Abstract
Mucosal-associated invariant T (MAIT) cells have been reported to play an important role in mucosal immunity. However, little is known about the roles of MAIT cells in chronic obstructive pulmonary disease (COPD). The aims of this study were to examine the levels of circulating MAIT cells and their subsets in COPD patients and to investigate the potential relationship between clinical parameters and MAIT cell levels. Forty-five COPD patients and 57 healthy control subjects were enrolled in the study. Circulating MAIT cells and their subset levels in the peripheral blood were measured by flow cytometry. Disease grades were classified according to the GOLD criteria for the assessment of severity of COPD. Circulating MAIT cell levels were found to be significantly reduced in COPD patients. In particular, this MAIT cell deficiency was more prominent in CD8+ and double-negative T cell subsets. Interestingly, elevated serum C-reactive protein level and reduced FEV1/FVC ratio were associated with MAIT cell deficiency in COPD patients. Furthermore, the circulating MAIT levels were found to be significantly lower in patients with moderate to severe COPD than in patients with mild COPD. Our data shows that MAIT cells are numerically deficient in the peripheral blood of patients with COPD. In addition, this MAIT cell deficiency was found to reflect inflammatory activity and disease severity. These findings provide important information for monitoring the changes in MAIT cell levels and for predicting the prognosis during the disease course.
Introduction
Chronic obstructive pulmonary disease (COPD) remains a major public health problem, and it is projected to be the fourth-leading cause of death worldwide by the year 2030 (Citation1). Global initiative for Chronic Obstructive Lung Disease (GOLD) experts have defined this disease as a common preventable and treatable disease with some extrapulmonary effects, characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases (Citation2). The primary risk factor for the development of COPD is cigarette smoking, but other factors such as burning biomass fuels for cooking and heating could also be important causes of this disease (Citation3, Citation4). The chronic airflow limitation characteristic of COPD is caused by a mixture of small airway disease (obstructive bronchiolitis) and parenchymal destruction (emphysema). Therefore, the diagnosis of COPD is functionally performed by using the spirometric criterion of the post-bronchodilator forced expiratory volume in 1 second (FEV1) to the forced vital capacity (FVC) fixed ratio of less than 70% (Citation2).
Mucosal-associated invariant T (MAIT) cells have recently emerged as one of the unique invariant T cell subsets, and they display their anti-microbial function in an innate-like manner (Citation5, Citation6). Human MAIT cells are relatively abundant in the peripheral blood, with a wide distribution ranging from 0.19% to 21.7% of the total T-cell population, as compared with invariant natural killer T (iNKT) cells, ranging from 0.01% to 5.15% of the total T-cell population (Citation7, Citation8). MAIT cells express an invariant T cell receptor (TCR) α-chain (Vα7.2-Jα33/12/20 in humans) paired with a limited set of Vβ chains (Citation9, Citation10).
In contrast to conventional T cells, which recognize peptide antigens bound to major histocompatibility complex (MHC) molecules, MAIT cells recognize bacteria-derived riboflavin (vitamin B2) metabolites presented by the MHC class 1b-like related protein (MR1) (Citation11, Citation12). Based on the expression of CD4 and CD8, MAIT cells can be subdivided into CD4+/CD8- (CD4+), CD4-/CD8+ (CD8+), and CD4/CD8 double-negative (DN) subsets (Citation8, Citation9, Citation13). Upon antigen recognition, MAIT cells rapidly produce Th1/Th17 cytokines, including interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and interleukin (IL)-17, in an innate-like manner (Citation14, Citation15).
Complex interactions between innate and adative immune responses to cigarette smoke can cause inflammatory cellular infiltration of the alveolar walls and remodeling of the small airways which can cause resistance to flow (Citation16). Such inflammation in the lung is known to be mostly mediated by CD4+ helper T cells (Citation16). Furthermore, an increased proportion of IFN-γ and TNF-α producing CD8+ T-cells and a decreased frequency of iNKT cells in the peripheral blood of COPD patients were reported (Citation17, Citation18). However, little is known about the relevance of MAIT cells in COPD. The aims of this study were to examine the levels of circulating MAIT cells and their subsets in COPD patients and to investigate the potential relationship between clinical parameters and MAIT cell levels.
Patients and Methods
Patients
The study cohort included 45 patients who had COPD (3 women and 42 men; mean age ± SD, 69.5 ± 6.9 years) according to the GOLD criteria (post-bronchodilator FEV1/FVC ratio < 70%) (Citation2), and 57 healthy control (HC) subjects (27 women and 30 men; mean age ± SD, 64.3 ± 9.1 years) who had no history of respiratory disorders such as COPD and pulmonary embolism, autoimmune diseases, infectious diseases, recent surgery, malignancies, left ventricular dysfunction, use of immunosuppressive drugs, and chronic liver, renal, or endocrine diseases. Inclusion criteria for HC subjects were: (i) current or former smokers with normal lung function (FEV1/FVC ratio ≥ 70% and FEV1 ≥ 80%); (ii) no pulmonary disease as determined by the history and chest X-ray; and, (iii) no fever during the 72 h prior to enrollment. All subjects were enrolled at Chonnam National University Hospital from March 2013 to June 2014. Pulmonary function tests were performed using a Sensormedics 2400 unit (Sensormedics, San Diego, CA, USA) that met the standards of the American Thoracic Society (Citation2). The study protocol was approved by the Institutional Review Board of Chonnam National University Hospital, and written informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Sample size estimation for comparing the MAIT cell frequency between COPD patients and HCs
We initially carried out a pilot study in a sample of 15 COPD patients and 20 HCs, and we assessed the means and standard deviations of MAIT cell frequency in COPD patients and healthy controls (for COPD patients, 1.0% and 1.5%; and for HCs, 2.3% and 3.0%, respectively). Based on the pilot study, sample sizes were estimated by SISA software online (http://www.quantitativeskills.com/sisa/calculations/samsize.htm) using the two-tailed t-test analysis with 80% power and α = 0.05. The estimated sample sizes were 42 and 55 for COPD patients and HCs, respectively, which were almost equal to slightly lower than our actual sample sizes (i.e., 45 and 57, respectively; ).
Table 1. Clinical and laboratory characteristics of 45 COPD patients
Monoclonal antibodies (mAbs) and flow cytometry
The following mAbs and reagents were used in this study: PE-Cy5-conjugated anti-CD161, FITC-conjugated anti-TCR γδ and PE-Cy7-conjugated anti-CD8α (all from Becton Dickinson, San Diego, CA, USA); APC-conjugated anti-TCR Vα7.2 (BioLegend, San Diego, CA, USA); and APC-Alexa Fluor 750-conjugated anti-CD3 and Pacific Blue-conjugated anti-CD4 (Beckman Coulter, Marseille, France). Dose-dependent titration analysis was performed to determine the optimal dilution point of APC-conjugated anti-TCR Vα7.2 for MAIT cell staining. The analysis showed that the optimal volume ratio of antibody to PBMC sample was 1 μL to 50 μL.
Furthermore, APC-conjugated IgG isotype (Becton Dickinson, San Diego, CA, USA) was used for the validation of background autofluorescence in peripheral blood. The validation assay using isotype control revealed that the background autofluorescence intensity was below the detection limit (0.01%) in the MAIT cell gate. Cells were stained with combinations of appropriate mAb for 20 min at 4°C. Stained cells were analyzed on a Navios flow cytometer using Kaluza software (Beckman Coulter, Brea, CA, USA).
Isolation of peripheral blood mononuclear cells (PBMCs) and identification of MAIT cells
Peripheral venous blood samples were collected in heparin-containing tubes, and PBMCs were isolated by density-gradient centrifugation using Ficoll-Paque Plus solution (Amersham Biosciences, Uppsala, Sweden). MAIT cells exhibit high expression of the C-type lectin CD161 (Citation13). Thus, human MAIT cells are phenotypically defined as CD3+TCRγδ-Vα7.2+CD161high cells by flow cytometry as previously described (Citation5, Citation14, Citation19). Flow-Check Pro Fluorospheres (Beckman Coulter, Marseille, France) were used daily for the verification of optical alignment and fluidic systems of a flow cytometer. All tests were performed on freshly isolated PBMC samples together with quality control (QC) materials. These test samples were not stored in liquid nitrogen. QC materials were made from 4% paraformaldehyde-fixed PBMC samples with a high level (about 10%) or a low level (about 1%) of MAIT cell frequency. The precision of our QC materials was tested at two levels, by running two replicates over 10 days to assess reproducibility (day-to-day variability), according to the modification of the CLSI EP15-A3 protocol (Citation20). Day to day variability was presented as the coefficient of variation (CV): for high level QC and low level QC, 7.20% and 4.99%, respectively.
Statistical analysis
Comparisons of MAIT cells were performed by analysis of covariance after adjusting for age and sex using the Bonferroni correction for multiple comparisons. Linear regression analysis was used to test associations between MAIT cell levels and clinical variables. P values less than 0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 18.0 software (SPSS, Chicago, IL, USA).
Results
Subject characteristics
The clinical and laboratory characteristics of the COPD patients are summarized in . Forty-five patients with COPD during 1-year period were included in this study. Another spirometric criterion (FEV1) can classify the severity of COPD into the following 4 grades: GOLD 1, mild COPD (FEV1 ≥ 80%); GOLD 2, moderate COPD (50% ≤ FEV1 < 80%); GOLD 3, severe COPD (30% ≤ FEV1 < 50%); and GOLD 4, very severe COPD (FEV1 < 30%) (Citation2). Of the 45 COPD patients tested by spirometry, 5 patients (11.1%) had mild COPD; 18 patients (40.0%) had moderate COPD; 17 patients (37.7%) had severe COPD; and 5 patients (11.1%) had very severe COPD. The number of current smokers, former smokers, and non-smokers was as follows: 19 current smokers, 20 former smokers, and 6 non-smokers among COPD patients; and 11 current smokers, 22 former smokers, and 24 non-smokers among HCs, respectively.
Reduced numbers of circulating MAIT cells in COPD patients
The percentages and absolute numbers of MAIT cells in the peripheral blood samples of the 45 COPD patients and the 57 healthy controls (HCs) were determined by flow cytometry. All comparisons of percentages and absolute numbers of MAIT cells were performed by analysis of covariance after adjusting for age and sex using the Bonferroni correction for multiple comparisons. MAIT cells were defined as CD3+TCRγδ- cells expressing TCR Vα7.2 and CD161high (). Percentages of circulating MAIT cells were significantly lower in COPD patients than in HCs (median 0.32% versus 1.17%, p < 0.05; ).
Figure 1. Reduced circulating mucosal-associated invariant T (MAIT) cell numbers in the peripheral blood samples of chronic obstructive pulmonary disease (COPD) patients. Freshly isolated peripheral blood mononuclear cells from 57 healthy controls (HCs) and 45 COPD patients were stained with allophycocyanin (APC)-Alexa Fluor 750-conjugated anti-CD3 and fluorescein isothiocyanate-conjugated anti-CD161 monoclonal antibodies and then analyzed by flow cytometry. Percentages of MAIT cells were calculated using a αβ T cell gate. (a) Representative MAIT cell percentages as determined by flow cytometry. (b) MAIT cell percentages among peripheral blood αβ T cells. (c) Absolute MAIT cell numbers (per microliter of blood). Symbols represent individual subjects and horizontal lines indicate median values. (d) MAIT cell subset percentages among peripheral blood αβ T cells. Data are shown as box plots. Each box represents the 25th and 75th percentiles. Lines inside the boxes represent the median. Whiskers represent the 10th and 90th percentiles. *p < 0.05, **p < 0.005.
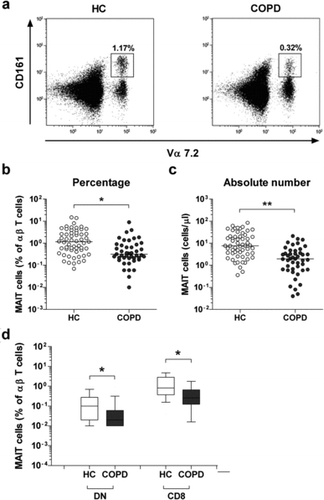
Absolute numbers of MAIT cells were calculated by multiplying MAIT fractions by CD3+TCRγδ- cell fractions and total lymphocyte numbers (per microliter of peripheral blood). COPD patients had significantly lower absolute numbers of MAIT cells than HCs (median 1.96 cells/μL versus 7.68 cells/μL, p < 0.005; ). As previously observed (Citation5, Citation9, Citation13, Citation19), the majority of MAIT cells in blood consisted of CD8+ and double-negative (DN) subsets. Percentages of CD8+ MAIT cell subsets of αβ T cells were significantly lower in COPD patients than in HCs (median 0.26% versus 0.82%, p < 0.05).
Moreover, COPD patients had significantly lower percentages of DN subsets as compared with HCs (median 0.02% versus 0.1%, p < 0.05; ). In addition, COPD patients and healthy controls were subdivided into the current smoker, former smoker, and non-smoker subgroups according to smoking status. However, no significant differences in circulating MAIT cell percentages were observed among current smokers, former smokers, and non-smokers in both the COPD and HC groups (Data not shown). These results suggest that circulating MAIT cell levels are not affected by smoking status
Relationship between circulating MAIT cell levels and clinical parameters in COPD patients
To evaluate the clinical relevance of MAIT cell levels in COPD patients, we investigated the relationship between circulating MAIT cell levels and clinical parameters by performing the regression analysis (). Because the distributions were skewed, the percentages of MAIT cells were log-transformed for the analysis. Univariate linear regression analysis showed that log-transformed percentages of MAIT cells were significantly correlated with post-bronchodilator FEV1/FVC ratio and CRP levels (p = 0.007 and p = 0.033, respectively). However, after multivariate analysis, only the FEV1/FVC ratio was found to be significantly correlated with log-transformed percentages of MAIT cells (p = 0.014; ).
Table 2. Regression coefficients for log-transformed percentage of MAIT cells with respect to clinical and laboratory parameters in the 45 COPD patients
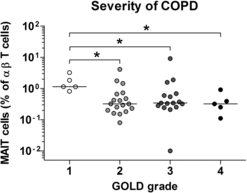
Furthermore, when the severity of COPD was subclassified according to the GOLD grading system (Citation2), percentages of circulating MAIT cells were significantly lower in patients with moderate to very severe COPD than in patients with mild COPD (median 0.32% versus 1.14% [p < 0.05]; 0.34% versus 1.14% [p < 0.05]; 0.32% versus 1.14% [p < 0.05], respectively; ). However, no significant correlation was found between MAIT cell frequencies and age, body-mass index (BMI), smoking, FVC, FEV1, total lung capacity (TLC), diffusing capacity of the lung for carbon monoxide (DLCO), ratio of DLCO to alveolar volume (DLCO/VA), PaO2, PaCO2, hemoglobin levels, leukocyte counts, neutrophil counts, lymphocyte counts, platelet counts, CD3+ T cell levels, or CD3+TCRγδ+ cell levels ().
Figure 2. Reduced circulating mucosal-associated invariant T (MAIT) cell frequency in patients with advanced stage of chronic obstructive pulmonary disease (COPD). Freshly isolated peripheral blood mononuclear cells from 45 COPD patients were stained with allophycocyanin (APC)-Alexa Fluor 750-conjugated anti-CD3 and fluorescein isothiocyanate-conjugated anti-CD161 monoclonal antibodies and then analyzed by flow cytometry. According to the Global initiative for Chronic Obstructive Lung Disease (GOLD) grading system, the severity of COPD can be subclassified into GOLD 1 (mild), GOLD 2 (moderate), GOLD 3 (severe), and GOLD 4 (very severe). Percentages of MAIT cells were calculated using a αβ T cell gate. Symbols represent individual subjects and horizontal lines indicate median values. *p < 0.05.
Discussion
To the best of our knowledge, this is the first study to investigate the levels of circulating MAIT cells and their clinical relevance in COPD patients. The present study showed that circulating MAIT cell levels were significantly lower in COPD patients compared with healthy controls. Interestingly, circulating MAIT cell levels were positively correlated with FEV1/FVC ratio, whereas circulating MAIT cell levels were inversely correlated with CRP levels, suggesting that MAIT cells may participate in enhancing the inflammatory response in the airways and the alveolar walls to noxious particles or gases in COPD patients. Taken together, these findings indicate that MAIT cells may play an important role in the pathogenesis of COPD.
In this study, we showed that circulating MAIT cell levels were reduced in COPD patients. MAIT cell deficiency in the peripheral blood has also been reported in several human diseases, such as infectious diseases and autoimmunity (Citation9, Citation21–Citation25). In particular, this MAIT cell deficiency was found to be due to the decline in the major MAIT cell subset (CD8+ MAIT cells) in COPD patients. These findings have also been previously demonstrated in patients with systemic lupus erythematosus, multiple sclerosis, or HIV infection (Citation9, Citation23, Citation24). Therefore, these findings suggest that the loss of circulating MAIT cells may be due to their trafficking to the inflamed airways and alveolar walls in COPD patients.
Recently, this notion has been supported by several lines of evidence, which suggest that MAIT cells express chemokine receptors which are preferentially homing to the peripheral tissues and that MAIT cells are reduced in the blood but are abundantly detected in the intestine of patients with inflammatory bowel disease or in the ascitic fluid from patients with ovarian cancer or active tuberculosis (Citation5, Citation14, Citation25). Therefore, concurrent measurement of MAIT cell levels in bronchial biopsies, bronchoalveolar lavage fluid, and peripheral blood is necessary to explain the “migration hypothesis” of MAIT cells in COPD.
The univariate analysis showed that elevated serum CRP level and reduced FEV1/FVC ratio were associated with MAIT cell deficiency in COPD patients. However, after multivariate analysis, only the FEV1/FVC ratio was identified as an independent determinant of MAIT cell deficiency. Collectively, these findings implicate an important role of MAIT cells in an inflammatory response to irritants such as cigarette smoke during the disease process. How such irritants trigger an innate immune response by involving MAIT cells is not known, but their ability to produce proinflammatory cytokines is a plausible explanation. Recent studies have reported that signaling via toll-like receptors can drive the expression of IL-12 and IL-18 in antigen-presenting cells (APCs), which can activate MAIT cells (Citation26, Citation27). In the present study, intracellular cytokine staining in MAIT cells and APCs was not performed, which would have helped to explain how different cytokine pathways contribute to the inflammatory process in COPD. Further investigation is needed to determine the role of MAIT cells in the pathogenesis of systemic inflammation in COPD.
Studies on biomarkers for risk or severity stratification of COPD are very important for identifying patients with poor prognosis and providing suitable treatment to such patients (Citation28). Therefore, we further investigated whether MAIT cell deficiency is associated with an increased risk of having exacerbations or with severity of COPD. Our study showed that MAIT cell levels were similar between stable conditions and exacerbations of COPD (data not shown). Contrary to our study, a previous study showed that circulating iNKT cell frequencies were significantly lower in patients with exacerbations of COPD than in those with stable COPD (Citation17).
Furthermore, the percentage of CD8+ T lymphocytes in the sputum was significantly increased at the onset of exacerbations as compared with the stable state (Citation29). Further investigation of this controversy is necessary to determine how these immune cells respond to irritants during exacerbations. The GOLD grade measured based on FEV1 is widely used for the assessment of disease severity of COPD. We also demonstrated that circulating MAIT cell levels were significantly lower in patients with relatively advanced stage of COPD than in patients with mild COPD. These findings suggest that screening of MAIT cell levels in the peripheral blood may be used as a predictor for the severity of COPD. However, no significant differences in MAIT cell frequencies were found among COPD patients with greater than GOLD grade 2, which may be due to the enrolment of a small number of each subgroup in this study and the above finding needs to be confirmed in a further study.
In conclusion, the present study shows that circulating MAIT cell levels are reduced in COPD patients and that this MAIT cell deficiency reflects inflammatory activity and disease severity. These findings provide important information for predicting the prognosis of COPD.
Declaration of Interest Statement
The authors declare that they do not have a conflict of interest. The authors alone are responsible for the content and writing of the paper.
ICOP_A_1069806_SUPP.doc
Download MS Word (310 KB)Additional information
Funding
References
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3(11):e442.
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4):347–365.
- Bruce N, Perez-Padilla R, Albalak R. Indoor air pollution in developing countries: a major environmental and public health challenge. Bull World Health Organ 2000; 78(9):1078–1092.
- Rivera RM, Cosio MG, Ghezzo H, et al. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int J Tuberc Lung Dis 2008; 12(8):972–977.
- Le Bourhis L, Martin E, Peguillet I, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol 2010; 11(8):701–708.
- Treiner E, Lantz O. CD1d- and MR1-restricted invariant T cells: of mice and men. Curr Opin Immunol 2006; 18(5):519–526.
- Kee SJ, Park YW, Cho YN, et al. Age- and gender-related differences in circulating natural killer T cells and their subset levels in healthy Korean adults. Hum Immunol 2012; 73(10):1011–1016.
- Lee OJ, Cho YN, Kee SJ, et al. Circulating mucosal-associated invariant T cell levels and their cytokine levels in healthy adults. Exp Gerontol 2014; 49:47–54.
- Cho YN, Kee SJ, Kim TJ, et al. Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus. J Immunol 2014; 193(8):3891–3901.
- Ussher JE, Klenerman P, Willberg CB. Mucosal-associated invariant T-cells: new players in anti-bacterial immunity. Front Immunol 2014; 5:450.
- Kjer-Nielsen L, Patel O, Corbett AJ, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012; 491(7426):717–723.
- Patel O, Kjer-Nielsen L, Le Nours J, et al. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat Commun 2013; 4:2142.
- Walker LJ, Kang YH, Smith MO, et al. Human MAIT and CD8alphaalpha cells develop from a pool of type-17 precommitted CD8+ T cells. Blood 2012; 119(2):422–433.
- Dusseaux M, Martin E, Serriari N, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 2011; 117(4):1250–1259.
- Le Bourhis L, Guerri L, Dusseaux M, et al. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol 2011; 32(5):212–218.
- Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med 2009; 360(23):2445–2454.
- Chi SY, Ban HJ, Kwon YS, et al. Invariant natural killer T cells in chronic obstructive pulmonary disease. Respirology 2012;17(3):486–492.
- Paats MS, Bergen IM, Hoogsteden HC, et al. Systemic CD4+ and CD8+ T-cell cytokine profiles correlate with GOLD stage in stable COPD. Eur Respir J 2012; 40(2):330–337.
- Martin E, Treiner E, Duban L, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol 2009; 7(3):e54.
- Clinical and Laboratory Standards Institute (CLSI). User verification of precision and estimation of bias; approved guideline. 3rd ed. Wayne, PA, USA: CLSI; 2014. CLSI document EP15-A3.
- Cosgrove C, Ussher JE, Rauch A, et al. Early and nonreversible decrease of CD161++ /MAIT cells in HIV infection. Blood 2013; 121(6):951–961.
- Jiang J, Wang X, An H, et al. Mucosal-associated invariant T-cell function is modulated by programmed death-1 signaling in patients with active tuberculosis. Am J Respir Crit Care Med 2014; 190(3):329–339.
- Leeansyah E, Ganesh A, Quigley MF, et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood 2013; 121(7):1124–1135.
- Miyazaki Y, Miyake S, Chiba A, et al. T. Mucosal-associated invariant T cells regulate Th1 response in multiple sclerosis. Int Immunol 2011; 23(9):529–535.
- Serriari NE, Eoche M, Lamotte L, et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014; 176(2):266–274.
- Jo J, Tan AT, Ussher JE, et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog 2014; 10(6):e1004210.
- Ussher JE, Bilton M, Attwod E, et al. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol 2014; 44(1):195–203.
- Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA 2013; 309(22):2353–2361.
- Makris D, Lazarou S, Alexandrakis M, et al. Tc2 response at the onset of COPD exacerbations. Chest 2008; 134(3):483–488.
