking in pack-years.
Statistical analyses
We have used multiple linear regressions to investigate the association of each adiposity marker (weight, BMI, waist circumference, WHR, and abdominal height) with FEV1% of predicted value, FVC% of predicted value, and FEV1/FVC separately for men and women. We have entered each adiposity marker individually in order to determine its contribution to the variation of FEV1% of predicted, FVC% of predicted, and FEV1/FVC as well as avoiding multicollinearity. We have defined a stronger association among the adiposity markers, when the yielded P-value was lower, based upon less variability and/or higher coefficients.
In addition, the association of FEV1/FVC with BMI was also evaluated using the aforementioned classification of BMI into five categories with normal weight (18.5–24.9 kg · m−2) as the reference group. We have investigated whether an effect modification was present with age, sex, smoking status, and cumulative tobacco consumption when BMI predicted FEV1/FVC. P-values for interaction were yielded from the Wald test.
We have used logistic regressions to investigate the probability of having airflow limitation defined according to the aforementioned criteria.
We have included age, sex, height, smoking status, and cumulative tobacco consumption as independent explanatory covariates taking possibility of confounding into account. A p-value < 0.05 was accepted as significant. Data were analyzed using STATA/SE 13.0 for Windows (StataCorp, College Station, Texas, USA).
Results
Table shows the general characteristics according to BMI classification. Women seemed to dominate in the lowest and highest end of the BMI spectrum. Underweight subjects were more likely to be current-smokers. However, cumulative tobacco consumption in current- and former-smokers for overweight, obese, and severely obese subjects were significantly higher in comparison with underweight and normal weight subjects (p = 0.001). The prevalence of airflow limitation according to a fixed ratio and LLN showed the same trend, highest among underweight subjects and decreasing stepwise with increasing BMI (p < 0.001).
Table 1. General characteristics of participants in the third examination of Copenhagen City Heart Study
Table shows the association of different adiposity markers with lung function. All adiposity markers were entered individually into the linear regression models. In both sexes, each adiposity marker was negatively associated with FEV1% of predicted and FVC% of predicted. However, not all were statistically significant. Both in men and women, almost all adiposity markers were positively and significantly associated with FEV1/FVC (only the association with WHR in women was not significant). Among all adiposity markers, BMI was the strongest predictor of FEV1/FVC in both sexes (Table ). FEV1/FVC increased with 0.04 in men and 0.03 in women, as BMI increased with 10 units (kg · m−2) independent of covariates. Figure shows the association of BMI with spirometric parameters illustrating the trends observed in Table . The association of BMI with FEV1% of predicted became significant when men and women were pooled (p = 0.03).
Figure 1. Association of BMI with lung function with 95% confidence intervals shown graphically. All models included sex, cumulative tobacco consumption and smoking status as independent explanatory covariates. Additionally, the model predicting FEV1/FVC has also included age and height as independent explanatory covariates. BMI = body mass index; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity.
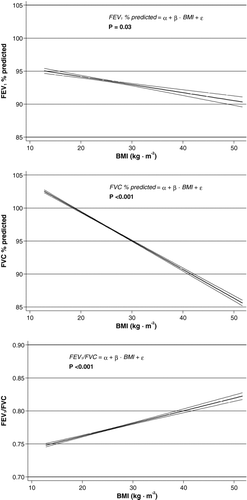
Table 2. Correlation of different adiposity markers with spirometric parameters
Figure shows the association of FEV1/FVC with BMI included according to the WHO classification. As seen, underweight subjects had significantly lower FEV1/FVC compared to normal weight subjects (0.04), whereas overweight, obese, and severely obese subjects had significantly higher FEV1/FVC (0.02, 0.03, and 0.03, respectively).
Figure 2. Association of BMI with FEV1/FVC according to classification by WHO with 95% confidence intervals. As independent explanatory covariates were age, height, sex, cumulative tobacco consumption, and smoking status included. p-value was yielded with Wald test. BMI = body mass index; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity.
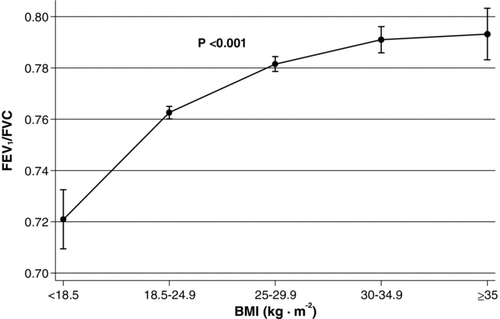
Figure shows influence of various parameters on the association between BMI and FEV1/FVC. We found a significant interaction of BMI and age when predicting FEV1/FVC ( p < 0.001), suggesting that the association of BMI with FEV1/FVC is more pronounced in the elderly. Similarly, a significant interaction between BMI and sex on FEV1/FVC was observed ( p = 0.006), suggesting that the association was more pronounced in men. Additionally, we also found a significant interaction between both smoking status ( p < 0.001) and cumulative tobacco consumption ( p = 0.002), suggesting that the association of BMI with FEV1/FVC is more pronounced in the subpopulation of current- and former-smokers and with increasing number of pack-years.
Figure 3. Importance of age, sex, smoking status, and cumulative tobacco consumption when BMI is predicting FEV1/FVC. In the interaction analyses regarding consumption, only current- and former-smokers were included. All models included if possible age, height, sex, cumulative tobacco consumption, and smoking status as independent explanatory covariates. p-values are for interaction and were yielded with Wald test. BMI = body mass index; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity.
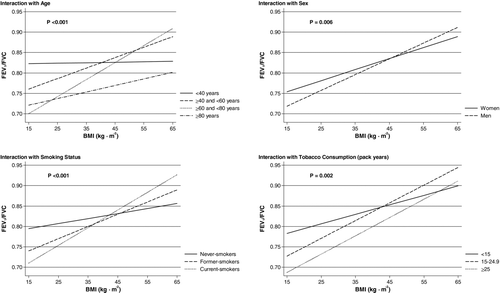
Table shows the relation between the presence of airflow limitation according to the four different criteria and BMI. In underweight subjects compared to normal weight subjects, the probability of airflow limitation was significantly higher with odds ratios (ORs) > 2.9. In contrast, among overweight, obese, and severely obese subjects, the probability of airflow limitation was significantly lower. The ORs were quite similar when airflow limitation was defined on the basis of fixed ratio or LLN, but weaker if our definition also required presence of respiratory symptoms and reduced FEV1% of predicted.
Table 3. Relation between BMI and the presence of airflow limitation according to different definitions
Discussion
We have found that weight, BMI, waist circumference, WHR, and abdominal height were positively and significantly associated with the FEV1/FVC ratio. Both in men and women, BMI was the strongest predictor of FEV1/FVC compared to other adiposity markers based upon R2 values obtained from the linear regression models. We could observe a difference of more than 0.07 in the FEV1/FVC ratio between the lowest and highest categories of BMI. As a consequence of this we suggest that individuals with overweight and obesity are at risk of being under-diagnosed with airflow limitation and thus also with COPD.
Additionally, the positive association of BMI with the FEV1/FVC ratio was most pronounced among current- and formers-smokers, which are subgroups with the highest á priori risk of COPD. We have also found a significant interaction with age, implying that the association was strongest among elderly, which could further add to the risk of under-diagnosis of COPD, as the diseases is most prevalent in this age-group. As the correct definition of airflow limitation is often a subject of dispute (Citation15–18), we have included four different definitions in the present analysis. Although we have observed a similar pattern with decreasing ORs for airflow limitation with increasing BMI using all four definitions in Table , the definitions based on FEV1/FVC < 0.70 and FEV1/FVC < LLN were most affected. Airflow limitation defined as the presence of both FEV1/FVC and FEV1% of predicted below LLN was least affected with increasing BMI. This suggests that in particular obese individuals with mild airflow limitation are at risk of remaining undiagnosed, whereas the risk is smaller for individuals with more severe grades of airflow limitation.
We believe that the positive association between BMI and the FEV1/FVC ratio mainly results from the mechanical effects of obesity. Obesity causes reductions in functional residual capacity and total lung capacity by making descent of the diaphragm difficult and compromises chest wall compliance, resulting in an extrathoracic restrictive ventilatory pattern (Citation19). Additionally, FVC decline is more pronounced compared to FEV1 resulting in an increase of FEV1/FVC (Citation8–12). Ochs-Balcom et al. (Citation11) found likewise a significant positive association of BMI with the FEV1/FVC ratio. In their study, among all adiposity markers including abdominal height, BMI was likewise the strongest predictor of FEV1/FVC.
It has been reported that increasing BMI was associated with restriction in men, while in women it was associated with obstruction in ventilatory pattern (Citation20), suggesting that variations in the amount and distribution of adipose tissue between men and women may affect the airways and lungs differently. In obese men, adipose tissue is often accumulated in the upper body (trunk, abdomen), whereas in obese women, adipose tissue is often accumulated in the lower body (hips, thighs) (Citation21). Perhaps with increasing BMI, men are more prone to a reduction in FVC than FEV1 compared to women due to the mechanical effects of increased truncal and abdominal obesity. In line with this, we could observe a significant interaction with sex, suggesting that men are more susceptible to the impact of BMI on the FEV1/FVC ratio compared to women, supporting previous findings (11, 22-27).
Previous longitudinal studies suggest that the association between BMI and lung function is reversible (20, 28–33). Whereas weight loss was associated with an improvement of both FEV1 and FVC, the opposite was seen in individuals who gained weight. These longitudinal findings are important and support that the mechanism linking BMI to the FEV1/FVC ratio is extrapulmonary restriction rather than intrapulmonary changes protecting overweight and obese individuals against development of airflow limitation and thus COPD. As overweight, obese, and severely obese individuals had higher levels of cumulative tobacco consumption compared to those with underweight and normal weight, we should have expected an even lower FEV1/FVC ratio and, additionally, increased prevalence of airflow limitation. However, the association between underweight and low FEV1/FVC might reflect a disease process within the lungs, as low BMI has been associated with a faster decline of FEV1 (Citation34), an increased risk of developing COPD (Citation35), and an increased respiratory mortality (Citation36–43). Yet, we cannot exclude the possibility of over-diagnosis of COPD in individuals with low or very low BMI.
Leone et al. (Citation27) had observed a significant association between metabolic syndrome and FEV1 and FVC after including different covariates (among others BMI). Interestingly, waist circumference which was the strongest predictor of the metabolic syndrome was both associated with obstructive and restrictive ventilatory pattern, which is in contradict with current finding as waist circumference was positively and significantly associated with the FEV1/FVC ratio. However, overweight and obesity might cause both restrictive ventilatory pattern by ways of mechanical effects and obstructive ventilatory pattern by ways of systemic inflammation contributed by deposition of visceral adipose tissue (Citation6).
We have also chosen not to exclude subjects with self-reported asthma in the present study. Obesity is considered as an independent risk factor for asthma (Citation44), although some have questioned the allegations of causality between obesity and asthma (Citation45). Lung volumes are reduced and asthma symptoms are increased in obesity, however, airflow limitation and airway hyperresponsiveness are not altered (Citation46). Additionally, weight loss has shown improvements in lung function and asthma symptoms (Citation47), but not necessarily in airflow limitation or airway hyperresponsiveness (Citation48). We have observed that the prevalence of self-reported asthma was highest in individuals with underweight, but also higher in individuals with overweight and obesity compared to individuals with normal weight (Table ). Yet, even in severe obesity, the prevalence of asthma was below 10% and as additional analyses excluding subjects with self-reported asthma did not change our results, we do not think that inclusion of individuals with asthma had an effect on our conclusions.
Our study has several potential limitations. We lack post-bronchodilatory values for FEV1 and FVC. Although this drawback has implications on the estimation of the prevalence of COPD in a population (Citation49), it does not weaken the notion of under-diagnosis in the overweight and obese individuals, as pre-bronchodilatory airflow limitation has to be present before suspicion of COPD can be raised and a bronchial reversibility test can be performed in order to confirm the diagnosis. Another limitation is the cross-sectional design, which weakens the conclusion on the possible mechanisms linking BMI with the FEV1/FVC ratio. Yet, the fact that previous longitudinal studies have documented the reversibility of spirometric indices following weight changes is in keeping with current results.
In conclusion, we have found a positive and significant association of adiposity markers, in particular BMI, with FEV1/FVC. High BMI reduces the probability of having airflow limitation. Ultimately, this may result in under-diagnosis and under-treatment of COPD among individuals with overweight and obesity. We encourage clinicians not to exclude the diagnosis of COPD in overweight and obese individuals with relevant tobacco exposure based solely on the absence of airflow limitation, in particular when FEV1/FVC ratio is in between 0.70 and 0.75.
Declaration of Interest Statement
The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and do not report anything relevant to this work.
Acknowledgments
The following authors are acknowledged: Study concept and design: Çolak, Marott, Vestbo, and Lange. Acquisition, analyses, or interpretation of data: Çolak, Marott, Vestbo, and Lange. Drafting of the manuscript: Çolak. Critical revision of the manuscript for important intellectual content: Çolak, Marott, Vestbo, and Lange. Statistical analyses: Çolak, Marott, and Lange. Obtained funding: Lange. Study supervision: Lange.
This study was funded by the Department of Internal Medicine, Herlev Hospital, Copenhagen University Hospital, and Department of Public Health, University of Copenhagen. The sponsors did not participate in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or in preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
References
- Mannino DM, Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2007 Oct 1; 4(7):502–506.
- Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA 2003 Jan 8; 289(2):187–193.
- Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 2009 May 26; 53(21):1925–1932.
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997 May 3; 349(9061):1269–1276.
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011 Feb 12; 377(9765):557–567.
- Franssen FM, O’Donnell DE, Goossens GH, Blaak EE, Schols AM. Obesity and the lung: 5. Obesity and COPD. Thorax 2008 Dec; 63(12):1110–1117.
- Cecere LM, Littman AJ, Slatore CG, Udris EM, Bryson CL, Boyko EJ, et al. Obesity and COPD: associated symptoms, health-related quality of life, and medication use. COPD 2011 Aug; 8(4):275–184.
- Lazarus R, Sparrow D, Weiss ST. Effects of obesity and fat distribution on ventilatory function: the normative aging study. Chest 1997 Apr; 111(4):891–898.
- Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Respir Dis 1983 Sep; 128(3):501–506.
- O’Donnell DE, Deesomchok A, Lam YM, Guenette JA, Amornputtisathaporn N, Forkert L, et al. Effects of BMI on static lung volumes in patients with airway obstruction. Chest 2011 Aug; 140(2):461–468.
- Ochs-Balcom HM, Grant BJ, Muti P, Sempos CT, Freudenheim JL, Trevisan M, et al. Pulmonary function and abdominal adiposity in the general population. Chest 2006 Apr; 129(4):853–862.
- van den Bemt L, van Wayenburg CA, Smeele IJ, Schermer TR. Obesity in patients with COPD, an undervalued problem? Thorax 2009 Jul; 64(7):640–641.
- Appleyard M ed. The Copenhagen City Heart Study. Østerbroundersogelsen. A book of tables with data from the first examination (1976-78) and a five year follow-up (1981-83). The Copenhagen City Heart Study Group. Scand J Soc Med 1989; 41 (Suppl.):1–160.
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894:i-253.
- Bhatt SP, Sieren JC, Dransfield MT, Washko GR, Newell JD, Jr., Stinson DS, et al. Comparison of spirometric thresholds in diagnosing smoking-related airflow obstruction. Thorax 2014 May; 69(5):410–415.
- Çolak Y, Løkke A, Marott JL, Lange P, Vestbo J. Impact of diagnostic criteria on the prevalence of COPD. Clin Respir J 2013 Jul; 7(3):297–303.
- Mohamed Hoesein FA, Zanen P, Lammers JW. Lower limit of normal or FEV1/FVC < 0.70 in diagnosing COPD: an evidence-based review. Respir Med 2011 Jun;105(6):907-15.
- van Dijk WD, Gupta N, Tan WC, Bourbeau J. Clinical relevance of diagnosing COPD by fixed ratio or lower limit of normal: a systematic review. COPD 2014 Feb; 11(1):113–120.
- O’Donnell DE, Ciavaglia CE, Neder JA. When obesity and chronic obstructive pulmonary disease collide. Physiological and clinical consequences. Ann Am Thorac Soc 2014 May; 11(4):635–644.
- Bottai M, Pistelli F, Di PF, Carrozzi L, Baldacci S, Matteelli G, et al. Longitudinal changes of body mass index, spirometry and diffusion in a general population. Eur Respir J 2002 Sep; 20(3):665–673.
- Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013 Jan; 93(1):359–404.
- Canoy D, Luben R, Welch A, Bingham S, Wareham N, Day N, et al. Abdominal obesity and respiratory function in men and women in the EPIC-Norfolk Study, United Kingdom. Am J Epidemiol 2004 Jun 15; 159(12):1140–1149.
- Chen R, Tunstall-Pedoe H, Bolton-Smith C, Hannah MK, Morrison C. Association of dietary antioxidants and waist circumference with pulmonary function and airway obstruction. Am J Epidemiol 2001 Jan 15; 153(2):157–163.
- Collins LC, Hoberty PD, Walker JF, Fletcher EC, Peiris AN. The effect of body fat distribution on pulmonary function tests. Chest 1995 May; 107(5):1298–1302.
- Harik-Khan RI, Wise RA, Fleg JL. The effect of gender on the relationship between body fat distribution and lung function. J Clin Epidemiol 2001 Apr; 54(4):399–406.
- Lazarus R, Gore CJ, Booth M, Owen N. Effects of body composition and fat distribution on ventilatory function in adults. Am J Clin Nutr 1998 Jul; 68(1):35–41.
- Leone N, Courbon D, Thomas F, Bean K, Jego B, Leynaert B, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med 2009 Mar 15; 179(6):509–516.
- Carey IM, Cook DG, Strachan DP. The effects of adiposity and weight change on forced expiratory volume decline in a longitudinal study of adults. Int J Obes Relat Metab Disord 1999 Sep; 23(9):979–985.
- Chen Y, Horne SL, Dosman JA. Body weight and weight gain related to pulmonary function decline in adults: a six year follow up study. Thorax 1993 Apr; 48(4):375–380.
- Chinn DJ, Cotes JE, Reed JW. Longitudinal effects of change in body mass on measurements of ventilatory capacity. Thorax 1996 Jul; 51(7):699–704.
- Chinn S, Jarvis D, Melotti R, Luczynska C, Ackermann-Liebrich U, Anto JM, et al. Smoking cessation, lung function, and weight gain: a follow-up study. Lancet 2005 May 7; 365(9471):1629–1635.
- Wang ML, McCabe L, Hankinson JL, Shamssain MH, Gunel E, Lapp NL, et al. Longitudinal and cross-sectional analyses of lung function in steelworkers. Am J Respir Crit Care Med 1996 Jun; 153(6 Pt 1):1907–1913.
- Wise RA, Enright PL, Connett JE, Anthonisen NR, Kanner RE, Lindgren P, et al. Effect of weight gain on pulmonary function after smoking cessation in the Lung Health Study. Am J Respir Crit Care Med 1998 Mar; 157(3 Pt 1):866–872.
- Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med 2008 Aug 15; 178(4):332–338.
- Harik-Khan RI, Fleg JL, Wise RA. Body mass index and the risk of COPD. Chest 2002 Feb; 121(2):370–376.
- Chailleux E, Fauroux B, Binet F, Dautzenberg B, Polu JM. Predictors of survival in patients receiving domiciliary oxygen therapy or mechanical ventilation. A 10-year analysis of ANTADIR Observatory. Chest 1996 Mar; 109(3):741–749.
- Chailleux E, Laaban JP, Veale D. Prognostic value of nutritional depletion in patients with COPD treated by long-term oxygen therapy: data from the ANTADIR observatory. Chest 2003 May; 123(5):1460–1466.
- Gray-Donald K, Gibbons L, Shapiro SH, Macklem PT, Martin JG. Nutritional status and mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996 Mar; 153(3):961–966.
- Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999 Dec; 160(6):1856–1861.
- Lindsted KD, Singh PN. Body mass and 26 y risk of mortality among men who never smoked: a re-analysis among men from the Adventist Mortality Study. Int J Obes Relat Metab Disord 1998 Jun; 22(6):544–548.
- Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998 Jun; 157(6 Pt 1):1791–1797.
- Sharp DS, Burchfiel CM, Curb JD, Rodriguez BL, Enright PL. The synergy of low lung function and low body mass index predicting all-cause mortality among older Japanese-American men. J Am Geriatr Soc 1997 Dec; 45(12):1464–1471.
- Singh PN, Lindsted KD. Body mass and 26-year risk of mortality from specific diseases among women who never smoked. Epidemiology 1998 May; 9(3):246–254.
- Ali Z, Ulrik CS. Obesity and asthma: a coincidence or a causal relationship? A systematic review. Respir Med 2013 Sep; 107(9):1287–1300.
- Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol 2005 May; 115(5):897–909.
- Schachter LM, Salome CM, Peat JK, Woolcock AJ. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax 2001 Jan; 56(1):4–8.
- Hakala K, Stenius-Aarniala B, Sovijarvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest 2000 Nov; 118(5):1315–1321.
- Aaron SD, Fergusson D, Dent R, Chen Y, Vandemheen KL, Dales RE. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest 2004 Jun; 125(6):2046–2052.
- Johannessen A, Omenaas ER, Bakke PS, Gulsvik A. Implications of reversibility testing on prevalence and risk factors for chronic obstructive pulmonary disease: a community study. Thorax 2005 Oct; 60(10):842–847.
