Abstract
Recent studies have recognized the genetic variants in the WW domain-containing oxidoreductase (WWOX) gene as genetic determinants of lung function, reflecting that the WWOX gene may be a susceptible factor of chronic obstructive pulmonary disease (COPD), which characters as poor lung function. We have previously showed that the copy number variation-67048 (CNV-67048) of WWOX was associated with lung cancer risk. Here, we hypothesized that the CNV-67048 affects COPD susceptibility. Based on a two-stage case-control study with a total of 1791 COPD patients and 1940 controls of southern and eastern Chinese, we found that the loss genotypes (0-copy and 1-copy) of CNV-67048 harbored a significantly increased risk of COPD, with an odds ratio (OR) as 1.29 (1.11–1.49) when compared with the common 2-copy genotype. The pre-forced expiratory volume in one second (pre-FEV1) to pre-forced vital capacity (pre-FVC) of carriers with loss genotypes (0.729 ± 0.130) was significantly lower than carriers with 2-copy genotype (0.747 ± 0.124; p = 7.93 × 10−5). However, no significant difference was observed on pre-FEV1, pre-FVC and the annual decline of pre-FEV1 between the loss genotypes and 2-copy genotype carriers. Our data suggest that the loss genotypes of CNV-67048 in WWOX predispose their carriers to COPD, which might be a genetic biomarker to predict risk of COPD in Chinese.
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most strikingly increasing lung diseases that threatens the life and health of world people. According to the latest WHO estimates, about 64 million people have COPD worldwide and more than 3 million people died of COPD in 2004–2005, which accounted for 5% of all deaths globally that year (Citation1). In China, approximate 8.2% of subjects who were 40 years and older suffer COPD (Citation2). COPD is now prevalent in China and it will grow exponentially in the coming years, driven by the high smoking rate and the aging of Chinese population, which is going to cause high burden on Chinese society.
Although COPD is most commonly caused by tobacco smoke, the ≠heritable factor has a determinant role on etiology of COPD, because only a small portion of smokers develop COPD (Citation3–5). Recently, multiple epidemiological studies, including genome-wide and candidate association studies, have revealed several susceptible loci in human genes for COPD (Citation6–8). These loci, most of which are single nucleotide polymorphisms (SNPs), are more or less associated with COPD or phenotypes of COPD, such as the forced expiratory volume in 1 second (FEV1), FEV1 to forced vital capacity ratio (FEV1/FVC) and decline of lung function. However, these SNPs only contribute to a small proportion of heritability of COPD as shown by the relatively small increments in risk they have (Citation9). Thus, a substantial proportion of heritability namely ìmissing heritabilityî remains unclear that may be due to other genetic variants such as copy number variation (CNV), which is a a form of structural variation covering more than 1 kb duplication or deletion, or insertion/deletion, which means the insertion or the deletion of bases in the DNA of an organism (Citation10).
CNV is a widespread and common phenomenon among humans, which accounts for roughly 12% of human genomic DNA (Citation11). Increasing evidences suggest that CNV might explain a large proportion of human ≠disease heritability (Citation12–14). For instance, the low copy number of FCGR3B can increase susceptibility to inflammatory autoimmune disorders (Citation15). We also have previously showed that the loss genotypes of WW domain-containing oxidoreductase (WWOX) CNV-67048 contributes to an increased risk of lung cancer (Citation16). However, until now, there is no study investigating the association between CNVs and COPD risk. It is well known that COPD and lung cancer are closely related with overlap of etiological factors on smoking and genetic susceptible loci (Citation17, 18).
It is worth noting that recent genome-association study identified the genetic variants in WWOX to influence lung function (Citation8). The variants in WWOX also affect susceptibility of lung cancer (Citation19). As an oxidoreductase, WWOX contains a short-chain dehydrogenase/reductase domain and participates in pathways involving oxidative stress (Citation20), and thus may play a role in development of COPD, because oxidative stress is a key etiological mechanism of COPD (Citation21). Oxidative stress is also a link between COPD and lung cancer (Citation21). Taken together, we hypothesized that the CNV-67048 in WWOX was associated with risk of COPD.
In the current study, we tested association between the CNV-67048 of WWOX and risk of COPD in a two-stage case-control study with a total of 1791 COPD patients and 1940 normal lung function controls of southern and eastern Chinese. We further assessed the effect of CNV-67048 on lung function.
Methods
Pulmonary function test and diagnosis of COPD
Pulmonary function test was performed by trained technicians with the EasyOne Spirometer (EasyOne Spirometer, ndd Medizintechnik AG, Switzerland) according to the criteria recommended by the American Thoracic Society (Citation22) and the Europe Respiratory Society (Citation23). The largest pre-bronchodilator FEV1 and FVC were chosen from at least three acceptable and two reproducible measurements which met the criteria for each individual. The post-bronchodilator Spirometry was obtained after inhalation of 400 μg of salbutamol for 20 minutes (≠Ventolin, GlaxoSmithKline) via a 500-ml spacer for those participants whose pre-FEV1/FVC <70%. Subjects with post-FEV1/FVC <70% and with at least one of some chronic airway symptoms, including chronic cough, dyspnea, sputum production or wheezing were identified to be COPD cases.
Study subjects
The study subjects have been described previously (Citation24, 25). In southern Chinese, we recruited 697 COPD patients from three communities (Liwan, Xicun and Zhanqian communities) in Guangzhou City, based on the cross-sectional surveys of COPD between February 2002 and June 2008 with a response rate of about 81%, and 328 cases from municipal hospitals in Guangzhou (the Guangzhou Chest Hospital, The Third Affiliated Hospital of Guangzhou Medical University, the Third Affiliated Hospital of Sun Yat-sen University) between December 2007 and June 2010 with a 91% response rate. We also randomly selected 1061 controls with age- (± 5 years) and sex-matched cases from the normal lung function individuals (i.e., pre-FEV1/FVC >70%) participating in the community cross-sectional survey of COPD.
In eastern Chinese, we enrolled 766 cases of COPD from the Second Affiliate Hospital of Soochow University in Suzhou city with a response rate of 83% during July 2009 to June 2011, and randomly picked up 879 normal pulmonary function controls, which were from a database consisting of 3,500 individuals based on a physical examination with an 81% response rate. In addition, the lung function of subjects recruited from Guangzhou community have been followed-up for years, and 427 subjects fully completed the 4-years’ follow-up with annual spirometric detection (Citation2, Citation26, Citation27). All the participants were genetically unrelated, ethnic Han Chinese. Having given a written informed consent, each participant was scheduled for an interview with a structured questionnaire that incorporated a standardized questionnaire revised from the international BOLD study and parts of questionnaires used in previous COPD studies in China (Citation28, 29).
The questionnaire covered demographic data, smoking status, biomass using, drinking status and respiratory symptoms as published previously (Citation2). Those participants who had smoked < 100 cigarettes in their lifetime were defined as never smokers; otherwise, they were classified as ever smokers. Similarly, participants who had consumed alcoholic beverages at least once a week for ≥ 1 year previously were defined as ever drinkers, and the remaining drinkers as never drinkers. Meanwhile, biomass as fuels was defined to those families who majorly used firewood to cook and get warm. The study was approved by the institutional review boards of Guangzhou Medical University and Soochow University.
CNV genotyping
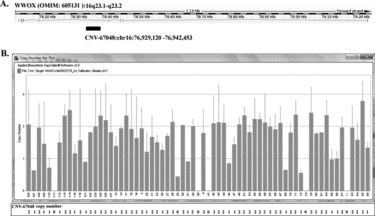
The CNV-67048 of WWOX (Figure ) was genotyped by the Taqman real-time quantitative PCR (qPCR) that has been previously described (Citation16). In brief, According to the protocol of Applied Biosystems (Citation30), a 10-μl ≠reaction system including the TaqMan gene copy ≠number assays for CNV-67048 (cat# Hs03922779), the control RNase P probe, the TaqMan PCR Master Mix and 10-ng DNA samples from each individual were ran on the ABI 7900HT fast real-time PCR System (Applied Biosystems) with the Sequence Detection Software (SDS, version 2.3, Applied Biosystems). Reactions were held at 95°C for 10 min and then cycled 40 times through 95°C for 15 s and 60°C for 1 min. Genotyping of each sample was done in triple at one time. The copy number of CNV-67048 was automatically determined using the software CopyCaller 2.0 (Applied BioSystems) (Figure ).
Statistical analysis
Comparisons in the distribution of CNV-67048 copy number as well as selected demographic variables between cases and controls were discussed by the chi-square tests. Association between CNV-67048 and COPD risk were tested using the unconditional logistic regression model without or with adjustment for age, sex, smoking status, biomass as fuels, drinking status and population. Trend test was also performed with the unconditional logistic regression model. A 10,000-times permutation test was used to estimate p values corrected after 10,000 times re-sampling to lower the sampling error. The REML model was used to assess the heritability explained by the genetic variants (Citation31). Briefly, the linear mixed-effects model with regard to unrelated individuals was applied to estimate the genetic variance attributable to the CNV-67048 based on the recessive model (i.e., the proportion of disease heritability explained by the variant, defined as hg2). hg2 was estimated as hg2 = Varg/(Varg+Vare), where Varg and Vare are the genetic and residual variance components estimated by the REML model using unrelated ≠individuals.
The Breslow–Day test was used to test the homogeneity of the results between stratum-ORs and frequency distribution of CNV-67048 genotypes between two populations. The sample size was estimated and the statistical power was calculated using the PS Software based on the formula for a case-control study (Citation32):
The sample size was calculated based on reported frequency of CNV-67048 genotypes in previously published lung cancer studies (the estimated frequency of loss genotypes of CNV-67048 in cases was 33.8% and in controls was 26.9% with a margin of error of less than 2%, 95% confidence intervals, 80% study power, and a design OR of 1.30) (Citation16). For 696 eligible cases, at least 696 controls are required. Furthermore, the student's t-test was used to analyze the effect of CNV-67048 on phenotypes of lung function, and the lineal regression with adjustment for potential confounding factors was introduced to assess the correlation between CNV-67048 copy number and lung function. All tests were two-sided by using the SAS software (version 9.3; SAS Institute, Cary, NC). p < 0.05 was considered to be ≠statistically significant.
Results
Demographics of the study population
As shown in Table , both the southern and eastern Chinese populations showed significant higher rate of smoking, more cigarette consumed and biomass fueling in cases than controls (p < 0.05 for all). No significant deviation was observed in distributions of age, sex and drinking status between cases and controls in both populations, except for the distribution of drinking status in the eastern Chinese (p = 0.028). Furthermore, the Breslow–Day test showed that the distributions of above factors in cases and controls were all in homogeneous between the two populations, except for biomass as fuels (p < 0.05), reflecting a minor different lifestyle between the two populations. In addition, according to the instruction of Global Initiative for COPD (Citation33), there were 359 (35.0%) cases of stage I, 356 (34.7%) cases of stage II, 217 (21.2%) cases of stage III, and 93 (9.1%) cases of stage IV in the southern Chinese, and 324 (42.5%) cases of stage I, 340 (44.6%) cases of stage II, 75 (9.9%) cases of stage III, and 23 (3.0%) cases of stage IV in the eastern Chinese.
Table 1. Frequency distributions of selected variables in COPD cases and controls
Association between the CNV-67048 copy number and COPD risk
Three genotypes, 2-copy, 1-copy and 0-copy were detected in all samples, and as shown in Table , there was a significant difference of CNV-67048 genotypes as well as alleles between COPD cases and controls in the southern Chinese population (p = 0.046 for genotypes; p = 0.017 for alleles). The copy number of CNV-67048 was significantly associated with increased COPD risks in a loss allele dose-dependent manner (p = 0.021). The dichotomic analysis further showed that the loss genotypes (1-copy + 0-copy) conferred a 23% increase in risk of COPD when compared with the common 2-copy (odds ratio (OR) =1.23, 95% confidence interval (95%CI) = 1.01–1.49; p = 0.038).
Table 2. Frequency distribution of CNV-67048 copy numbers and their associations with COPD risk
Similar results were observed in the eastern Chinese population, which were homogeneous with the results from the southern Chinese population (≠Breslow–Day test: p = 0.550). Compared with individuals with 2-copy, carriers with loss genotypes harbored 1.36-fold risk of COPD (OR = 1.36, 95%CI = 1.09–1.70; p = 0.007). We then combined the two populations to increase the study power and found that the loss genotypes of CNV-67048 exerted a 29% increase in risk for developing COPD (OR = 1.29, 95%CI = 1.11–1.49; p = 8.00 × 10−4). The permutation test confirmed the above association after correction for 10,000 times of re-sampling (corrected p = 0.001). Furthermore, the heritability test showed that the CNV could explain about 0.79% of COPD heritability.
WWOX CNV-67048 genotypes and lung function*
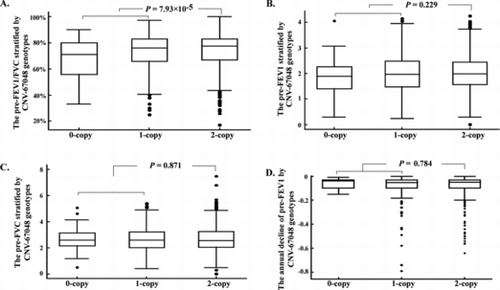
We analyzed correlation between the CNV-67048 copy numbers and phenotypes of COPD that are pre-FEV1, pre-FVC, pre-FEV1/FVC and the annual decline of pre-FEV1. We found that the individuals carrying loss genotypes (1-copy + 0-copy) had a significantly lower pre-FEV1/FVC than individuals carrying 2-copy genotype (0.729 ± 0.130 vs. 0.747 ± 0.124; t test: p = 7.93 × 10−5), and the copy number of CNV-67048 was negatively associated with pre-FEV1/FVC (linear ≠regression: p = 2.10 × 10−4; Figure ). However, no other significant differences in pre-FEV1 and pre-FVC between the genotypes of CNV-67048 (p > 0.05 for all; Figure , 2C). Moreover, although the mean decline of pre-FEV1 in carriers with loss genotypes (−0.099 ± 0.132) was higher in carriers with 2-copy genotype (−0.089 ± 0.107), the difference was not significant (p = 0.784; Figure ).
Discussion
In this two-stage case-control study investigating an association between the CNV-67048 of WWOX and COPD risk, we showed that the loss genotypes of CNV-67048 conferred a significant increase in risk for developing COPD. Meanwhile, the loss genotypes carriers were more likely to have a poor lung function than the 2-copy genotypes carriers. The CNV could explain about 0.79% of COPD heritability. To the best of our knowledge, this is the first study of revealing a CNV to be a susceptible marker for developing COPD.
Previous genome-wide association study had identi≠fied the genetic variants in WWOX to be associated with lung function, suggesting WWOX to be a candidate susceptible gene for COPD. WWOX functions as an oxidoreductase, which plays an important role in the regulation of a wide variety of cellular functions such as protein degradation, transcription, and RNA splicing. WWOX is important for antioxidant defense during oxidative stress, which is a major pathogenesis of COPD (Citation34).
Stimulus such as tobacco smoking could directly trigger oxidative stress by virtue of decrease in WWOX expression (Citation35, 36). WWOX also can interact with P53 (Citation37), which is an important regulator involving the pathogenesis of COPD (Citation38). In previous study, we have reported that the CNV-67048 was functional that the loss genotypes could decrease the WWOX expression in lung tissues (Citation16). Here we found that the CNV-67048 was associated with COPD risk. Also, individuals ≠carrying the loss genotypes exerted significant lower pre-FEV1/FVC than subjects carrying the 2-copy genotype. The loss genotypes of CNV-67048 can cause a down-regulation of WWOX expression, thus influence the antioxidant role of WWOX on resisting oxide ≠stimulation, and finally predispose their carriers to develop COPD. Anyway, all these suggested that the CNV-67048 in WWOX may be a genetic biomarker to predict risk of COPD.
Multiple studies have identified several susceptible loci (i.e., SNPs) of COPD. However, ìmissing heritabilityî still exists because these SNPs only explain a small proportion of heritability of COPD (Citation9). Compared to SNP, CNV is a form of structural variation that covers more than 1 kb duplication or deletion. CNV can directly alter gene dosage, disrupt gene structure and thus influence gene expression and phenotypic variation, and indirectly regulate gene function through position effects, predispose to deleterious genetic changes, or provide substrates for chromosomal change in evolution and thus confer risk to complex disease traits (Citation39–41). Several CNVs have been reported to be associated with susceptibility and prognosis to various human diseases. Up to now, the only research object of CNV and COPD is that, the authors used the comparative genomic hybridization arrays (aCGH) techniques to detect CNVs with different frequencies in lung cancer patients with and without COPD, and they supported that the losses of 8p23 region is a predictor for prognosis of COPD (Citation42). However, the loss of 8p23 is just a somatic mutation in cancer tissues. Therefore, to the best of our knowledge, the current study is the first study to show a germline CNV to be associated with COPD risk.
Based on a case-control study, there were some limitations in the current study. On the account of the fact that part of the COPD cases were recruited from the communities and information on selected factors were collected by questionnaire survey, bias including prevalence-incidence bias, recall bias and interviewer bias may exist. Meanwhile, COPD diagnosis based on post-FEV1/FVC < 0.7 might lead to overdiagnosis in elderly subjects, and thus cause selection bias. These biases may cause error results on association between the CNV and COPD risk. Moreover, this study is also restricted to the Chinese population, so it is uncertain whether our findings can be generalized to other populations. However, our study has some strength. The results from southern and eastern Chinese subjects were consistent. The study power is strong as 93.1% (two-sided test, α = 0.05) to detect an OR of 1.29 for the loss genotypes of CNV-67048 (which occurred at a frequency of 26.1% in the controls) compared with the 2-copy genotype. In addition, the CNV-67048 exerted an effect on phenotype of lung function. All these support that our finding that the CNV's significant association with COPD was not achieved by chance.
Conclusion
This study revealed the functional CNV-67048 in WWOX to be associated with risk of COPD as well as lung function in Chinese. The CNV-67048 may be a genetic biomarker for susceptibility to COPD. Validations with larger population-based studies in different ethnic groups and mechanism studies are warranted.
| Abbreviations | ||
| C.I. | = | Confidence interval |
| CNV | = | copy number variation |
| WWOX | = | WW domain-containing oxidoreductase |
| LOH | = | Loss of heterozygosis |
| MAF | = | minor allele frequency |
| OR | = | odds ratio |
| PCR | = | polymerase chain reaction |
Declaration of Interest Statement
This study was supported by the National Natural ≠Scientific Foundation of China grants 30671813, 30872178, 81072366, 81273149, 81473040 (J. Lu), 81402753 (L. Yang), and partly by 81001278 and 81171895 (Y. Zhou); the Guangdong Provincial Scientific Research Grants 8251018201000005(J. Lu), Guangdong Provincial High Level Experts Grants 2010-79, Changjiang Scholars and Innovative Research Team in University Grant IRT0961 and Guangdong Natural Science Foundation team grant 10351012003000000 (J. Lu). The authors alone are responsible for the content and writing of the paper.
Acknowledgements
We thank Dr. Zhanhong Xie, Ms. Wanmin Zeng and Ling Liu for their assistance in recruiting the subjects.
References
- Mathers C, Boerma T, Fat DM, et al. The global burden of disease: 2004 update, 2008. World Health Organization.
- Zhong N, Wang C, Yao W, Chen P, Kang J, Huang S, Chen B, Wang C, Ni D, Zhou Y, Liu S, Wang X, Wang D, Lu J, Zheng J, Ran P. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med 2007; 176:753–760.
- Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet 2012; 379: 1341–1351.
- Bosse Y. Genetics of chronic obstructive pulmonary disease: a succinct review, future avenues and prospective clinical applications. Pharmacogenomics 2009; 10:655–667.
- Sandford AJ, Joos L, Pare PD. Genetic risk factors for chronic obstructive pulmonary disease. Curr Opin Pulm Med 2002; 8:87–94.
- Wilk JB, Shrine NR, Loehr LR, Zhao JH, Manichaikul A, Lopez LM, Smith AV, Heckbart SR, Smolonska J, Tang W, Loth DW, and over 50 other authors. Genome-wide association studies identify CHRNA5/3 and HTR4 in the development of airflow obstruction. Am J Respir Crit Care Med 2012; 186:622–632.
- Cho MH, Castaldi PJ, Wan ES, Siedlinski M, Hersh CP, Demeo DL, Himes BE, Sylvia JS, Klanderman BJ, Ziniti JP, Lange C, Litonjus AA, Sparrow D, Regan EA, Make BJ, Hokanson JE, Murray T, Hetmanski JB, Pillai SG, Kong X, Anderson WH, Tal-Singer R, Lomas DA, Coxson HO, Edwards LD, MacNee W, Vesto J, Yates JC, Augusti A, Calverley PMA, Celli B, Crim C, Rennard S, Wouters E, Bakke P, Gulsvik A, Crapo JD, Beaty TH, Silverman EK, and on behalf of the ICGN, ECLIPSE, and COPDGene Investigaotrs. A genome-wide association study of COPD identifies a susceptibility locus on chromosome 19q13. Hum Mol Genet 2012; 21:947–957.
- Soler Artigas M, Loth DW, Wain LV, Gharib SA, Obeidat M, Tang W, Zhai G, Zhao JH, Xmith AV, Huffman JE, Albrecht E, Jackson CM, Evans DM, Cadby G, Fornage M, Manichaikul A, Lopez LM, Johnson T, Aldrich MC, Aspelund T, and over 100 more. Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet 2011; 43:1082–1090.
- So HC, Gui AH, Cherny SS, Sham PC. Evaluating the heritability explained by known susceptibility variants: a survey of ten complex diseases. Genet Epidemiol 2011; 35:310–317.
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravartai A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature 2009; 461:747–753.
- Stankiewicz P, Lupski JR. Structural variation in the human genome and its role in disease. Annu Rev Med 2010; 61:437–455.
- Zhang F, Seeman P, Liu P, Weterman MAJ, Gonzaga-Jauregui C, Towne CF, Batish SD, DeVriendt E, De Jonghe P, Rautenstrauss B, Krause K-H, Khajavi M, Posadka J, Vandenberghe A, Palau F, Can Maldergem L, Baas F, Timmerman V, Lupski JR. Mechanisms for nonrecurrent genomic rearrangements associated with CMT1A or HNPP: rare CNVs as a cause for missing heritability. Am J Hum Genet 2010; 86:892–903.
- Eichler EE, Flint J, Gibson G, Kong A, Leal SM, Moore JH, Nadeau JH. Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet 2010; 11:446–450.
- Krepischi AC, Pearson PL, Rosenberg C. Germline copy number variations and cancer predisposition. Future Oncol 2012; 8:441–450.
- Aitman TJ, Dong R, Vyse TJ, Norsworthy PJ, Johnson MD, Smith J, Mangion J, Roberton-Lowe C, Marshall AJ, Petretto E, Hodges MD, Bhangal G, Patel SG, Sheehan-Rooney K, Duda M, Cook PR, Evans DJ, Domin J, Flint J, Boyle JJ, Pusey Cd, Cook HT. Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature 2006; 439:851–855.
- Yang L, Liu B, Huang B, Deng J, Li H, Yu B, Qiu F, Cheng M, Wang H, Yang R, Yang X, Zhou Y, Lu J. A functional copy number variation in the WWOX gene is associated with lung cancer risk in Chinese. Hum Mol Genet 2013; 22:1886–1894.
- Schwartz AG, Ruckdeschel JC. Familial lung cancer: genetic susceptibility and relationship to chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173:16–22.
- Young RP, Hopkins RJ. How the genetics of lung cancer may overlap with COPD. Respirology 2011; 16:1047–1055.
- Huang D, Qiu F, Yang L, Li Y, Cheng M, Wang H, Ma Gn, Wang Y, Hu M, Ji W, Zhou Y, Lu J. The polymorphisms and haplotypes of WWOX gene are associated with the risk of lung cancer in southern and eastern Chinese populations. Mol Carcinog 2013; 52 Suppl 1: E19–27.
- O'Keefe LV, Colella A, Dayan S, Chen Q, Choo A, Jacob R, Price G, Venter D, Richards RI. Drosophila orthologue of WWOX, the chromosomal fragile site FRA16D tumour suppressor gene, functions in aerobic metabolism and regulates reactive oxygen species. Hum Mol Genet 2011; 20:497–509.
- Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer 2013; 13:233–245.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegl G, Wanger J. Standardization of spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995; 152:1107–1136.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J 2005; 26:948–968.
- Huang D, Yang L, Liu Y, Zhou Y, Guo Y, Pan M, Wang Y, Tan Y, Zhong H, Hu M, Lu W, Ji W, Wang J, Ran P, Zhong N, Zhou Y, Lu J. Functional polymorphisms in NF-κB1/IκBα predict risks of chronic obstructive pulmonary disease and lung cancer in Chinese. Hum Genet 2013; 132:451–460.
- Yang L, Qiu F, Lu X, Huang D, Ma G, Guo Y, Hu M, Zhou Y, Pan M, Tan Y, Zhong H, Ji W, Wei Q, Ö Lu J. Functional polymorphisms of CHRNA3 predict risks of chronic obstructive pulmonary disease and lung cancer in Chinese. PLoS One 2012; 7:e46071.
- Zhou Y, Hu G, Wang D, Wang S, Wang Y, Liu Z, Hu J, Shi Z, Peng G, Liu S, Lu J, Zheng J, Wang J, Zhong N, Ran P. Community based integrated intervention for prevention and management of chronic obstructive pulmonary disease (COPD) in Guangdong, China: cluster randomised controlled trial. Br Med J 2010; 341:c6387.
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Manning DM, Menezes AMB, Sullivan SD, Lee TA, Weiss KB, Jensen RL, Marks GB, Gulsvik A, Nizankowska-Mogilnicka E, and the BOLD Collaborative Research Group. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007; 370:741–750.
- Buist AS, Vollmer WM, Sullivan SD, et al. The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD 2005; 2:277–283.
- Cheng X, Li J, Zhang Z. [Analysis of basic data of the study on prevention and treatment of COPD and chronic cor pulmonale]. Zhonghua Jie He He Hu Xi Za Zhi 1998; 21:749–752.
- Mayo P, Hartshorne T, Li K, et al. CNV analysis using TaqMan copy number assays. Curr Protoc Hum Genet 2010; 67: 2.13:2.13.1–2.13.10.
- Vattikuti S, Guo J, Chow CC. Heritability and genetic correlations explained by common SNPs for metabolic syndrome traits. PLoS Genet 2012; 8:e1002637.
- Dupont WD, Plummer Jr WD. Power and sample size calculations for studies involving linear regression. Contr Clin Trials 1998; 19:589–601.
- Vestbo J, Hurd SS, AgustÌ AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347–365.
- Rosanna DP, Salvatore C. Reactive oxygen species, inflammation, and lung diseases. Curr Pharm Des 2012; 18:3889–3900.
- Godschalk R, Curfs D, Bartsch H, Van Schooten FJ, Nair J. Benzo[a]pyrene enhances lipid peroxidation induced DNA damage in aorta of apolipoprotein E knockout mice. Free Radic Res 2003; 37:1299–1305.
- Thavathiru E, Ludes-Meyers JH, MacLeod MC, Aldaz CM. Expression of common chromosomal fragile site genes, WWOX/FRA16D and FHIT/FRA3B is downregulated by exposure to environmental carcinogens, UV, and BPDE but not by IR. Mol Carcinog 2005; 44:174–182.
- Chang NS, Pratt N, Heath J, Schultz L, Sleve D, Carey GB, Zevotek N. Hyaluronidase induction of a WW domain-containing oxidoreductase that enhances tumor necrosis factor cytotoxicity. J Biol Chem 2001; 276:3361–3370.
- Dagouassat M, Gagliolo JM, Chrusciel S, Bourin MC, Duprez C, Caramelle P, Boyer L, Hue S, Stern JB, Validire P, Longrois D, Norel X, Dubois-Randè J, Le Gouvello S, Adnot S, Bockzkowski J. The cyclooxygenase-2-prostaglandin E2 pathway maintains senescence of chronic obstructive pulmonary disease fibroblasts. Am J Respir Crit Care Med 2013; 187:703–714.
- Feuk L, Carson AR, Scherer SW. Structural variation in the human genome. Nat Rev Genet 2006; 7:85–97.
- McCarroll SA, Hadnott TN, Perry GH, Sabeti PC, Zody MC, Barrett JC, Dallaire S, Gabriel SB, Lee C, Daly MJ, Altshuler DM, International HapMap Consortium. Common deletion polymorphisms in the human genome. Nat Genet 2006; 38:86–92.
- Feuk L, Marshall CR, Wintle RF, Scherer SW. Structural variants: changing the landscape of chromosomes and design of disease studies. Hum Mol Genet 2006; 15 Spec No 1: R57–66.
- Lin H, Zheng Y, Ping Z, Li A. Using comparative genomic hybridization arrays (aCGH) techniques to detect chronic obstructive pulmonary disease related susceptibility regions. Intern J Bio-Sci Bio-Technol 2013; 5:121–130.