Abstract
Background: Lung transplantation is a therapeutic option for patients with end-stage lung disease and a survival benefit has been described in patients with alpha-1-antitrypsin deficiency (A1ATD). The aims of the current study were to determine the survival and health benefits of lung transplantation in UK patients with A1ATD compared to carefully matched non-transplant patients.
Methods: Patients with the PiZZ (alpha-1-antitrypsin deficiency) genotype who had undergone lung transplantation between 1996 and 2011 were identified from the UK A1ATD registry. Lung physiology, health status and survival were compared pre- and post-transplant using carefully matched non-transplant patients.
Results: Thirty-two A1ATD patients who had undergone lung transplant were identified. Lung function decline pre-transplant was not different to the closely matched non-transplanted cohort. The transplant group pre-transplant, although matched for FEV1, had lower gas transfer measurements, (mean KCO% predicted 41.0% SE ± 3.86 vs 55.6% SE ± 3.10 p < 0.001) and worse health status (SGRQ mean score 64.2 SE ± 2.5 vs 55.3 SE ± 2.0, p < 0.001). Post-transplant, physiology and health status improved significantly (p < 0.002). However, the post-operative mortality over 5 years was no better than for a second group of non-transplant patients further matched for gas transfer or a third group also matched for SGRQ.
Conclusion: Patients who underwent lung transplant had lower gas transfer and quality-of-life pre-transplant compared to non-transplant patients matched for FEV1, age and sex, suggesting that these parameters provide extra information helpful in decision making. Lung transplantation for A1ATD patients significantly improves quality-of-life but not survival.
Introduction
Alpha-1-antitrypsin deficiency (A1ATD) is an autosomal co-dominant serine proteinase inhibitor deficiency, which predisposes to the early onset of emphysema, through the unopposed action of excess neutrophil elastase in the lungs (Citation1). It may account for 1–2% of younger (<50 years) cases of chronic obstructive pulmonary disease (COPD) particularly in northern Europe (Citation2). Patients with A1ATD have a wide spectrum of clinical phenotypes as is seen in usual COPD, but with increased levels of inflammation (Citation3), and a predisposition to develop panacinar, predominantly basal emphysema (Citation4). Patients with A1ATD generally develop symptoms at a younger age, (in their 40s) and with less cigarette exposure (Citation5) compared to usual COPD. As a result, patients generally progress to severe disease with respiratory failure at a younger age than is typically seen in usual COPD.
Lung transplantation is a final therapeutic option for patients who continue to decline despite optimal medical therapy, including augmentation therapy, where this is available. The role of transplantation in usual COPD patients with emphysema remains unclear; Charman and colleagues reviewed 653 transplants undertaken at a single centre, and showed a significant increase in survival compared to COPD patients matched for FEV1 (Citation6). Thabut et al. confirmed this and identified several factors predictive of survival post-transplantation, which included systolic pulmonary artery pressure, FEV1 and BMI (Citation7). Conversely, Hosenpud and colleagues found no survival benefit for patients with emphysema listed for lung transplantation in the United States between 1992 and 1994 (Citation8), although during this period organ allocation was based upon waiting list time rather than clinical need. Another study in Norway also failed to demonstrate a survival advantage over a 13-year period, for either single or bilateral lung transplant (Citation9).
The reasons for the variable outcomes may, in part, be explained by the heterogeneity of the phenotypes seen in COPD, as some patients decline more rapidly than others, and many patients have co-morbidities, which preclude lung transplantation. Patient selection for transplantation is based on lung function criteria, lack of co-morbidity, exercise capacity and exercise induced desaturation, as well as subjective assessment of likely compliance post-transplantation and the pre-transplant quality of life (QOL).
There is very little data on transplantation in patients with A1ATD. However, a recent Swedish study suggested a clear survival benefit of lung transplantation compared to conventional medical therapy with median survival increasing from 8 years to 11 years (Citation10), importantly, in patients who were not receiving augmentation therapy. However, because of the variable nature of COPD in A1ATD, matching with a non-transplanted cohort presents a major practical problem. More recently survival post-lung transplant in patients with A1ATD was compared to usual COPD and found no difference in survival rates although no data for non-transplanted A1ATD patients was provided (Citation11).
The UK database includes extensive patient characteristics and follow up data over many years for approximately 1000 A1ATD patients. This facilitates matched patient analysis to determine factors other than spirometry and subjective assessment that may influence transplant decision making, and, importantly, post-transplant survival. The current study investigates these issues.
Materials and Methods
Patient selection
We retrospectively reviewed the notes of all patients attending the ADAPT Programme (Antitrypsin Deficiency Assessment and Programme for Treatment), which is the UK national centre for patients with A1ATD, established in May 1996. Patients who had attended the programme between May 1996 and 12th December 2011 and who had undergone a single or double lung transplant were included in the analysis. All patients had the PiZZ (alpha-1-antitrypsin deficiency) genotype, and none had received augmentation therapy.
Patients were assessed in detail yearly, including lung physiology measurements (spirometry, lung volumes and gas transfer measured using the single breath diffusion of carbon monoxide; according to the ARTP/BTS guidelines), documentation of detailed demographic data and assessment of health status using the St George's Respiratory Questionnaire (SGRQ). All data was collected in the stable clinical state and both before and after transplant for the transplant group.
In the initial analysis baseline parameters for each patient who underwent transplantation were matched as closely as possible for age, smoking status and pack-year history, body mass index (BMI), forced expiratory volume in 1 second (FEV1) and lack of co-morbidity, to patients from the registry who did not undergo transplantation. In subsequent analyses measures of gas transfer (second analysis) and SGRQ (third analysis) were also included as part of the matching process.
Survival and causes of death
Data on the date of transplant and transplant centre were collected for all patients. For patients who died during the follow-up period, information on the date and cause of death was obtained from the patients’ primary or secondary care physician and (where undertaken) autopsy results were obtained. Several patients died in hospital post-transplant due to complications, and any death which occurred within 90 days of surgery was classified as post-operative mortality (Citation12). Because transplant could occur at any time after the patient had been recruited to the ADAPT registry, a second group of patients was matched for the last data collected pre-transplant (FEV1, FVC, lung function decline, BMI, smoking status, pack-year history, age, gender and lack of co-morbidity) but also to include gas transfer, for those who had survived the 90 days’ post-transplant to obtain the best matched survival control group.
Data analysis
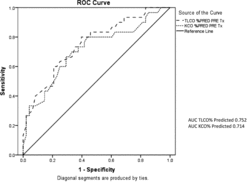
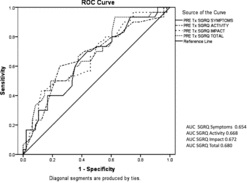
Data analysis was carried out using PASW Statistics 18 (IBM New York). Annual lung function decline, including FEV1, total transfer factor for carbon monoxide (TLCO) and transfer factor corrected for alveolar volume (KCO), was calculated using at least 4 consecutive annual data points. Normally distributed data are reported as means with standard error, and that not normally distributed as medians with interquartile ranges. A p value of ≤ 0.05 was pre-set to determine any significant differences between the groups. Calculations on overall survival post-transplant started on the day of transplant and continued until either the patient died, or until the 12th December 2011 (closing date of study). Receiver Operating Characteristics (ROC curves) were calculated (using PASW) to assess the sensitivity and specificity of any objective measures that influenced lung transplantation.
All patients attending the ADAPT Project provide written informed consent, and the ADAPT Research Project has been approved by the South Birmingham Ethics Committee (LREC number 3359).
Results
Thirty-two patients underwent either a single (4 patients), or bilateral (28 patients) lung transplant during the study period, and the demographic data is summarised in . The patients’ baseline characteristics were matched as closely as possible with 48 non-transplant patients as the first control group and their data is also summarised in . The mean age at transplant in this patient group was 54 years, which is similar to that of the International Registry, and the Swedish A1ATD cohort (Citation10, Citation12) though older than the recent U.S. cohort (Citation11).
Table 1. Demographic data of the transplant group of patients and the first matched group of patients who did not undergo lung transplantation
There were no significant differences in baseline FEV1% predicted or FEV1/FVC ratios between the two groups of patients or other chosen matching parameters, confirming the validity of the matching process for these key parameters. There were, however, significant differences in gas transfer measurements and health status defined by the SGRQ, with the patients in the transplanted group having significantly worse measured values.
Follow-up data of at least 4 measurements over 3 consecutive years was available for 16 patients in the transplant group, from which decline in lung function pre-lung transplant was calculated. The decline was not different to that observed for the matched patients.
CT scan reports were available for 30 of the 32 transplanted patients. All had evidence of both upper and lower zone emphysema with 9 (30%) also having clear evidence of bronchiectasis, similar to the incidence seen in both usual COPD (Citation13) and A1ATD (Citation14).
Selection criteria for transplant in COPD
As part of the criteria for transplant selection depends up on symptomatic assessment, the data for the SGRQ is consistent with this approach. The total score and individual domain scores were higher for those patients who were selected for, and subsequently underwent, transplantation. This, at least in part, probably reflects the worse gas transfer measurements (). The sensitivity and specificity of these measures for selection are summarised in .
Health status pre- and post-transplant
SGRQ scores, collected at 1-year post-transplant, were available for 14 patients. The pre- and post-transplant SGRQ Scores for these patients are summarised in . Total and individual domain scores improved significantly post-transplant, at least in those patients who survived 1 year.
Table 2. Pre- and post-transplant health status scores measured using the St George's Respiratory Questionnaire
The improvement in health status was associated with significant improvements in lung function parameters. summarises the pre- and post-transplant spirometry, where this was available, in the stable clinical state at 1 year post-transplant.
Table 3. Pre- and post-transplant lung physiology
Survival
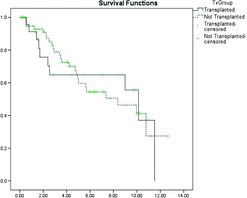
The Kaplan–Meier curve () shows the survival post-transplantation. In this patient group 90-day, 1-year and 5-year survival rates were 81.3% 74.2% and 52.9%, respectively, which is comparable with that reported in the International Registry (Citation12). The 10-year survival (45.2%) however, was slightly better than the 30% reported by the International Registry (Citation12) and significantly higher than that reported in the U.S. cohort of approximately 20% (Citation11).
Figure 3. Kaplan–Meier survival curve for patients with A1ATD who underwent lung transplantation. The vertical cross-lines represent censored data.
Sixteen patients died during the follow up period, of whom, 6 were in the 90 day post-operative period, and 9 died subsequently of complications relating to lung disease and the transplant. The remaining 1 death was attributed to complications unrelated to their lung disease and transplant.
In patients who survived more than 8 years post-transplant, the causes of death were either completely unrelated, such as motor neurone disease or attributed/related to the transplant and its treatment, including and post-transplant lymphoproliferative disorder (2 patients), pneumonia and ARDS and pulmonary fibrosis and pneumonia (1 patient) and pancreatitis (1 patient).
There were no significant differences in the pre-transplant demography of patients who died during the early post-operative period or the first year post-transplant when compared to those who survived beyond a year.
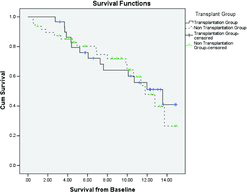
Survival starting 90 days’ post-transplant (to exclude early post-operative mortality) was compared between the first matched group (for FEV1) and those patients who underwent transplantation, and demonstrated no significant survival benefit. The Kaplan–Meier curve for this analysis is shown in .
Figure 4. Kaplan–Meier survival curve for the transplanted (excluding those who died in the post-operative period) and a cohort matched for FEV1 and FEV1/FVC ratio. The vertical cross-lines represent censored data.
The patients in the transplanted group were subsequently re-matched at the closest time point to their transplant to a further group of patients who did not undergo transplantation. This included age, smoking pack-year history, BMI, and spirometry (FEV1, FVC and FEV1/FVC ratio) but also gas transfer (TLCO and KCO % predicted) and previous physiological decline. shows that there were no significant differences in any of these parameters between these 2 groups of patients except for the quality-of-life scores, again confirming the validity of the objective matching process, but confirming that patients who proceeded to transplantation were more symptomatic than those who did not.
Table 4. A summary of the demographic data for the transplant group and the second group of patients matched at the time point closest to transplant
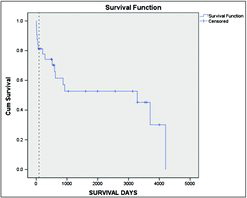
Survival starting 90 days post-transplant was then compared between these 2 groups of patients, and the results are presented in the Kaplan–Meier curve in . The results indicate that once the immediate post-operative mortality has passed, there was still no survival advantage of the transplant patients with median survival times of 10.1 vs 8.4 years for the transplant and non-transplant patients, respectively (p = 0.954).
Figure 5. Kaplan–Meier survival curve for the transplanted (excluding those who died in the post-operative period) and a cohort matched for age, spirometry and gas transfer at the time of transplant. The vertical cross-lines represent censored data.
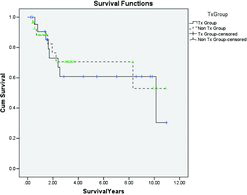
As there remained a significant difference in QoL between the 2 patient groups once matched for gas transfer a further matching was undertaken to include the SGRQ. The matching became more difficult when all parameters were used and so the data was limited to 30 patients where this was possible. The data for these final 2 groups is summarised in (p > 0.1 for all parameters). The Kaplan–Meier curve for this analysis is shown in . Once the 90-day perioperative mortality had been excluded, there was no difference in survival demonstrated between patients who did or did not undergo transplantation.
Table 5. A summary of the demographic data for the transplant group and the third group of patients matched including HRQoL at the time point closest to transplant
Discussion
The extensive information available from the UK A1ATD registry has allowed unique information to be assessed for patients undergoing lung transplant. In particular, it highlights gas transfer as an objective measure that explains (at least in part) the worse symptomatology that provides part of the patient selection process for transplantation. The rate of initial lung function decline pre-transplant was comparable between the transplanted and non-transplanted patient groups, suggesting that a more rapid rate of decline was not a factor in determining patients’ symptoms pre-transplant. The data uniquely describes the improvement in health status post-lung transplantation, providing evidence of the immediate clinical benefit of transplantation in this patient group. Following transplantation, patients are closely followed up by the relevant transplant centre, hence not all were continually assessed post-transplant within the ADAPT Project, as some wished to be reviewed by a single transplant centre only.
Survival in patients with A1ATD post-lung transplantation has recently been reviewed, and a survival benefit in patients undergoing transplant was demonstrated compared to those not being transplanted (Citation10). In our series, 6 (18.8%) of the transplant patients were never discharged from hospital and died of post-operative complications. The published figure from the International Registry for all-cause post-operative mortality following lung transplantation is 12% at 3 months, suggesting the current figure is higher than expected. Patients attend the ADAPT project from all over the United Kingdom, and are referred to 1 of 5 transplant centres based on their geographical location. The distribution of patients between these centres resulted in numbers for each that were too small to determine an individual centre effect.
The work, however, included patients who had undergone lung transplants from mid 1990s up to December 2011, during which time there have been changes in surgical techniques and immunosuppressive therapy, which may be reflected in the observed early post-surgical mortality. However, in general, the survival after discharge was comparable with that currently reported in the literature and may even be better after 5 years.
Historically, patients with COPD were considered to be potential candidates for lung transplantation if they met certain criteria, which included an FEV1 25% predicted without significant bronchodilator reversibility, and/or a PC02 > 7.3 kPa (55 mmHg) and/or elevated pulmonary artery pressures with progressive deterioration (Citation15). Patients with usual COPD are now assessed for lung transplantation with other objective measures such as the BODE index integrated into the decision making process. This allows a multi-dimensional assessment of patients, rather than relying on FEV1 alone. The International Guidelines for Lung Transplantation (Citation16) suggest that a patient who has a BODE Index score of greater than 5 could be considered for referral for transplant, and with a score of 7 or greater could be considered for transplantation (in the absence of contra-indications) if one or more of the following are present; 1) history of hospitalisation with acute hypercapnia, 2) pulmonary hypertension or cor pulmonale (or both) despite long-term oxygen therapy, and 3) FEV1 < 20% and either TLCO < 20% or homogenous distribution of emphysema (Citation16).
One limitation of our current study is that much of it predates the introduction of the BODE index, and therefore BODE indices could not be calculated (Citation17). However, we have demonstrated that patients with A1ATD who received lung transplantation had worse health status and gas transfer measurements than a group matched for FEV1. Although these parameters are not included in the BODE assessment, they are consistent with features beyond FEV1 that influence decision making. In the UK, patients are assessed for suitability for listing for transplantation by transplant centres based on the severity of lung physiology and the perceived effect on quality-of-life.
The data presented in the current study are consistent with this approach, and provide evidence that not only gas transfer but also the SGRQ could be used as a more stratified approach pre-transplant in this group of patients. A further limitation is that we were unable to assess outcome in patients who declined referral for transplantation, patients referred for transplantation and not accepted or accepted for transplantation but never underwent the procedure due to allocation of organs. It is possible that such patients may have had a worse survival than the transplant patients. However the careful objective matching of patient groups within our large data base provides a cohort with similar demographic features and will partly circumvent this drawback.
In contrast to the recently published data from the Swedish study, we did not demonstrate a survival benefit in patients with A1ATD following lung transplantation. Survival rates once the post-operative mortality data were removed from the analysis did not differ between the transplant group and a second highly matched group taking gas transfer into account or the third matched group that also included SGRQ scores. The reasons for this difference to the Swedish data are not clear. There were fewer subjects reported here compared to the 83 patients reported from Sweden, which may influence the statistical power of the data (Citation12). However, in our study we were able to closely match patients not only for spirometry but also gas transfer. Health Status and lack of co-morbidity (in particular the absence of clinically significant liver disease, which was not present in any of the patients included in this study), all of which influence mortality (Citation18).
Furthermore, a less stringent matching process was used for the controls in the Swedish study and included patients who had features associated with poor outcome and may therefore explain (at least in part) differences with the results reported here. The difference between the 2 studies may therefore reflect other physiological or clinical factors in the Swedish cohort that were not assessed, or had a negative effect on survival of the control group. Alternatively the better survival in the non-transplanted patients may reflect differences in the prevailing health care systems between the 2 countries. Nevertheless, the initial improvement in spirometry and particularly quality of life reported here is clearly a benefit post-transplant. Importantly, the data reported here provides patients and health care professionals with clearer explanations of the current risks and benefits of lung transplantation in A1ATD especially in the United Kingdom.
Conclusions
We have demonstrated a clear improvement on HRQoL assessed using SGRQ following lung transplantation in patients with end-stage disease secondary to A1ATD. When compared to a matched group of patients, gas transfer measurements and HRQoL were worse at baseline in those who subsequently proceeded to transplantation, and these parameters should be included in pre-transplant assessments of patients with A1ATD. Survival, however, was not significantly different from a carefully matched non-transplant cohort (once peri-operative mortality had been taken into account).
Funding
Grifols provided an unrestricted grant to the ADAPT (Antitrypsin Deficiency Assessment and Programme for Treatment) project to support this and ongoing research into alpha-1-antitrypsin deficiency, including HS's time as a clinical research fellow.
Declaration of Interest Statement
RAS serves on advisory boards for many pharmaceutical companies with treatments related to COPD (GSK, AstraZeneca, Almirall, Novartis, Boehringer Ingelheim, CSL Behring). RAS has also received lecture fees and travel fees to International meetings funded by Grifols, Takeda, Novartis, Almirall and Boehringer Ingelheim. HS has received travel funding and lecture fees from GlaxoSmithKline.
We declare that none of the authors have any other conflicts of interest regarding the contents of this manuscript. The authors alone are responsible for the content and writing of the paper.
Authors Stone and Edgar are joint first authors.
References
- Stockley RA. Alpha 1 antitrypsin deficiency: what next? Thorax 2000; 55:614–618.
- Devereux G. COPD: Definition, epidemiology and risk factors. BMJ 2006; 332(7550):1142–1144.
- Woolhouse IS, Bayley D, Stockley RA. Sputum chemotactic activity in chronic obstructive pulmonary disease; effect of alpha-1-antitrypsin deficiency and the role of leukotriene B4 and interleukin 8. Thorax 2002; 57:709–714.
- Stockley RA. Neutrophils and the pathogenesis of COPD. Chest 2002; 121:151S–155S.
- Dowson LJ, Guest PJ, Stockley RA. Longitudinal changes in physiological, radiological, and health status measurements in alpha-(1)-antitrypsin deficiency and factors associated with decline. Am J Respir Crit Care Med 2001; 164:1805–1809.
- Charman SC, Sharples LD, McNeil KD, Wallwork J. Assessment of survival benefit after lung transplantation by patient diagnosis. J Heart Lung Transpl 2002; 21(2):226–232.
- Thabut G, Ravaud P, Christie JD, Castier Y, Fournier M, Mal H, Leseche G, Porcher R. Determinants of the survival benefit of lung transplantation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177:1156–1163.
- Hosenpud JD, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet 1998; 351:24–27.
- Stavem K, Bjortuft O, Borgan O, Geiran O, Boe J. Lung transplantation in patients wih chronic obstructive pulmonary disease in a national cohort is without survival benefit. J Heart Lung Transpl 2006; 25:75–84.
- Tanash HA, Riise GC, Hansson L, Nilsson PM, Piitulainen E. Survival benefit of lung transplantation in individuals with severe α1-antitrypsin deficiency (PiZZ) and emphysema. J Heart Lung Transplant 2011; 30(12) 1342–7.
- Banga A, Gildea T, Rajeswaran A, Rokadia H, Blackstone EH, Stoller JK. The natural history of lung function following lung transplantation for alpha-1-antitrypsin deficiency. Am J Respir Crit Care Med 2014; 190(3):247–281.
- Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Benden C, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI. The registry of the international society for heart and lung transplantation: 28th adult lung and heart-lung transplant report – 2011. J Heart Lung Transplant 2011; 30 (10):1104–1122.
- O'Brien C, Guest PG, Hill SL, Stockley RA. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax 2000; 55(8):635–642.
- Parr DG, Guest PG, Reynolds JH, Dowson LJ, Stockley RA. Prevalence and impact of bronchiectasis in alpha-1-antitrypsin deficiency. Am J Respir Crit Care Med 2007; 176(12):1215–1221.
- International Guidelines for the Selection of Lung Transplant Candidates. The American Society for Transplant Physicians (ASTP)/American Thoracic Society (ATS)/European Respiratory Society (ERS)/International Society for Heart and Lung Transplantation (ISHLT). Am J Respir Crit Care Med 1998; 158:335–339.
- Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ, Egan T, Keshavjee S, Knoop C, Martinez FJ, Nathan S, Palmer S, Patterson A, Singer L, Snell G, Studer S, Vachiery JL, Ganville AR. International guidelines for the selection of lung transplant candidates: 2006 update —Consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transpl 2006; 25:745–755.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body mass index, airflow obstruction, dyspnoea and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:1005–1012.
- Dawkins PA, Dowson LJ, Guest PJ, Stockley RA. Predictors of mortality in α1-antitrypsin deficiency. Thorax 2003; 58:1020–1026.