Abstract
The alpha-1 antitrypsin deficiency (AATD) targeted screening program, together with the National Registry, were established in Poland in 2010 soon after the AATD diagnostics became available. Between 2010 and 2014 a total of 2525 samples were collected from respiratory patients countrywide; 55 patients with severe AAT deficiency or rare mutations were identified and registered, including 36 PiZZ subjects (65%). The majority of AATD patients were diagnosed with COPD (40%) or emphysema (7%), but also with bronchial asthma (16%) and bronchiectasis (13%). Therefore, the registry has proved instrumental in setting-up the AATD-dedicated network of respiratory medical centres in Poland. Since augmentation therapy is not reimbursed in our country, the smoking cessation guidance, optimal pharmacotherapy of respiratory symptoms as well the early detection, and effective treatment of exacerbations is absolutely essential.
Introduction
Although in Western European countries algorithms for identification and management of alpha-1 antitrypsin (AAT) deficient patients are well established, in most of the Central Eastern Europe awareness of the inherited AAT deficiency (AATD) is still quite low among physicians and general population. Similarly, the diagnostic protocols are neither known nor sufficiently implemented. Due to the lack of reliable epidemiological data from this region, the AATD is usually not recognized as an issue by the national healthcare systems. Consequently, in most countries, no systemic approach on the regional or country level for AATD diagnostics and medical care has been developed.
In this regard Poland has not been different until very recently. Diagnostic of AATD was not possible; therefore no AATD patients were identified. The general understanding of the problem among medical professionals was either poor or non-existent.
Scarce epidemiological data regarding AATD prevalence in Poland did not help to change this situation. AATD incidence in overall Polish population of 38 million had been randomly studied, mostly in the 1990s (Citation1–5). However, the number of subjects analysed per study (n = 423–859) was noticeably low, research programs were restricted mostly to two regions in Western and Southern Poland, and laboratory methods applied for deficiency confirmation differed significantly. Accordingly, the incidence of AAT deficiency alleles reported by respective studies varied considerably. For example, the expected prevalence of PI*Z allele ranged between 6.7 to 14.2 per 1.000, while for the PI*S allele, the range was between 9.4 to 17.5 per 1000. Not surprisingly, these facts precluded reliable interpretation and therefore affected any significant medical or public impact. It was generally accepted that the incidence of the severe AAT deficiency in the Polish population did not warrant any specific detection programs.
Thus prior to 2009, AATD identification was available in Poland only as a part of scientific research projects. No routine diagnostic programs were active nor were AATD oriented management plans established. This situation changed when the AATD Reference Diagnostic Centre was established by our laboratory at the National Institute of Tuberculosis and Lung Diseases in Warsaw. From the very beginning, the complete range of diagnostic methods was offered to ensure reliable identification of a wide range of SERPINA 1 gene mutations, i.e., nephelometry, pheno- and genotyping and sequencing. In 2010 the targeted screening program for patients with respiratory obstructive diseases was launched in cooperation with 6 major respiratory university hospitals countrywide. The parallel screening project targeting patients with severe liver disorders had been active at the Warsaw Liver Transplant Centre between 2010–2012 (Citation6).
The National Registry for AAT patients in Poland has been established in 2010. It is based at the Department of Genetics and Clinical Immunology, National Institute of Tuberculosis and Lung Diseases in Warsaw, which serves as well as the core AATD laboratory for the country. Here we present the current status of the National Registry, including patients identified within the respiratory targeted screening in Poland between 2010–2014.
Methods
The registry
The registry resources include a principal coordinator, consultant committee consisting of 6 respiratory specialists and computer support staff. The registry complies with all existing data protection legislation and has been approved by respective Polish authorities. The registry is financially supported by its home institution, the National Institute of Tuberculosis and Lung Diseases as well as by the Polish Foundation for the Patients with Alpha-1 Antitrypsin Deficiency.
Material
Peripheral blood serum and/or dry blood spot (DBS) samples on filter paper (Whatman #903) were prepared as described previously (Citation7).
Diagnostic methods
AAT concentration assessment
The analysis of alpha-1 antitrypsin level in serum and DBS material was performed by the nephelometric method (Immage 800, Beckman Coulter).
Phenotyping
AAT phenotype analysis in serum/DBS was performed by isoelectrophocusing on polyacrylamide gel using an Multiphor II Electrophoresis System (GE Healthcare Bio-Sciences AB) (2010–2012) or on agarose gel, using the Hydrasys electrophoresis system (Sebia) and the Hydragel 18 A1AT Isofocusing kit (Sebia) utilising immunofixation and specific antibody to AAT (2012–2014).
Genotyping
Genomic DNA was extracted from DBS using a commercially available kit Extract-N-Amp Blood PCR Kits (Sigma-Aldrich). Genetic material present in the DBS eluate was directly used for A1AT genotyping without need of DNA purification from blood.
The identification of two most common mutations of the A1AT gene (Z, S) was performed in a single reaction by real-time PCR method in the LightCycler 480 II instrument (Roche Diagnostics Ltd., Switzerland) using hydrolysing probes coupled with fluorescent dyes (VIC or FAM) complementary to the mutant variants (PI*S or PI*Z). Primer and probe sequences as well as PCR reaction conditions were previously described by Struniawski et al. (Citation7).
AAT gene sequence analysis
Diagnosis of rare AAT variants was established by direct sequencing. Sequence analysis of AAT exons 2–4 was performed at 16-capillary 3130xl Genetic Analyzer (Applied Biosystems, USA) (Genomed, Poland).
Diagnostic algorithm
The diagnostic algorithm was adopted from the Polish Respiratory Society guidelines (Citation8). Accordingly, all samples were routinely analysed for AAT concentration, followed by AAT phenotyping if AAT level proved below cut-off level of 120 mg/ml in serum or DBS equivalent. Presence of Z and S alleles was confirmed by genotyping, while rare mutations were confirmed by sequencing.
Results
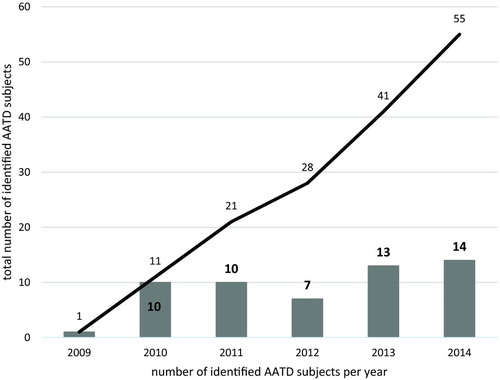
In total, 2525 samples have been evaluated within the ongoing targeted screening program for respiratory patients between 2010–2014 (Figure ).
As of December 31, 2014, the registry database included 55 patients with severe AATD phenotype, mostly PiZZ (n = 36, 65%) or with rare AAT mutations (Table ). Individuals with FM (n = 9, 16.4%) or IM (n = 5, 9.1%) heterozygosity had been registered as well to follow up their geographical distribution as well as the natural history of disease. Incidentally, most of the identified FM patients originate from the north or north-eastern part of Poland.
Table 1. Baseline demographics for the main groups of patients in the registry
The average age at diagnosis for all AATD patients in the registry was 54.6 ± 13.2 years, while for ZZ subjects it was 49.4 ± 14.5 years. More than a half (55.8%) of AATD subjects in the database has a history of smoking. Again, ZZ homozygotes differ significantly with 78% of ever-smokers.
Accordingly, the majority of ZZ patients was diagnosed with COPD (43% vs 40% in all registered AATD subjects) and emphysema (11% vs 7%), but also bronchial asthma (13% vs 16%), bronchiectasis (11% vs 13%) and other disorders, (granulomatosis with poliangitis, spontaneous pneumothorax, panniculitis) (16.5% vs 24%). Three PiZZ subjects (5.5%) did not present any clinical symptoms, nor lung fuction or liver abnormalities. Consequently, the mean FEV1/FVC ratio in PiZZ group was 45 ± 20%. Interestingly, diagnosis of COPD prevailed in the FM and IM subgroups (66% in both) with FEV1/FVC of 55 ± 25% and 49 ± 24%, respectively. Although family screening is available and generally accepted by the next of kin of AATD patients, only 2% of registered AATD were non-index cases.
Discussion
The respiratory targeted screening program in Poland has been launched in 2010 soon after establishment of the core AATD laboratory in Warsaw. In parallel, the Polish Respiratory Society published recommendations on AATD diagnostics and treatment, the joint effort of specialists in respiratory and liver disorders (Citation8). It was the very first document to provide Polish medical professionals with clear guidelines regarding the indications for AAT targeted screening and diagnostic algorithm. Widely presented at that time, the recommendations proved to be invaluable in increasing awareness of AATD in Poland.
The next milestone in the targeted screening program transpired in 2011 when the new method of blood collection for AATD diagnostics was introduced (Citation7). The Dry blood spot (DBS) on filter paper technique enabled low-invasive sampling, helped decrease the costs and simplified material shipment. Yet, it was the fact that Warsaw laboratory performed full AATD diagnostics from DBS samples, including the very first step, AAT blood concentration assessment, that proved to be the fundamental change. The AAT serum concentration measurement has been apparently problematic in many parts of Poland, particularly outside major medical centres. Consequently, DBS introduction enabled access to the screening program for the considerable number of regional medical practices countrywide.
The interim analysis of the Polish targeted screening program performed in June 2014 demonstrated that in this cohort approximately 13% subjects had AATD alleles. The detection frequency for S and Z alleles was 4 times that of the general population in Poland, while the frequency of homozygous Pi*ZZ was 16 times that of the general population (Citation9). The comparative analysis was based on the published data regarding AATD incidence in general Polish population. According to the meta-analysis of 5 major epidemiological studies ever performed in Poland, the calculated average prevalence of PI*Z allele was established at 14.5 per 1.000 and that of PI*S allele at 10.9 per 1.000 in a combined population of 2.653 subjects yet analysed by means of a wide range of laboratory techniques (Citation5). Consequently, this part of the interim analysis should be accepted with certain caution. Moreover, the preliminary data from recently concluded large-scale research screening program in newborns from Central Poland accomplished between 2011–2014 suggest that the prevalence of the main deficiency phenotype PiZZ (1 per 5.345) in the general Polish population might be significantly higher, as compared to previously published values (1 per 9.110) (Citation5, Citation10).
Although the Polish AATD registry is still limited in numbers, the patients’ demographic and clinical characteristics do not seem to differ significantly from other European registries. Male patients prevail, both in general (64%) and PiZZ (67%) groups. Likewise, most of the registered individuals present the history of smoking, respectively 55.8% and 78%. The ever-smokers’ proportion within respective phenotypic subgroups is quite clearly related to the gender distribution. The higher male proportion the lower the number of never-smokers in the group. Incidentally, in Poland, the smoking habit is more prevalent in men (34%) than women (22%), particularly in the middle-aged (40–59 years) generation (37%) (Citation11). Not surprisingly COPD/emphysema are the most prevalent clinical presentations both in all (47%) and ZZ homozygous (54%) individuals. Interestingly, the average age at diagnosis in Polish AATD COPD patients (49.4 ± 14.5 years) is very similar to some other European countries. According to recent reports in Italy, the average age at diagnosis is 49 ± 14. Still, in Spain and Germany newly-diagnosed AATD patients are younger, 45.4 ± 14.6 and 45.5 ± 10.9 respectively (Citation12, 13).
Two facts are of considerable concern. The extremely low number of non-index cases, only 1 subject registered so far, suggest potential problems in communication between medical personnel and patients, as well as remaining very low awareness in general Polish population regarding AATD.
Likewise, the number of registered AATD patients ever treated with augmentation therapy is highly inadequate. The augmentation therapy has been registered in Poland in 2007 but until today has not been reimbursed. Consequently, it is available for the patients mostly within the clinical trials. Accordingly, only 3 out of 36 PiZZ patients had been ever on the augmentation therapy.
This situation determines the key role of the registry in providing Polish AATD patients with the best medical care available. The currently existing network enables severe AATD patients to have direct access to pre-determined respiratory centres to ensure the pharmacotherapy optimization, early detection and effective treatment of exacerbations. A considerable number of patients are cared for by the Warsaw Institute; however, considerable effort has been made to ensure the involvement of other respiratory centres to build up the AATD – dedicated network (Citation14).
In summary, the Polish experience with AATD registry is relatively short. We are still at the starting point towards creating an effective system of medical care for patients with AATD deficiency. Consequently, apart from the well-organized diagnostic and clinical network, much effort needs to be invested into increasing awareness of medical professionals as well as healthcare providers. In those fields should the AATD registry prove to play an instrumental role.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Titenko-Holland NV, Kowalska A. Alpha-1-antitrypsin (PI) subtypes in Russians and Poles. Hum Hered 1992; 42:384–386.
- Walter H, Danker-Hopfe H, Lemmermann M, Lorenz M. Investigations on the variability of four genetic serum protein markers in Poland. Z Morphol Anthropol 1992; 79:203–214.
- Kowalska A, Rujner J. Polymorphism locus Pi (alpha1-antitrypsin) of residents in the Poznan province. Pol Tyg Lek 1994; 49:195–197.
- Kowalska A, Rujner J, Titenko-Holland NV, Pilacik B. Alpha-1-antitrypsin subtypes in Polish newborns. Hum Hered 1995; 45:351–354.
- Kaczor MP, Sanak M, Libura-Twardowska M, Szczeklik A. The prevalence of alpha1-antitrypsin deficiency in a representative population sample from Poland. Respir Med 2007; 101:2520–2525.
- Górska K, Korczyński P, Struniawski R, et al. Heterozygous alpha-1 antitrypsin deficiency in patients evaluated as live transplant condidates. Pol Arch Int Med 2013; 123:14–20.
- Struniawski R., Szpechcinski A., Poplawska B, et al. Rapid DNA extraction protocol for the alpha-1 antitrypsin deficiency detection from dried blood spots by real-time PCR. Adv Exp Med Biol 2012; 756:29–37.
- Chorostowska-Wynimko J, Nizankowska-Mogilnicka E, et al. Diagnosis and treatment of patients with alpha-1 antitrypsin (alpha-1 AT) deficiency. Pneumonol Alergol Pol 2010; 78:348–355.
- Chorostowska-Wynimko J. Looking beyond COPD: improving the management of alpha-1 patients. Eur Resp Rev 2015 (in press).
- Chorostowska-Wynimko J, Struniawski R, Popławska B, et al. The incidence of alpha-1-antitrypsin (A1AT) deficiency alleles in population of Central Poland– preliminary results from newborn screening. Pneumonol Alergol Pol 2012; 80:450–453.
- Włodarczyk A, Raciborski F, Opoczyńska D, et al. Daily tobacco moking atterns in rural and urban areas of oland–the results of the GATS study. Ann Agric Environ Med 2013; 20:588–594.
- Piras B, Ferrarotti I, Lara B, et al. Clinical phenotypes of Italian and Spanish patients with α1-antitrypsin deficiency. Eur Respir J 2013; 42:54–64.
- Köhnlein T, Janciauskiene S, Welte T. Diagnostic delay and clinical modifiers in alpha-1 antitrypsin deficiency. Ther Adv Respir Dis 2010; 4:279–287.
- Chorostowska-Wynimko. Believe in miracles–Central-Eastern European Alpha-1 Antitrypsin Network. Pneumonol Alergol Pol 2013; 81:285–287.