Abstract
Patients' preference is an important factor in selecting an inhaler treatment for COPD. The DISKUS® dry powder inhaler (DPI), which has been available to deliver several COPD medications for a decade, and the ELLIPTA® DPI, developed for the delivery of newer once-daily medications for patients with COPD, were studied in terms of patient preference and inhaler-specific attributes.
We conducted a randomized, open-label, crossover study in patients with COPD. Patients used placebo ELLIPTA DPI once daily and placebo DISKUS DPI twice daily, for ∼1 week each, while continuing their COPD medications. Endpoints were: inhaler preference based on size of the numbers on the dose-counter (primary); the number of steps needed and inhaler size (secondary); and based on comfort of the mouthpiece, ease of opening, overall preference, and dosing regimen preference (‘other’). Safety assessments included adverse events (AEs).
A total of 287 patients were randomized. A significantly (p < 0.001) larger proportion of patients preferred the ELLIPTA DPI over DISKUS DPI for each of the tested attributes and overall, and preferred once-daily over twice-daily dosing. AEs were reported for 36 patients (13%); one (dry mouth) was considered to be related to the placebo-containing DISKUS DPI. Three patients had five non-fatal serious AEs, none were deemed inhaler-related.
This study demonstrated that more patients with COPD preferred five specific inhaler attributes of the ELLIPTA DPI over DISKUS DPI and overall, and preferred once-daily versus twice-daily dosing. Safety profiles were consistent with those expected for COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of poor health, resulting in millions of deaths annually (Citation1) and contributing significantly to healthcare costs and morbidity (Citation2). According to the World Health Organization, COPD was the fifth leading cause of death worldwide in 2002, and is estimated to be the third leading cause by 2030 (Citation3).
First-line options for the pharmacological management of COPD are all inhaler-based medicines. Current inhalers range from traditional patient-activated, pressurized, metered-dose inhalers, to single- and multi-dose dry powder inhalers (DPIs). Patients' experience of and attitudes towards an inhaler are important aspects of inhaler selection (Citation4,Citation5). Factors, including arthritis, poor eyesight, difficulty in preparing a dose, poor timing of respiratory intake, inspiratory flow profile, confusion regarding the number of doses remaining, and problems with lockout mechanisms can impair adequate patient utilization of an inhaler and could lead to poor adherence and suboptimal disease control. The prevalence of COPD rises with age (Citation5), and many of these factors are more likely to represent a hindrance to adequate inhaler use in elderly patients.
Bateman recommended that three factors are considered when selecting inhalation therapy for COPD patients. These are 1) characteristics of the inhaler, 2) patient's knowledge, attitude and preference, and 3) physician familiarity with inhalers and their skill in understanding patient needs and preferences (Citation6). Additionally, a less frequent dosing schedule has also been indicated as one way to improve adherence to COPD therapy (Citation7,Citation8).
Use of the DISKUS®Footnote DPI is well established for the delivery of medications such as the fixed combination inhaled corticosteroid/long-acting beta-2 receptor agonist (ICS/LABA) fluticasone propionate/salmeterol 250/50 mcg in the treatment of asthma and COPD (Citation9). The ELLIPTA®a DPI is a multi-dose inhaler, which has been approved by the U.S. Food & Drug Authority (FDA) for delivery to patients with COPD of the once-daily fixed combination ICS/LABA fluticasone furoate/vilanterol in 2013(10), the long-acting anticholinergic/long-acting beta-2 receptor agonist umeclidinium/vilanterol in 2013 (Citation11), and the long-acting anticholinergic umeclidinium monotherapy in 2014 (Citation12). These have subsequently been approved in the European Union and Canada (Citation13–Citation18).
This article reports a phase IIIb study that was designed to assess patients’ preferences for selected attributes of the ELLIPTA DPI and the DISKUS DPI after experiencing each one separately for approximately 1 week; therefore, for the purposes of the study, both inhalers were loaded with placebo – so that the therapeutic effect from a pharmacologic agent would not influence patients' preference regarding the inhaler-specific attributes – in an open-label fashion. The primary objective of the study was to evaluate whether more patients with COPD preferred the ELLIPTA DPI based on the size of numbers on the dose counter. The secondary objectives were to evaluate patient preferences between the two inhalers based on the number of steps needed to use the inhaler and the size of the inhaler.
Methods
Study population
Patients included in the study were males and non-pregnant females ≥40 years of age, current or former smokers, with a diagnosis of COPD, a post-albuterol forced expiratory volume in one second (FEV1) ≤70% of the predicted normal, and a measured FEV1/forced vital capacity ratio of ≤0.70. Patients could not have used either the ELLIPTA DPI or the DISKUS DPI within 6 months of screening, or have had a current diagnosis of asthma, poorly controlled COPD, or other diseases or abnormalities that would have put them at risk through study participation. The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines (ICH, 1996) and all applicable regulatory requirements. Written informed consent was obtained from each participant prior to start of any study-specific procedures. All patients continued their COPD medications throughout the study while additionally using the placebo-containing study inhalers.
Study design
This was a phase IIIb, randomized, open-label, crossover study performed at 19 study centers in the United States between 28 May and 15 July 2013 (GSK study number: RLV116669; ClinicalTrials.gov identifier: NCT01868009).
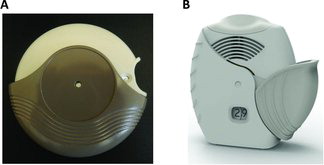
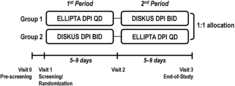
Eligible patients were randomized (1:1) to one of two placebo inhaler sequences: 1) ELLIPTA DPI once daily in Period 1 followed by DISKUS DPI twice daily in Period 2, or 2) DISKUS DPI twice daily in Period 1 followed by ELLIPTA DPI once daily in Period 2. The inhalers used in the study are shown in .
Each period was 5–9 days in duration (). At the dispensing visits (Visits 1 and 2), site personnel educated each patient in how to use the inhaler with which they were provided, using the demonstration inhaler in accordance with the step-by-step instructions provided in the patient information leaflet. Patients were given an opportunity to familiarize themselves with the inhaler, and were asked to demonstrate correct use of the demonstration inhaler. Following this training, patients took their first dose from the study inhaler at the investigator site and were instructed to continue daily administration until the next clinic visit (Visits 2 and 3).
For each inhalation, DISKUS DPI requires 4 steps (open, slide the lever, inhale, and close; see online supplement for further details) and ELLIPA DPI 3 steps (open, inhale, and close; see online supplement for further details). At the end of Period 2, patients independently provided responses to seven questions that were designed to assess their preference for inhaler attributes and dosing regimens. Randomization also determined which one of the two preference question versions the patient completed. These versions differed only by the order of response options (i.e., ‘ELLIPTA DPI’, ‘DISKUS DPI’ or ‘No preference’ versus ‘DISKUS DPI’, ‘ELLIPTA DPI’ or ‘No preference’ for inhaler preference questions; ‘Once a day,’ ‘Twice a day’ or ‘No preference’ versus ‘Twice a day,’ ‘Once a day’ or ‘No preference’ for dosing preference questions). Patients were randomized using the Registration and Medication Ordering System (RAMOS, GSK, UK), a computer-generated randomization schedule and an Interactive Voice Response System. Patients' compliance with study inhaler use was assessed using the inhalers' built-in dose counters, based upon the study start and stop dates.
Development of the questions
The questions were developed using item solicitation and validation methods applicable to the generation of inhaler preference items. Specifically, relevant inhaler attributes were derived through the analysis of results from individual in-depth exit interviews conducted with clinical trial participants from studies in which patients had used both the ELLIPTA DPI and DISKUS inhalers prior to approval: HZC112206 (NCT01053988) or HZC112207 (NCT01054885) (total n = 25). The interviews assessed the participants' general impressions, likes, and dislikes of both inhalers. Attributes of either inhaler that patients perceived to be beneficial were identified from the interview.
Based on the analysis of the interview results, seven questions were initially developed assessing attributes that differed between the ELLIPTA and the DISKUS DPIs. The final inhaler preference questions and final wording were refined through three rounds of cognitive 1:1 interviews with 22 patients with COPD who had experience in using the DISKUS inhaler for administration of fluticasone propionate/salmeterol. These patients were recruited from three regions of the United States (Detroit, MI; San Antonio, TX; Raleigh, NC) and were demographically and culturally diverse.
The final preference questions used in the study were:
| 1) | Which device do you prefer based on the size of the numbers on the dose counter? | ||||
| 2) | Which device do you prefer based on the number of steps needed to take your COPD medication? | ||||
| 3) | Which device do you prefer based on the size of the device? | ||||
| 4) | Which device do you prefer based on the comfort of the mouthpiece? | ||||
| 5) | Which device do you prefer based on the ease of opening the device? | ||||
| 6) | Which device do you prefer overall? | ||||
| 7) | Would you prefer to take your COPD medication once or twice a day? | ||||
Questions 1 through 5 were attributes that patients from the 1:1 interviews believed were most important to them regarding how they assess preference. Questions 6 and 7 were not part of the original item solicitation but were determined to be important and were cognitively tested to ensure patient understanding of the items.
Study outcomes
The primary endpoint of the study was inhaler preference based on the patients' response to the question ‘Which device do you prefer based on the size of the numbers on the dose counter?’ Secondary endpoints were inhaler preference based on the patients' response to the questions ‘Which device do you prefer based on the number of steps needed to take your COPD medication?’ and ‘Which device do you prefer based on the size of the device?’ Other endpoints were preference based on the responses to question numbers 4–7 above, i.e., comfort of the mouthpiece, ease of opening, overall preference, and dosing regimens (once daily versus twice daily).
Safety assessments
Although the study did not include active pharmacological agents as an intervention, AEs were monitored to ensure the safety of participating patients. Protocol stopping criteria included COPD exacerbation, diagnosis of pneumonia, an AE that would put the patient at an unacceptable risk, and use of any medication delivered by the DISKUS or ELLIPTA DPI. Safety assessments, performed to assess stopping criteria, included incidence of adverse events (AEs), and COPD exacerbations; AEs were coded using company standard dictionaries, Medical Dictionary for Regulatory Activities (MedDRA) and GSKDrug.
Statistical analysis
Randomization of 278 patients was planned to provide 90% power to detect an odds ratio of 1.5 using a two-sided test with significance level of 0.05. An odds ratio of 1.5 corresponds to 60% of patients expressing a preference for a specific attribute of the ELLIPTA DPI and 40% of patients expressing either a preference for the DISKUS DPI or no preference over the same attribute. It was estimated that approximately 5% of patients randomized may not complete the study. Preference endpoints were analyzed using the per-protocol (PP) population, which comprised all patients from the intent-to-treat (ITT) population (randomized patients who received one dose from at least one study inhaler) who completed at least one preference question. The response for each specific attribute was analyzed using a Cochran-Mantel-Haenszel test that accounted for inhaler use sequence and question version. The Cochran-Mantel-Haenszel test served as a stratified approximation to Prescott's test, a variation of a one-sample Chi-square test that accounted for study inhaler sequence, preference question version, and patients who indicated no preference (Citation19,Citation20). Inhaler use sequence was accounted for in these tests to assess the impact of period effect on preference questions. A step-down statistical approach (from primary to secondary and from secondary to other endpoints) and Hochberg's method (Citation21) (across secondary endpoints) were applied to adjust for multiplicity across comparisons. AEs were summarized in the ITT Population.
Sensitivity analysis
A sensitivity analysis was performed for each preference endpoint by combining the ‘preference for the DISKUS DPI’ and ‘no preference’ responses versus preference for the ELLIPTA DPI using the methods described above, thus representing a conservative estimate of preference for the ELLIPTA DPI.
Results
Patient disposition and baseline characteristics
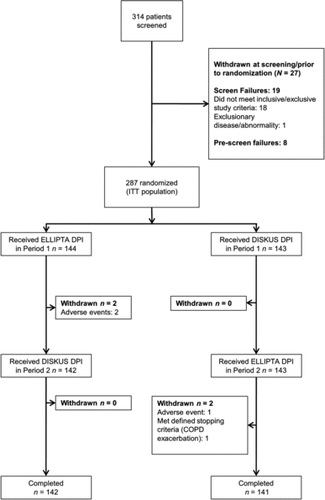
Of 314 patients screened, 287 were randomized and included in the ITT population; 283 patients completed the study. Two patients from the ITT Population were excluded from the PP Population because they were unable to complete the preference questions due to AEs that led to withdrawal during Period 1 ().
Four patients withdrew prematurely; three due to AEs and one due to meeting protocol-defined stopping criteria (COPD exacerbation).
The demographics and baseline characteristics of the study population were representative of a general COPD population ().
Table 1. Patient demographics, COPD history, and tobacco use
The majority of patients (84%) in the ITT Population had comorbidities, most commonly hypertension (67%), hypercholesterolemia (57%), coronary artery disease (22%) and diabetes mellitus (20%). Fifty-one percent of patients reported prior medical conditions, most frequently pneumonia (28%), cataract (15%), and myocardial infarction (9%).
Concurrent COPD medications used most frequently during the study were salbutamol (58%), tiotropium bromide (39%), budesonide/formoterol fumarate (23%), and salbutamol sulphate/ipratropium bromide (11%). Fluticasone propionate and fluticasone propionate/salmeterol combination were used by seven and four patients respectively; these were administered via a metered-dose inhaler. Fifteen percent of patients reported using oxygen.
Exposure and compliance
Mean exposure to each of the inhalers was 7.2 days. Inhaler compliance rates were slightly higher with the ELLIPTA DPI (used once daily) compared with the DISKUS DPI (used twice daily). A greater proportion of patients had >120% compliance when using the ELLIPTA DPI (7%) compared with the DISKUS DPI (3%) (), indicating that the ELLIPTA mouthpiece cover was opened more frequently than instructed.
Table 2. Exposure and inhaler use compliance (ITT population)
Inhaler and dosing regimen preference
A larger proportion of patients preferred the ELLIPTA DPI over the DISKUS DPI for each of the five selected attributes and overall, and preferred a once-daily dosing regimen over a twice-daily dosing regimen.
All comparisons achieved statistical significance at α = 0.05 level after accounting for the pre-defined multiple testing strategy due to the small p-values for the comparisons (all p < 0.001). Neither inhaler sequence nor question version had an impact on preference.
Preference for the ELLIPTA DPI was highest for the size of the numbers on the dose counter (68% of patients) and the number of steps needed to use the inhaler (67%). The highest preference rates for the DISKUS DPI were for overall preference (31%), size of the inhaler (27%), and comfort of the mouthpiece (27%) and the highest proportion of ‘No preference’ responses were for comfort of the mouthpiece (26%) and size of the inhaler (22%); preference for the ELLIPTA DPI was higher in all of these categories ().
Table 3. Analysis of inhaler attribute and dose regimen preference (PP population)
Sensitivity analyses
In the sensitivity analysis, which was designed to obtain a more conservative estimate of preference for the ELLIPTA DPI, a statistically significantly (p < 0.001) larger proportion of patients preferred the ELLIPTA DPI versus those who preferred the DISKUS DPI or had ‘no preference’ based on the size of the numbers on the dose counter (68% versus 32%), and based on the number of steps to take the COPD medication (67% versus 33%). No statistically significant difference was observed between the proportion of patients who preferred the ELLIPTA DPI versus those who preferred the DISKUS DPI or had ‘no preference’ based on the size of the inhaler (51% versus 49%; p = 0.724). The use of the pre-defined, step-down multiple testing procedure meant that no statistical testing could be applied to the remaining endpoints. However, a numerically larger proportion of patients preferred the ELLIPTA DPI based on ease of opening the inhaler (60% versus 40%), and overall inhaler preference (63% versus 37%). Additionally, a numerically larger proportion of patients preferred once-daily dosing versus twice-daily dosing, or no preference (67% versus 33%).
Safety
AEs were reported by 36 (13%) patients (). The most common system organ class AEs were nervous system disorders (n = 10; 3%), infections and infestations (n = 8; 3%), and gastrointestinal disorders (n = 7; 2%). One AE (dry mouth) was considered by the investigator to be related to the placebo-containing DISKUS DPI.
Table 4. Adverse events occurring in more than one patient (ITT Population)
Three patients experienced a total of five non-fatal SAEs (deep vein thrombosis, esophageal candidiasis, and liver metastases, n = 1 [patient withdrawn as a result]; bronchitis, n = 1 [patient withdrawn as a result]; vertebrobasilar insufficiency, n = 1). These SAEs were not considered to be related to use of the inhalers. All SAEs except for the liver metastases were resolved and there were no deaths during the study period.
Two patients experienced COPD exacerbations during the study. Both patients were treated with corticosteroids and antibiotics and were withdrawn from the study (one recorded as having been withdrawn from the study due to an AE). One additional patient was withdrawn due to a non-serious wrist fracture.
Discussion
The availability of an increasing number of inhalers, which offer a variety of therapy choices and delivery designs, means that selecting an inhaled maintenance therapy for COPD patients can be challenging. It is recommended that the physician considers several aspects when selecting such therapy, including inhaler features and the patient's attitude and preference toward a specific inhaler, in addition to the appropriate pharmacological agents (Citation6).
In the United States, the ELLIPTA and DISKUS DPIs are available to deliver fixed-dose combination ICS/LABA treatments indicated for long-term maintenance treatment of airflow obstruction and for reducing exacerbations in COPD patients: 1) once-daily fluticasone furoate/vilanterol 100/25 mcg delivered via the ELLIPTA DPI and 2) twice-daily fluticasone propionate/salmeterol 250/50 mcg delivered via the DISKUS DPI. Studies comparing the efficacy and safety of these two treatments in patients with COPD were previously published (Citation22,Citation23).
This study assessed patient preference of inhaler-specific features using placebo-containing ELLIPTA and DISKUS DPIs. Placebo-containing DPIs were used in this study rather than inhalers containing active medication, while the participating patients separately continued their COPD treatment, to eliminate the confounding therapeutic effect of a pharmacologic agent which could influence patients' preference. The results demonstrate that more patients with COPD consistently preferred the ELLIPTA DPI over the DISKUS DPI based on five of the selected attributes: the size of the numbers on the dose counter, the number of steps needed to use the inhaler, the size of the inhaler, the comfort of the mouthpiece, and the ease of opening the inhaler.
More patients also preferred the ELLIPTA DPI overall. The sensitivity analysis revealed similar trends for all attributes and reached statistical significance for the proportion of patients preferring the ELLIPTA DPI based on the size of the numbers on the dose counter and the number of steps required to take the COPD medication. These findings are consistent with a recently published study in which patients with asthma and COPD compared the ELLIPTA DPI to their other currently prescribed inhalers including the DISKUS DPI (Citation24).
Patients' preference for an inhaler depends on multiple factors including durability, ergonomics (e.g., size, shape, and comfort), and ease of use (e.g., number of steps, ability to track unused doses, hand-mouth coordination) (Citation23). Therefore, it is important to assess the specific attributes affecting preference of the inhaler and not only overall preference. This process of elucidating patient preference for a specific attribute, either in absolute terms or in relation to another attribute, may help focus patient education and improve prescriber–patient dialogue, thereby engaging patients in their treatment selection, which could positively influence their medication adherence (Citation6,Citation26,Citation27).
This study also showed that more patients reported a preference to take their COPD medication once daily versus twice daily. Although this finding is not related to preferences for the ELLIPTA or DISKUS DPIs, and, as an “Other” endpoint, was not adjusted for multiple testing, it is important to establish patients' overall preference for their dosing regimen as this is one of the factors that can affect patient adherence in COPD (Citation28). The treatments delivered via the ELLIPTA DPI have been developed specifically for once-daily delivery in patients with COPD.
Improved drug delivery afforded by next-generation inhalers, coupled with an awareness of inhaler- and patient-specific variables affecting inhaler use, may improve clinical outcomes in the treatment of COPD (Citation29). A recent large, multinational, cross-sectional survey of 1443 patients with COPD showed that patients' overall satisfaction with their inhaler was significantly associated with treatment compliance, and that there was a direct association between inhaler satisfaction and fewer exacerbations (Citation25). However, the impact of patient preference on actual treatment compliance, adherence, persistence, and clinical outcomes was not tested in this trial and cannot be inferred.
In addition to patient preference and dosing regimen, other factors should be considered when choosing an inhaled maintenance therapy for COPD, including access, cost, inspiratory flow profiles, efficacy and safety. These factors are the focus of high-quality clinical practice guidelines, but it has been found that patient preferences are poorly integrated into these guidelines (Citation30). By increasing awareness of patient preferences for specific attributes of the ELLIPTA and DISKUS DPIs, the evidence provided by well-designed studies like this one could provide physicians with useful information when considering an inhaled maintenance therapy for COPD.
Study limitations and strengths
During the development of the study concept, studying the preference of inhaler-specific attributes among current users of DISKUS DPI who have not previously used ELLIPTA DPI was considered. However, in order to rule out any potential biases in patients who had experience with DISKUS DPI, the study excluded anyone with the use of a DISKUS DPI within 6 months of randomization. Patients were asked if they had used DISKUS DPI within the last 6 months, but historical use of DISKUS DPI beyond 6 months was not assessed in this study. Therefore, the study was not able to address whether previous DISKUS use would have influenced patients' preference between ELLIPTA DPI and DISKUS DPI.
This study was not designed to assess correct use and ease-of-use of either inhaler. Therefore, it cannot be inferred on average how many instructions were needed for each inhaler to observe the correct inhalation technique at the dispensing visits. Compliance assessment for the study was based on the number on the dose counter at the clinic visits and therefore, it cannot be absolutely certain that the patients have used the study inhalers on a daily basis as instructed between clinic visits. Because patients were instructed to use ELLIPTA DPI once daily and DISKUS DPI twice daily, the issue of over-compliance was more pronounced when using ELLIPTA DPI during the study.
For future studies, in addition to training on how to administer the study inhaler (including how frequently), it is suggested that patients would be reminded of the instruction not to open the inhaler (that action will change the number on the dose counter) unless dosing is needed. The cross-over design, short treatment periods, and use of placebo-containing inhalers did not allow for assessment of clinical endpoints and long-term treatment compliance. However, there are strengths to the study design that support the robustness of the findings.
These include methodological aspects such as the systematic approach used to select the preference questions, the randomized, crossover study design, the sample size of the study to support statistical validity of outcomes, and the conservative statistical approach used to assess the sensitivity of the findings. The one-week length of the treatment periods was chosen in light of the crossover design of the study. It was considered that treatment periods of a longer duration could potentially bias patients' preference towards the more recently-used inhaler. In this study, the sequence in which the inhalers were used did not significantly affect patient preference. Because in real-world practice patients will not be using two inhalers containing an ICS/LABA combination concurrently, a crossover design entailing sequential use was deemed the most appropriate design for this study. Finally, the open-label design of this study was essential to compare the physical features of these two DPIs and is reflective of real-world situation where the patient is shown two devices at the doctor's office and asked to select based on their preference.
Conclusions
The results of this study demonstrate that significantly more patients with COPD preferred five selected inhaler-specific attributes (size of the numbers on the dose counter, number of steps needed, inhaler size, comfort of the mouthpiece, and ease of opening) of the ELLIPTA DPI compared with the DISKUS DPI, and that two thirds of patients with COPD preferred to take their COPD medications once-daily rather than twice-daily. The results of the safety assessments performed in this study were consistent with health conditions typically observed in patients with COPD.
Declaration of Interest Statement
This study was funded by GSK (study number RLV116669; ClinicalTrials.gov number NCT01868009). Suyong Yun Kirby, Chang-Qing Zhu and Richard H Stanford are employees of GSK and own stock in the company. George Georges is an ex-employee of GSK and owns stock in the company. Edward M. Kerwin has served on advisory boards, speaker panels, or received travel reimbursement for Amphastar, AstraZeneca, Boehringer Ingelheim, Forest, Ironwood, Merck, Mylan, Novartis, Pearl, Pfizer, Sanofi Aventis, Sunovion, Targacept, Teva and Theravance, and has also conducted multicenter clinical research trials for approximately 50 pharmaceutical companies.
Editorial support in the form of editorial suggestions to the first draft of this paper, assembling tables and figures, collating author comments, copyediting, fact checking, referencing and graphic services were provided by Laura Maguire, MChem and Ian Grieve, PhD at Gardiner-Caldwell Communications (Macclesfield, UK); this support was funded by GSK.
The authors alone are responsible for the content and writing of the paper.
Acknowledgment
The authors would like to thank the RLV116669 study team, study participants, investigators and site staff for their contribution to the study.
Author George Georges is formerly of GSK, North Carolina.
Notes
a DISKUS and ELLIPTA are trademarks of the GSK group of companies.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of Chronic Obstructive Pulmonary Disease. Updated 2014. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf ( accessed: 21 November 2014).
- Chapman KR, Mannino DM, Soriano JB, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J 2006; 27:188–207.
- World Health Organization. Chronic Respiratory Diseases: Burden of COPD. Available from: http://www.who.int/respiratory/copd/burden/en/index.html (accessed: 21 November 2014).
- Hodder R, Price D. Patient preferences for inhaler devices in chronic obstructive pulmonary disease: experience with Respimat Soft Mist inhaler. Int J Chron Obstruct Pulmon Dis 2009; 4:381–390.
- Barrons R, Pegram A, Borries A. Inhaler device selection: special considerations in elderly patients with chronic obstructive pulmonary disease. Am J Health Syst Pharm 2011; 68:1221–1232.
- Bateman ED. Improving inhaler use in COPD and the role of patient preference. Eur Respir Rev 2005; 14:85–88.
- Ágh T, Inotai A, Mészáros Á. Factors associated with medication adherence in patients with chronic obstructive pulmonary disease. Respiration 2011; 82:328–334.
- Lareau SC, Yawn BP. Improving adherence with inhaler therapy in COPD. Int J Chron Obstruct Pulmon Dis 2010; 5:401–406.
- Fuller R. The Diskus: a new multi-dose powder device–efficacy and comparison with Turbuhaler. J Aerosol Med1995; 8(Suppl 2):S11–S17.
- US Food, Drug Administration. Medication Guide: BREO ELLIPTA. 2013. Available from: http://www.fda.gov/downloads/drugs/drugsafety/ucm352347.pdf ( accessed 21 November 2014).
- US Food, Drug Administration. Medication Guide for ANORO ELLIPTA. 2013. Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM380278.pdf ( accessed 21 November 2014).
- US Food, Drug Administration. Highlights of Prescribing Information: INCRUSE ELLIPTA. 2014. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205382s000lbl.pdf ( accessed 21 November 2014).
- European Medicines Agency. Summary of product characteristics: RELVAR ELLIPTA. 2013. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002673/WC500157633.pdf ( accessed 21 November 2014).
- Health Canada. Summary Basis of Decision (SBD): BREO ELLIPTA. 2013. Available from: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/sbd-smd/drug-med/sbd_smd_2013_breo_ellipta_157301-eng.php ( accessed 21 November 2014).
- European Medicines Agency. Summary of product characteristics: ANORO ELLIPTA. 2014. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002751/WC500168424.pdf ( accessed 21 November 2014).
- Health Canada. Summary Basis of Decision (SBD): ANORO ELLIPTA. 2014. Available from: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/sbd-smd/drug-med/sbd_smd_2014_anoro_ellipta_161585-eng.php ( accessed: 21 November 2014).
- European Medicines Agency. Summary of product characteristics: INCRUSE ELLIPTA. 2014. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002809/WC500167430.pdf ( accessed: 21 November 2014).
- GlaxoSmithKline. GSK announces approval in Canada for Incruse Ellipta (umeclidinium) as a treatment for COPD. 2014. Available at: http://www.gsk.com/en-gb/media/press-releases/2014/gsk-announces-approval-in-canada-for-incruse-ellipta-umeclidin ium-as-a-treatment-for-copd/ ( accessed 21 November 2014).
- Prescott RJ. The comparison of success rates in cross-over trials in the presence of an order effect. Appl Stat 1981; 30:9–15.
- Senn, S. Cross-over Trials in Clinical Research, 2nd ed. Chichester, UK: John Wiley & Sons, Ltd., 2002: 128–131, 202–203.
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1988; 75:800–802.
- Agustí A, de Teresa L, De Backer W, Zvarich MT, Locantore N, Barnes N, Bourbeau J, Crim C. A comparison of the efficacy and safety of once-daily fluticasone furoate/vilanterol with twice-daily fluticasone propionate/salmeterol in moderate to very severe COPD. Eur Respir J 2014; 43:763–772.
- Dransfield MT, Feldman G, Korenblat P, LaForce CF, Locantore 5, Pistolesi M, Watkins ML, Crim C, Martinez FJ. Efficacy and safety of once-daily fluticasone furoate/vilanterol (100/25 mcg) versus twice-daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patients. Respir Med 2014; 108:1171–1179.
- Svedsater H, Dale P, Garrill K, et al. Qualitative assessment of attributes and ease of use of the ELLIPTA™ dry powder inhaler for delivery of maintenance therapy for asthma and COPD. BMC Pulm Med 2013; 13:72.
- Chrystyn H, Small M, Milligan G, et al. Impact of patients' satisfaction with their inhalers on treatment compliance and health status in COPD. Respir Med 2014; 108:358–365.
- Bridges JFP, Onukwugha E, Johnson FR, Hauber ABN. ISPOR Connections 2007; 13:4–7. Available from: http://www.ispor.org/news/articles/ISPORConnections_Vol13No6_Dec15.pdf ( accessed 21 November 2014).
- Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients' preferences matter. Br Med J 2012; 345:e6572.
- Bourbeau J, Bartlett SJ, Patient adherence in COPD. Thorax 2008; 63:831–838.
- Fink JB, Colice GL, Hodder R. Inhaler devices for patients with COPD. COPD 2013; 10:523–535.
- Chong CA, Chen IJ, Naglie G, Krahn MD. How well do guidelines incorporate evidence on patient preferences? J Gen Intern Med 2009; 24:977–982.