abstract
A systematic review and meta-analysis was performed to assess the safety, efficacy and cost of Early Supported Discharge (ESD) and Hospital at Home (HAH) compared to Usual Care (UC) for patients with acute exacerbation of COPD (AECOPD). The structure of ESD/HAH schemes was reviewed, and analyses performed assuming return to hospital during the acute period (prior to discharge from home treatment) was, and was not, considered a readmission. The pre-defined search strategy completed in November 2014 included electronic databases (Medline, Embase, Amed, BNI, Cinahl and HMIC), libraries, current trials registers, national organisations, key respiratory journals, key author contact and grey literature. Randomised controlled trials (RCTs) comparing ESD/HAH to UC in patients admitted with AECOPD, or attending the emergency department and triaged for admission, were included. Outcome measures were mortality, all-cause readmissions to 6 months and cost. Eight RCTs were identified; seven reported mortality and readmissions. The structure of ESD/HAH schemes, particularly selection criteria applied and level of support provided, varied considerably. Compared to UC, ESD/HAH showed a trend towards lower mortality (RRMH = 0.66; 95% CI 0.40–1.09, p = 0.10). If return to hospital during the acute period was not considered a readmission, ESD/HAH was associated with fewer readmissions (RRMH = 0.74, 95% CI: 0.60–0.90, p = 0.003), but if considered a readmission, the benefit was lost (RRMH = 0.84; 95% CI 0.69–1.01, p = 0.07). Costs were lower for ESD/HAH than UC. ESD/HAH is safe in selected patients with an AECOPD. Further research is required to define optimal criteria to guide patient selection and models of care.
Introduction
The burden of chronic obstructive pulmonary disease (COPD) is increasing and is predicted to be the fourth-leading cause of death and seventh-leading cause of disability-adjusted life years worldwide by 2030 (Citation1). In the United Kingdom (UK), patients with acute exacerbations of COPD (AECOPD) account for one in eight emergency hospital admissions (Citation2), and have high in-hospital mortality (7.7%) and 90-day readmission (∼33%) rates (Citation3). The estimated annual healthcare cost of COPD is: 38.6 billion Euros in the European Union; 49.4 billion dollars in the United States (Citation4); and £817.5 million (Citation5) to £982 million (Citation6) in the United Kingdom, of which 30% to 50% is due to inpatient care.
Hospital at Home (HAH) “provides active treatment by health care professionals in the patient's home for a condition that would require acute hospital inpatient care” (Citation7). Early Supported Discharge (ESD) (Citation8–Citation10) aims to shorten length of stay. However, the definition of HAH and ESD varies across healthcare systems; in some settings HAH refers exclusively to admission avoidance (Citation11), whilst elsewhere HAH simply implies a higher level of care than ESD (Citation12). In published RCTs in COPD, the terms HAH and ESD are variably used to refer to similar services, and individual studies often include schemes aimed at both admission avoidance and shortening length of stay. Such schemes also vary substantially in the level of clinical and social support provided. Loan equipment, such as oxygen concentrators and nebulisers, is typically available and patients are supported by visiting respiratory specialist nurses, with medical supervision.
Some services will provide intravenous therapy and short-term social services input. ESD/HAH may encourage greater mobility and independence, and should include education on self-management, which may improve outcomes. Contrary to national guidelines, most hospitals in the United Kingdom do not offer ESD/HAH (Citation3). In AECOPD, previous meta-analyses concluded that ESD/HAH is safe (Citation13–Citation15). Two recent meta-analyses were published at similar times, but differed in their conclusions in regard to readmission risk, reflecting differences in trial selection and interpretation of the event rates and risk of bias (Citation15, Citation16).
It is advised that treatment reviews and meta-analyses are updated bi-annually (Citation17). Our meta-analysis includes an RCT published subsequent to earlier reports and compares the efficacy of ESD/HAH to usual care with respect to mortality, readmission and cost. We describe how the benefit of ESD/HAH depends on whether return to hospital during the period of acute care is considered a readmission. In contrast to other studies, in our primary analysis we excluded patients lost to follow-up. Including such patients assumes their event rate is zero and introduces bias, particularly when there are substantial differences in the proportion lost to follow-up between arms. Some RCTs included patients who did not present through accident and emergency (A&E) departments (or equivalent) (Citation18) or were not triaged for admission at the time of randomisation (i.e., patients in UC were discharged directly from A&E) (Citation19). Such patients are likely to be experiencing milder exacerbations and may have been well enough for immediate discharge from A&E; to ensure consistency and avoid bias they have been excluded.
We also review the structure of ESD/HAH schemes, and assess costs, whilst recognising the problems of comparing cost across different countries and healthcare structures.
Materials and Methods
Selection criteria and outcome measures
RCTs of ESD/HAH compared to UC in patients with a primary diagnosis of AECOPD, triaged for admission, were considered for inclusion. Studies were only included if, without ESD/HAH, all patients would have been admitted; patients could receive ESD/HAH directly from A&E provided this criterion was met. The reported outcomes are: number of patients experiencing one or more readmissions, mortality and cost.
Search strategy and selection of studies
The pre-planned search strategy included combining search terms COPD, pulmonary disease, lung disease, respiratory disease, airway disease, airway obstruction, airflow limitation, hospital at home, home care, home base, home support, early supported discharge, early discharge, hospitalisation, hospital base, hospital care, usual care and acute care. Searches using all available date ranges were conducted on databases including Medline, Embase, Amed, Cinahl and HMIC and in web-based libraries (e.g., British Library, United States National Library of Medicine and Institute of Health Economics), relevant national organisations (e.g., National Institute for Health and Clinical Excellence (NICE), BTS, Global Initiative for Chronic Obstructive Lung Disease) and current research registers (e.g., Clinical Trials Register, Current Controlled Trials Register, Centre for Health Economics). Hand-searching of relevant journals was performed to ensure abstracts and conference proceedings were retrieved and the bibliography of each trial was screened to identify any additional RCTs not retrieved in the initial search. All searches were completed by November 2014. Abstracts were screened and, if potentially eligible, full papers reviewed. All reports identified were independently assessed by three reviewers (CE, KB, SB). Disagreements were resolved with discussion.
Methodological quality assessment (Risk of bias)
Bias was assessed and reported using The Cochrane Collaboration six core risks of bias (Citation20). Bias was assessed independently by at least 2 authors for all trials (CE, KB, SB).
Data extraction
Data were extracted onto a data abstraction table. When available, characteristics of the trial (e.g., author, year of publication and journal citation, country, setting, design, methodology), study population (e.g., total number enrolled, patient characteristics, age, other important baseline characteristics), interventions (ESD/HAH and UC details), risk of bias in trials, duration of follow-up and outcomes (including outcome definition, unit of measurement) were recorded. Where possible, all data extracted were those relevant to an intention-to-treat analysis. The time points at which outcomes were collected and reported were noted. All authors were contacted to clarify reported data.
Statistical analysis
Trials with similar outcome measures were pooled in meta-analyses. For time to event (hospital readmission) data, it was not possible to extract the log of the hazard ratio [log(HR)] and its standard error from trial reports or approximate using the methods of Parmar et al. (Citation21). Consequently, we analysed readmission outcomes at the specific time points reported to estimate the risk ratio (RR). A fixed effect risk ratio was calculated for each trial using the Mantel–Haenszel approach (RRMH) and these were pooled in sub-groups of trials with similar durations of follow-up. Fixed effect models were chosen (unless otherwise stated) as most trials were similar in methodology, setting and population.
Ninety-five percent confidence intervals (CI) were calculated for the summary effect size and P < 0.05 was deemed statistically significant. Heterogeneity between trials was assessed by visual inspection of forest plots and estimation of the percentage heterogeneity between trials that cannot be ascribed to sampling variation (Citation22). If there was evidence of substantial heterogeneity, potential reasons for this were assessed.
Results
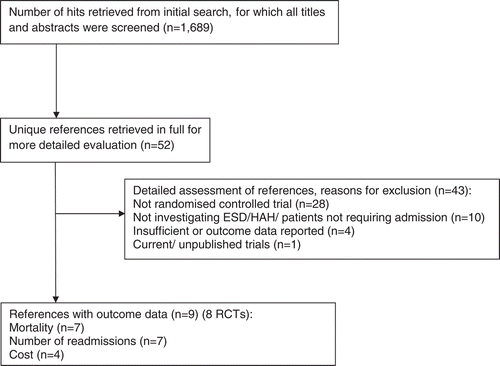
From the literature search, 1,689 references were screened. Including hand-searching, we identified 52 unique references that were retrieved in full. Eight RCTs were included, all published within the last 15 years. The majority of excluded studies were not RCTs or did not involve a comparison of ESD/HAH and usual care (see ). Seven RCTs (Citation8–Citation10, Citation12, Citation23–Citation25) were included in the mortality and readmissions review. Four trials included a cost analysis (Citation9, Citation23, Citation26, Citation27). One RCT published separate clinical (Citation10) and cost (Citation27) analyses.
Description of selected studies
Four RCTs were conducted in the UK (Citation8, Citation9, Citation12, Citation24), two in the Netherlands (Citation10, Citation25, Citation27), one in Australia (Citation26) and one in Italy (Citation23). The inclusion and exclusion criteria are summarised in . All trials randomised patients to ESD/HAH or UC and provided some description of the ESD/HAH service offered. Clinical responsibility for patients in the ESD/HAH arm remained with the hospital team until discharge from the scheme, with the exception of one trial in which the General Practitioner provided care out-of-hours and for “other medical problems” (Citation8). Reported outcomes include mortality (Citation8–Citation10, Citation12, Citation23–25), readmissions (Citation8–Citation10, Citation12, Citation23–Citation25), cost (Citation9, Citation23, Citation26, Citation27), length of in-hospital stay for UC (Citation8, Citation9, Citation12, Citation24, Citation25) and ESD/HAH (Citation8, Citation23, Citation25), total length of care (in hospital and at home) for ESD/HAH (Citation8, Citation9, Citation12, Citation23, Citation25), patient preference (Citation9, Citation12) and service satisfaction (Citation9, Citation12, Citation23, Citation26).
Methodological quality of included studies
Seven trials (Citation8–Citation10, Citation12, Citation23–Citation25) reported the method of randomisation. Two trials (Citation9, Citation10) employed computer-generated random numbers and three trials used random numbers (Citation8, Citation23, Citation25). Four trials reported allocation concealment using sealed envelopes (Citation10, Citation12, Citation24, Citation25).
Blinding of participants and treating clinicians is not possible. Only one RCT was single blind (Ricauda et al.) (Citation23); clinicians who performed baseline assessments and the researcher assessing outcomes were unaware of allocation. For subjective outcomes (e.g., quality of life) the risk of bias was considered moderate for Ricauda et al., and high in the remaining trials. Mortality is an objective outcome with low risk of bias regardless of blinding (Citation20). Readmission was regarded as objective in the Cochrane 2012 meta-analysis (Citation15). On balance, we agree, although subjective influences may affect patient behaviour.
Six trials (Citation8–Citation10, Citation12, Citation23, Citation24) included patients who were lost to follow-up, withdrew consent or were excluded from analysis (e.g., due to incorrect diagnosis). In total, 726 and 688 patients were included in the mortality and readmission analyses, respectively. The baseline characteristics of patients and risk of bias, other than blinding, in the included trials are shown in . The recruitment process and structure of ESD/HAH services outlined in . Readmission and mortality rates are shown in .
Table 1. Inclusion and exclusion criteria for trials included in the mortality and readmission analysis
Table 2. Baseline characteristics of patients and risks of bias in trials included in the mortality and readmission analysis
Table 3. Recruitment process and structure of care for ESD/ HAH services in trials
Table 4. Readmission and mortality rate in trials.
Organisational structure of ESD/HAH schemes
Two trials offered ESD/HAH from Monday to Friday only (Citation8, Citation9) and two trials provided a 7-day service (Citation23, Citation24). Utens et al. (Citation10), Nicholson et al. (Citation26) and Ojoo et al. (Citation12) provided telephone support 7 days a week, but it is unclear if patients were visited at the weekends. Patients were recruited from the A&E department (Citation23, Citation24, Citation26), or from general or speciality wards (Citation8–Citation10, Citation12, Citation25).
In most trials, patients returned home within 24 (Citation9, Citation23, Citation24) to 48 (Citation12, Citation25) hours of hospital admission. Patients were usually visited at home within 24 hours of discharge (Citation8–Citation10, Citation23, Citation24), though Nicholson et al. (Citation26) and Nissen et al. (Citation25) were unclear in this regard.
Most ESD/HAH services involved home visits from hospital-based nurses with respiratory experience, but not physicians (Citation8, Citation9, Citation12, Citation24). In one trial of patients aged 75 years and over, home visits were performed by both geriatricians and nurses; although not respiratory specialists, they are experienced in delivering HAH treatment (Citation23). In other trials, visits were performed by “generic community nurses” (Citation10) or community based nurses and General Practitioners (Citation26). Within most ESD/HAH services, the nurses could obtain medical advice from respiratory physicians (Citation8, Citation9, Citation24, Citation26).
Out of hours support varied, and included district nurses (Citation24), out of hours GP (Citation8), the on call respiratory team/ medical chest unit/ respiratory ward (Citation9, Citation12, Citation28), or the HAH team (which included nurses and physicians) (Citation23); in one study this information was unclear (Citation25). Five trials (Citation8, Citation9, Citation23–Citation25) reported the number of home visits, which ranged from a mean of 2.6 visits (Citation25), to 14.1 visits from nurses and 9.9 visits from geriatricians (Citation23). The trial with the least number of nurse home visits also offered a telephone support service. The mean (SD) number of telephone calls from patient to nurse was 0.76 (1.34) and from nurse to patient was 1.56 (1.31).
Two trials offered patient and carer education (Citation12, Citation23), including recognition and management of AECOPD, to the ESD/HAH group only. Other support offered includes social support (Citation23, Citation24), physiotherapy (Citation23, Citation25), and counselling and occupational therapy (Citation23).
Mortality
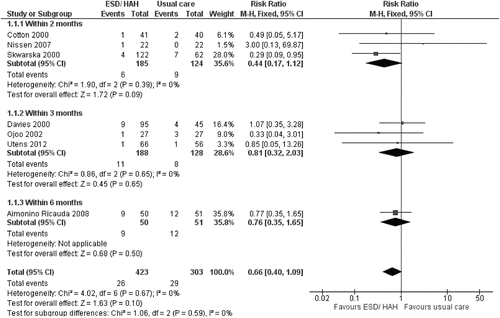
Meta-analysis of seven RCTs (Citation8–Citation10, Citation12, Citation23–Citation25), assessing 726 participants showed a trend towards lower risk of death within 2 to 6 months favouring ESD/HAH (RRMH = 0.66, 95% CI: 0.40–1.09, p = 0.10) (Figure 2). The percentage of the variability in effect estimates due to heterogeneity rather than sampling error (chance) was not important (I2 = 0%).
The results using a random effects model were similar (RRMH = 0.67, 95% CI: 0.40–1.11, p = 0.12), which suggests a small amount of between trial variation. To investigate the possibility of publication bias, the analysis was performed only including the largest trials, which did not reduce the treatment effect but widened the confidence interval (0.60, 95% CI: 0.28–1.25, p = 0.29) (Citation9, Citation10, Citation24). We performed a sensitivity analysis excluding the trial with the most select population (patients aged 75 and over) (RRMH = 0.60, 95% CI: 0.32–1.16, p = 0.13) (Citation23). Finally, the analysis was repeated including all patients lost to follow-up (assuming zero event rate) to allow comparison to previous meta-analyses, which adopted this approach (RRMH = 0.67, 95% CI: 0.41–1.10, p = 0.11) (Citation15, Citation16).
Readmissions
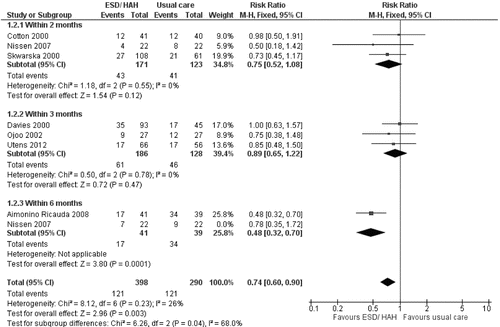
Meta-analysis of seven RCTs (Citation8–Citation10, Citation12, Citation23–Citation25), assessing 688 participants, assuming return to hospital during ESD/HAH was not a readmission, showed ESD/HAH was associated with a lower risk of readmission within 2 to 6 months than UC (RRMH = 0.74, 95% CI: 0.60–0.90, p = 0.003). This, and the time periods for readmission, are shown in . The percentage of the variability in effect estimates that was due to heterogeneity rather than chance was I2 = 26%. An analysis with random effects was similar (RRMH = 0.72, 95% CI: 0.57–0.92, p = 0.010).
The results were not robust to a sensitivity analysis that excluded the trial of Aimonino Ricauda et al. (Citation23), in which patients were limited by age (greater or equal to 75 years) (RRMH = 0.83, 95% CI: 0.65–1.05, p = 0.11, I2 = 0%).
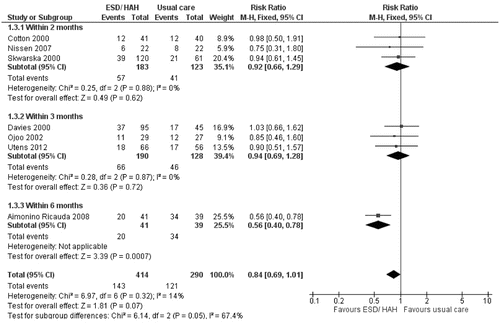
The benefit was also not seen when including all trials with return to hospital during the period care within ESD/HAH classed as a readmission (RRMH = 0.84; 95% CI 0.69–1.01, p = 0.07) ().
Finally, the analysis was repeated including patients lost to follow-up. Once again ESD/HAH was associated with fewer readmissions (RRMH = 0.75, 95% CI: 0.61–0.93, p = 0.007), but not if return to hospital during ESD/HAH was considered a readmission (RRMH = 0.88 95% CI: 0.72–1.07, p = 0.21).
Service costs
Three trials performed cost analyses (Citation9, Citation23, Citation26), and one trial performed cost-effectiveness and cost utility analysis (Citation27). There is substantial variation in the costs that were included in the analyses, and how these costs were assessed.
Although the trials were conducted in different countries with different healthcare systems, the cost per episode of healthcare associated with ESD/HAH was consistently lower than UC (UK ESD/HAH = £877, UC = £1753 (Citation9); Italy ESD/HAH = ∃1,175.9, UC = ∃1,390.9 (Citation23); Netherlands ESD/HAH = €1,219, UC = €1,463 (Citation27); and Australia ESD/HAH = Aus∃745, UC = Aus∃2543) (Citation26).
Only one trial assessed costs beyond the acute event; both healthcare and societal costs were reported over three months (Citation27). Healthcare costs for the acute period (the period receiving in hospital or home treatment)) and the acute and follow-up periods combined (ESD/HAH = €4,129, UC = €4,297) marginally favoured ESD/HAH. However, during the follow-up period alone, usual care was less expensive (ESD/HAH = €2,910, UC = €2,834). The largest costs during the follow-up period were due to community nursing and readmissions. Readmission costs were equal in both arms (€941), however in the usual care arm a larger proportion of patients were lost to follow-up. This may have underestimated the readmission cost in UC by underestimating the readmission rate. UC patients had a marginally lower mean change in their Clinical COPD questionnaire, reflecting a smaller deterioration in symptoms.
The UC group had marginally higher QALYs, though the difference was small and statistically non-significant. Therefore, from a healthcare perspective HAH was associated with a savings per QALY lost of €31,111. This is not consistent with other studies that tend to show improved quality of life with ESD/HAH though do not report in-depth economic evaluations. When costs from a societal perspective were also considered, including formal and informal carer costs and production losses for the patient, over the acute and follow-up periods combined, ESD/HAH was more expensive than UC (ESD/HAH = €6,304, UC = €5,395).
Discussion
Principal findings
Compared to UC, ESD/HAH was associated with a trend towards lower mortality in trials reporting outcome between 2 and 6 months after discharge from hospital or ESD/HAH.
ESD/HAH was associated with a lower rate of all-cause readmission than UC at 2 to 6 months provided all trials were included and that return to hospital during the period of acute care within ESD/HAH was not considered a readmission. Of importance, the trial by Aimonino Ricauda et al. (Citation23) was age restrictive, education was only routinely provided in the ESD/HAH arm and there was a high event rate in the UC arm (after correction for age and co-morbidity).
If this trial is excluded from the analysis, the difference in readmission rates is no longer significant. However, this trial (Citation23) provides strong evidence that patients aged 75 and over may be safely included in ESD/HAH schemes. Most patients hospitalised with AECOPD are elderly (Citation3) and older patients are most at risk of readmission (Citation29), and death (Citation29–Citation31).
Conceptually, if patients receiving ESD/HAH remain fully under the care of the specialist hospital based team, return to hospital during the period of acute care may be regarded as a transfer to a higher level of care within the same episode, rather than a readmission. This may also be of interest to commissioners and inform service tariffs. Whilst the distinction between HAH and ESD is blurred, return to hospital during a period of ESD is more typically regarded as a readmission. Regardless of the service description and level of care provided, the patient and their carers may regard return to hospital as a failure of ESD/HAH and the event as a readmission, which may have a negative impact on quality of life and service satisfaction. If this approach is adopted, readmission rates were similar for ESD/HAH and UC.
Compared to UC, ESD/HAH is associated with a shorter in-hospital stay, although the total period of care tends to be longer. This does not necessarily mean that patients receiving ESD/HAH are kept under review unnecessarily. Pressures to reduce length of stay in hospital may have led to patients being discharged earlier than is optimal. If return to hospital is regarded as a readmission, this favours UC because UC patients cannot be ‘readmitted’ during their inpatient stay. Conversely, as the total period of care is longer in ESD/HAH, and the risk of readmission is highest in the early discharge period, not defining return to hospital as a readmission may favour ESD/HAH. In common with earlier reviews (Citation15, Citation16), we estimate that 23% of patients could be safely treated at home.
Service costs relating to health during the initial treatment phase favour ESD/HAH over UC. For most studies, a description and breakdown of the cost calculations are not provided. Due to this, and heterogeneity of studies, identifying the most cost-effective model is not possible. Goossens et al. (Citation27) provide a detailed cost analysis, and when considering health and social costs combined, ESD/HAH is more expensive. In this study all patients spent three days in hospital, and so this model is closer to ESD than HAH, and the results may have been different if the patients had returned home soon after admission or hospital admission was avoided. Patient preference favours ESD/HAH over UC, whilst service satisfaction appears to be similar although further robust trials are required.
Strengths and weaknesses of meta-analysis and comparison of included studies
This review and meta-analysis has provided an up-to-date analysis of ESD/HAH compared to UC for AECOPD and includes a trial (Citation10) not published at the time of previous reviews (Citation15, Citation16). We employed a comprehensive search strategy and contacted the corresponding authors to verify data when necessary.
The included trials were conducted in different countries with different healthcare systems. The diagnostic criteria for AECOPD were similar, but there were important variations in inclusion criteria and the structure and organisation of ESD/HAH services. Some trials did not offer enrolment at the weekends (Citation8, Citation9, Citation12), reducing both the cost of, and the number of patients who could access, ESD/HAH. In two trials (Citation23, Citation24) patients were recruited directly from A&E or the Emergency Department, facilitating quicker discharge home, whilst in other trials (Citation8–Citation10, Citation12, Citation25) patients were recruited from wards, allowing a period of stabilisation and observation as an inpatient. It is likely that offering both pathways, tailored to the individual patient, would optimise costs and the proportion of patients suitable to access the service.
The structure of ESD/HAH services varied, including the healthcare professionals involved, the number of home visits, telephone support, access to medical services such as home oxygen, and provision of temporary social support. Differences in selection criteria and service structure may, in part, explain the striking variation in the level of support provided in the ESD/HAH arm; the mean number of home visits ranged from 2.6 nurse visits (Citation25) to 14.1 nurse and 9.9 physician home visits (Citation23).
Amongst patients with AECOPD who require in-hospital or ESD/HAH treatment, those with more severe exacerbations and/or poor performance status may require greater clinical and social support at home. A more comprehensive service, offering frequent visits from professionals, home oxygen therapy and temporary social services if required, will allow inclusion of a broader spectrum of patients. Although this will increase the cost of ESD/HAH, it may still be less expensive than UC.
In some trials there were differences in the elements of care provided in each arm. For example, in one trial, education, including exacerbation self-management, was provided to patients and carers in the ESD/HAH group, but not to the UC group (Citation23).
The period of follow-up varied; this influenced the event rate. To address this, we initially planned to analyse results using hazard ratios, but the data required were not available and could not be estimated using Parmar's methods (Citation21); therefore, risk ratios were calculated.
Differentiating between ESD and HAH is challenging, and in this review we have considered both together. HAH is an appropriate term for patients that have their entire episode treated at home, without admission. The term HAH is also used in some healthcare systems for patients who are assessed in the medical admission unit and return home for treatment the same day or the following morning if admitted overnight.
For patients who deteriorate at home, during the period of care under ESD/HAH, an overnight stay in hospital is often defined as a readmission. However, equally this may be considered an escalation in level care within a single acute episode, and alternatively defined as “return to hospital.” We have analysed the data separately, where possible, to reflect this variation. Some patients may have a brief assessment in an emergency department or ambulatory setting without an overnight stay; this would typically not be considered a readmission, and we consider that the RCTs described were consistent in this respect.
Comparison with previous meta-analyses
Trial selection
Two meta-analyses comparing ESD/HAH and UC published in 2012 came to different conclusions with regards to outcome. Jeppesen et al. (Citation15) reported moderate quality evidence that ESD/HAH was associated with lower readmission risk than UC (RR 0.65, 95% CI 0.59–0.99, p = 0.04) (Citation15), which was further strengthened following exclusion of the trial deemed to have the highest risk of bias (CI 0.58 to 0.91; p = 0.006), and moderate evidence of a trend towards a reduction in mortality. In contrast, McCurdy (Citation16) found no significant difference in readmission and mortality rates. The evidence was regarded as low to very low in quality, with a need for further research.
We did not include the study by Nicholson et al. (Citation26), which primarily compares costs, in our meta-analysis of readmission. We shared the concerns reported by Jeppesen et al., which is why they excluded this paper in a sensitivity analysis. Nicholson et al. may have included patients referred by the outpatient department. No information was provided on baseline function, the randomisation process, allocation concealment, mortality or readmissions. Data on readmissions was obtained at the time of the Cochrane review, but the period of follow-up is unclear. In the ESD/HAH arm the risk ratio for readmission was high compared to other trials (Citation8, Citation9, Citation12, Citation19, Citation24), however due to the small number of subjects, the confidence intervals are wide (RR = 2.77, 95% CI:0.69 to 11.17).
We excluded Hernandez et al. (Citation19), which was included by Cochrane, because patients attending A&E with an AECOPD without the need for admission were considered eligible; 38.6% of the patients in the UC arm were discharged directly from A&E. A similar proportion of those treated within ESD/HAH would be expected to not otherwise require admission, thus this structure of care does not meet the definition of ESD/HAH for all included patients. Whether or not this group of patients benefit from home support is of importance, but is not the subject of this review.
Patient events
McCurdy differs from Jeppesen et al. (15) in the number of events because McCurdy classes return to the hospital during ESD/HAH as a readmission. Neither adjusted their analyses for patients who die prior to discharge, yet such patients are not at risk of readmission.
McCurdy discusses the issues surrounding missing data, but, like Cochrane, did not make any adjustment in the analysis. Both performed an intention to treat analysis, but included those patients lost to follow-up in whom outcome data was not available. This assumes their event rate is zero, though other RCTs suggest that patients with missing data have a higher event rate than the population with complete data (Citation32). The ideal approach to missing data is multiple imputation, but this requires raw trial data.
We analysed readmission rates with and without return to hospital counting as a readmission and with and without patients lost to follow-up. We are grateful to all authors who clarified data.
Implications for clinicians and policymakers
AECOPD are associated with substantial morbidity, mortality and healthcare costs. It is imperative that clinically and cost effective methods to reduce admissions and readmissions are considered and implemented. In selected patients presenting with AECOPD, ESD/HAH schemes substantially reduce length of stay, with similar or lower mortality and readmission rates compared to conventional inpatient care. Despite this, many Trusts currently do not offer such services.
Future research
We recommend that future RCTs of ESD/HAH clearly define readmission, and provide data on patients who return to hospital during ESD/HAH and whether these same patients are readmitted during the follow-up period.
The optimal selection criteria and structure of care for ESD/HAH services is unclear. Selection of patients should be based on their chance of surviving the acute episode, among other factors. The application of a robust prognostic tool for use in AECOPD would potentially be very useful in this respect (Citation33). It is likely that a tailored approach to ESD/HAH, depending on the clinical and social dependency and performance status of each patient would be most efficient. Compared to basic ESD/HAH schemes primarily reliant on specialist respiratory nurses, multi-disciplinary interventions including higher levels of clinical support, temporary social support and input from occupational therapists and physiotherapists may allow a broader spectrum of patients to access ESD/HAH.
Incorporating services such as early pulmonary rehabilitation and education for both patients and carers within ESD/HAH is likely to confer additional benefits. The clinical outcomes and costs associated with different models of ESD/HAH warrant further study. A better understanding of patients, carers and clinicians views of ESD/HAH may help inform the refinement and expansion of these services. Cost analyses should be based on actual costs rather than tariff and include all direct and indirect costs, including temporary social care, primary care and readmission costs. ESD/HAH schemes may foster greater independence and reduce the risk of subsequent readmission, particularly if combined with education, including self-management. Consequently, ESD/HAH could be provided for readmissions as well as the index admission, and costs analysed across all episodes.
Declaration of Interest Statement
There is no financial, personal, academic, employment, consultancy, ownership or intellectual conflicts of interest to be declared. No sponsorship was received for conducting this review. The authors alone are responsible for the content and writing of the paper.
References
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3(11):442. PubMed PMID: 17132052. Pubmed Central PMCID: PMC1664601. Epub 2006/11/30. eng.
- Healthcare Commission. Clearing the air. A national study of chronic obstructive pulmonary disease. Great Britain: Commission for Healthcare Audit and Inspection. 2006.
- Royal College of Physicians, British Thoracic Society, British Lung Foundation. Report of the National Chronic Obstructive Pulmonary Disease Audit 2008: Clinical audit of COPD exacerbations admitted to acute NHS trusts across the UK. London: Royal College of Physicians, 2008.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4):347–365. PubMed PMID: 22878278. Epub 2012/08/11. eng.
- Guest JF. The annual cost of chronic obstructive pulmonary disease to the UK's National Health Service. Dis Mgmt Health Outcom 1999; 5(2):93–101.
- Britton M. The burden of COPD in the U.K.: results from the Confronting COPD survey. Respir Med 2003; 97 Suppl C:S71–79. PubMed PMID: ovid.com:/bib/medline/12647945.
- Shepperd S, Doll H, Broad J, Gladman J, Iliffe S, Langhorne P, et al. Hospital at home early discharge. Cochrane Database System Rev 2009 (1):CD000356. PubMed PMID: 19160179. Pubmed Central PMCID: PMC4175532. Epub 2009/01/23. eng..
- Cotton MM, Bucknall CE, Dagg KD, Johnson MK, MacGregor G, Stewart C, et al. Early discharge for patients with exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Thorax 2000; 55(11):902–906.
- Skwarska E, Cohen G, Skwarski KM, Lamb C, Bushell D, Parker S, et al. Randomized controlled trial of supported discharge in patients with exacerbations of chronic obstructive pulmonary disease. Thorax 2000; 55(11):907–912.
- Utens CM, Goossens LM, Smeenk FW, Rutten-van Molken MP, van Vliet M, Braken MW, et al. Early assisted discharge with generic community nursing for chronic obstructive pulmonary disease exacerbations: results of a randomised controlled trial. BMJ Open 2012; 2(5). PubMed PMID: 23075570. Pubmed Central PMCID: PMC3488726. Epub 2012/10/19. eng.
- Leff B, Burton L, Mader SL, Naughton B, Burl J, Inouye SK, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med 2005; 143(11):798–808. PubMed PMID: 16330791. Epub 2005/12/07. eng.
- Ojoo JC, Moon T, McGlone S, Martin K, Gardiner ED, Greenstone MA, et al. Patients' and carers' preferences in two models of care for acute exacerbations of COPD: Results of a randomised controlled trial. Thorax 2002; 57(2):167–169.
- Ram FS, Wedzicha JA, Wright J, Greenstone M. Hospital at home for patients with acute exacerbations of chronic obstructive pulmonary disease: systematic review of evidence. Br Med J 2004; 329(7461). doi:10.1136/bmj.38159.650347.55 (published 8 July 2004)
- Ram FSF, Wedzicha JA, Wright JJ, Greenstone M, Lasserson TJ. Hospital at home for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database System Rev 2009 (4). 20 OCT 2003. DOI: 10.1002/14651858.CD003573
- Jeppesen E, Brurberg KG, Vist GE, Wedzicha JA, Wright JJ, Greenstone M, et al. Hospital at home for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database System Rev 2012 (5). 16 MAY 2012. DOI: 10.1002/14651858.CD003573.pub2
- McCurdy BR. Hospital-at-home programs for patients with acute exacerbations of chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ontario Health Technol Assess Ser 2012; 12(10):1–65. PubMed PMID: 23074420. Pubmed Central PMCID: PMC3384361. Epub 2012/10/18. eng.
- Higgins JPT GS, Scholten RJPM. Maintaining reviews: updates, amendments and feedback. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, 1st ed Chichester, UK: John Wiley and Sons, Ltd.; 2008: 32.
- Shepperd S, Harwood D, Jenkinson C, Gray A, Vessey M, Morgan P. Randomised controlled trial comparing hospital at home care with inpatient hospital care. I: three month follow up of health outcomes. Br Med J 1998; 316(7147):1786–1791. PubMed PMID: PMC28578.
- Hernandez C, Casas A, Escarrabill J, Alonso J, Puig-Junoy J, Farrero E, et al. Home hospitalisation of exacerbated chronic obstructive pulmonary disease patients. Euro Respir J 2003; 21(1):58–67.
- Higgins JPT Assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. 1st ed. Chichester, UK: John Wiley and Sons Ltd, 2009: 196.
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17(24):2815–2834. PubMed PMID: 9921604. Epub 1999/01/28. eng.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J 2003; 327(7414):557–560. PubMed PMID: 12958120. Pubmed Central PMCID: PMC192859. Epub 2003/09/06. eng.
- Aimonino Ricauda N, Tibaldi V, Leff B, Scarafiotti C, Marinello R, Zanocchi M, et al. Substitutive “hospital at home” versus inpatient care for elderly patients with exacerbations of chronic obstructive pulmonary disease: A prospective randomized, controlled trial. J Amer Geriatr Soc 2008; 56(3):493–500.
- Davies L, Wilkinson M, Bonner S, Calverley PM, Angus RM. “Hospital at home” versus hospital care in patients with exacerbations of chronic obstructive pulmonary disease: prospective randomised controlled trial. Br Med J 2000; 321(7271):1265–1268.
- Nissen I, Jensen MS. Randomised controlled trial of nurse-supported discharge of patients with exacerbation of chronic obstructive pulmonary disease [Danish] Sygeplejeassisteret hjemmebehandling af eksacerbation i kronisk obstruktiv lungesygdom. Ugeskrift for Laeger 2007; 169(23):2220–2223.
- Nicholson C, Bowler S, Jackson C, Schollay D, Tweeddale M, O'Rourke P. Cost comparison of hospital- and home-based treatment models for acute chronic obstructive pulmonary disease. Austral Health Rev 2001; 24(4):181–187.
- Goossens LM, Utens CM, Smeenk FW, van Schayck OC, van Vliet M, van Litsenburg W, et al. Cost-effectiveness of early assisted discharge for COPD exacerbations in The Netherlands. Value in Health 2013; 16(4):517–528. PubMed PMID: 23796285. Epub 2013/06/26. eng.
- Utens CM, Goossens LM, Smeenk FW, van OCO, van W, Janssen A, et al. Effectiveness and cost-effectiveness of early assisted discharge for chronic obstructive pulmonary disease exacerbations: the design of a randomised controlled trial. BMC Public Health 2010;10: 618.
- McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest 2007; 132(6):1748–1755. PubMed PMID: ovid.com:/bib/medline/17890477.
- Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med 2003; 163(10):1180–1186. PubMed PMID: ovid.com:/bib/medline/12767954.
- Roche N, Zureik, M, Soussan, D, Neukirch, F, Perrotin, D, Urgence BSC. Predictors of outcomes in COPD exacerbation cases presenting to the emergency department. Euro Respir J 2008; 32(4):953–961. PubMed PMID: ovid.com:/bib/medline/18508819.
- Akl EA, Briel M, You JJ, Sun X, Johnston BC, Busse JW, et al. Potential impact on estimated treatment effects of information lost to follow-up in randomised controlled trials (LOST-IT): systematic review. Br Med J 2012; 344:e2809. PubMed PMID: 22611167. Epub 2012/05/23. eng.
- Steer J, Gibson J, Bourke SC. The DECAF Score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax 2012; 67(11):970–976. PubMed PMID: 22895999. Epub 2012/08/17. Eng.