Abstract
Type 2 Diabetes Mellitus (T2DM) and cardiovascular diseases (CVD) are common in patients with chronic obstructive pulmonary disease (COPD). Prevention of these co-morbidities in COPD requires knowledge on their risk factors. Metabolic syndrome (MetS) predisposes to the development of T2DM and CVD but its prevalence in COPD remains unclear. The aim of this review was to assess the prevalence of MetS and its components in COPD patients compared to controls and to investigate the contribution of clinical characteristics to MetS prevalence. We systematically searched PubMed and EMBASE for original studies in COPD that have investigated the prevalence of MetS and its components. In total, 19 studies involving 4208 COPD patients were included. The pooled MetS prevalence was 34%. Compared to controls, the prevalence was higher in COPD (10 studies, 32% and 30%, p = 0.001). The three most prevalent components in both COPD and controls were arterial hypertension (56% and 51%), abdominal obesity (39% and 38%) and hyperglycemia (44% and 47%). Compared to COPD patients without MetS, those with MetS had higher body mass index (BMI) (29.9 and 24.6 kg/m2, p < 0.001), higher forced expiratory volume in 1 second (FEV1) % predicted (54 and 51, p < 0.001) and were more frequently female (31% and 25%, p = 0.011). In conclusion, the prevalence of MetS in COPD patients is high and hypertension, abdominal obesity and hyperglycemia are the most prevalent components. Further studies are needed to evaluate the impact of lifestyle factors and medications on MetS in COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) represents a major cause of morbidity and mortality worldwide and is estimated to become the fourth leading cause of death in 2030 Citation(1). Although COPD is primarily characterized by airflow obstruction and pulmonary inflammation, its effects reach beyond the lungs. Systemic manifestations such as osteoporosis, depression, cardiovascular disease (CVD) and Type 2 Diabetes Mellitus (T2DM) are highly prevalent in these patients and significantly contribute to symptom burden and health status Citation(2, 3). CVD and T2DM are present across all COPD disease stages Citation(2) and increase the risk of hospitalization and mortality Citation(4). In fact, in COPD patients with mild-to-moderate airflow obstruction, CVD is even the leading cause of mortality Citation(5).
Prevention of incident T2DM and CVD in patients with COPD requires a detailed understanding of their risk factors, which could be generic or could reflect an interaction between lifestyle and disease specific determinants. Metabolic syndrome (MetS) is a constellation of metabolic risk factors that increase the risk developing T2DM and CVD. The diagnosis of MetS is based on the presence of central obesity, hypertension, dyslipidemia, and hyperglycemia. Various MetS definitions are available and differ in specific cut points of the components (). The prevalence of MetS in the general population varies from 21%−31% in Asia Citation(6, 7), to 34% in the USA Citation(8), and increases with increasing age and body mass index (BMI) Citation(9). Predisposing factors associated with MetS development are smoking Citation(10, 11) and a sedentary lifestyle Citation(12, 13), which are well-described features in COPD patients Citation(3). Moreover, specific factors relating to COPD as a primary lung disease, such as relative hypoxaemia and steroid use may also contribute to the MetS Citation(14). Therefore, the prevalence of MetS in COPD is hypothesized to be higher compared to the general population. Furthermore, knowledge on predisposing factors may aid in characterizing the patients with the highest risk of developing MetS and it may give more insight into targets for interventions aiming to reduce MetS prevalence, development of T2DM and eventually CVD mortality in COPD.
Table 1. Most widely used definitions of metabolic syndrome.
In this review we systematically searched the literature for observational studies that analyzed MetS prevalence in COPD patients and preferentially in control populations as well. Furthermore, we investigated the prevalence of the individual MetS components and studied associations with general clinical characteristics including age, gender, BMI, disease severity, inflammatory profile, medication use and lifestyle characteristics.
Methods
Data sources and search strategy
This systematic review was performed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Citation(22). Pubmed and EMBASE databases (through April 2015) were used to find relevant articles. The search strategy consisted of terms on MetS and COPD (see Supplementary information appendix 1 for terms used and search strategies). In addition, reference lists of retrieved articles were scanned for additional publications.
Study selection and data extraction
A first screening was independently done by two researchers (NCL and RB) based on title and abstract. In case of disagreement, a third person (BB) decided whether to include or exclude the study. Articles were considered for inclusion when they were original studies in patients with COPD, reported on the definition and prevalence of MetS and were written in English. MetS definition, prevalence of MetS and its individual components, age, gender, BMI, forced expiratory volume in 1 second (FEV1), Global initiative for Obstructive Lung Disease (GOLD) stage, information about systemic inflammation, lifestyle characteristics (smoking and physical inactivity), and medication use were extracted. Original authors were contacted in case of missing data. If we were unable to gain information on the MetS definition applied, the study was excluded.
Statistics
The overall pooled prevalence was calculated by summing the number of COPD patients with MetS divided by the total number of COPD patients. Means were weighted by sample size to calculate the pooled means. We compared the prevalence of MetS, prevalence of its components and characteristics (i.e., age, gender, BMI and FEV1%) between COPD patients and controls as well as between COPD patients with and without MetS using Chi-square test for discrete variables and Welch test for continuous variables due to heterogeneity of variance. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 22. A p−value < 0.05 was considered statistically significant.
Results
Study selection
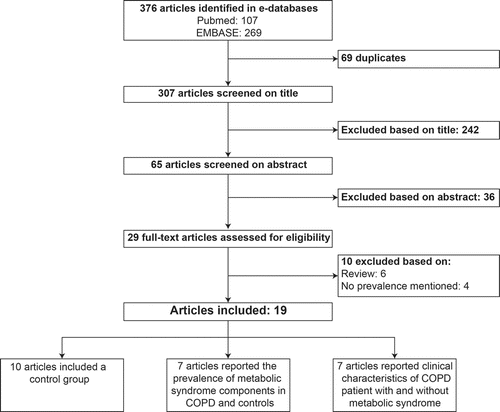
A flow chart of article selection is presented in . The searches in Pubmed and EMBASE yielded 107 and 269 hits, respectively. Of these 376 articles, 69 were duplicates and 278 were excluded based on title and abstract. Of the remaining 29 articles, 6 were excluded because they were not original studies, and 4 were excluded because no prevalence of MetS was mentioned. Finally, 19 observational studies were included, of which 10 included a control group.
General characteristics and prevalence of MetS in COPD patients
Characteristics of the COPD patients of the included 19 studies are presented in . In total, studies reported on 4208 COPD patients. These patients had a pooled mean age of 65 ± 4 years (mean ± standard deviation), a BMI of 25.1 ± 2.0 kg/m² a FEV1% predicted of 66 ± 14% and were mostly men (72%). The overall mean prevalence of MetS was 34% (21–58%) and varied between geographical areas (North America 53%, South America 36%, Europe 41%, Middle East 38% and East and Northeast Asia 28%). Studies differed in the MetS definition used: 6 studies applied NCEP ATP III 2001, 5 studies used NCEP ATP III 2005, 5 studies used IDF and 4 studies applied Alberti et al.Citation(21).
Table 2. Characteristics of COPD patients in included papers and prevalence of metabolic syndrome and its components.
General characteristics of COPD patients with MetS and without Mets
In total 10 studies compared characteristics of COPD patients with MetS to COPD patients without MetS Citation(24, 25, 27, 29–31, 34–36, 42). Patients with MetS had a higher BMI and had higher FEV1% predicted compared to patients without MetS (). The prevalence of male patients was significantly lower in the MetS group compared to the non-MetS group. Furthermore, four studies reported higher serum C-reactive protein Citation(31, 34–36) and serum interleukin-6 Citation(31) in COPD patients with MetS compared with patients without MetS (data not shown).
Table 3. General characteristics of chronic obstructive pulmonary disease patients with MetS and without MetS.
Differences in prevalence of MetS and its components between COPD patients and controls
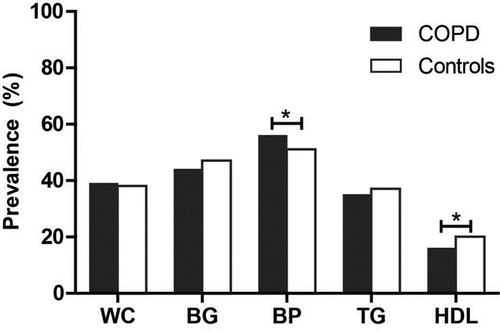
In total, 10 studies compared COPD patients (n = 2864) with a control group (n = 24532) Citation(25, 30, 34, 36–41). Characteristics of COPD patients are shown in , characteristics of the controls are presented in Supplemental Table S1. COPD patients were significantly older, had a significantly lower BMI and were more often males (data not shown). As presented in , a higher prevalence of MetS among COPD patients compared to controls was found in 9 out of 10 studies Citation(23, 25, 30, 34, 36–39, 41), and was significant in 5 studies Citation(23, 30, 34, 37, 38). The overall mean prevalence of MetS in COPD patients was 32% (23–58%) versus 30% (17–54%) in controls (p = 0.001). In total, 7 studies reported the prevalence of MetS components in COPD patients (N = 1725) and controls (N = 18380). As shown in , the three most prevalent components in both COPD patients and controls were hypertension (56% and 51%), hyperglycemia (44% and 47%) and abdominal obesity (39% and 38%). Prevalence of hypertension and low HDL cholesterol was significantly different between COPD patients and controls while prevalence of other components was comparable between both groups.
Figure 2. Prevalence of metabolic syndrome in patients with COPD versus controls. Absolute numbers included in the studies: Marquis32 n = 38 COPD patients n = 34 controls; Park21 n = 94 COPD patients and n = 3661 controls, Breyer17 n = 228 COPD patients, n = 156 controls; Akpinar24 n = 91 COPD patients, n = 42 controls; Ozgen Alpaydin25 n = 50 COPD patients, n = 40 controls; Hosny29 n = 50 COPD patients, n = 35 controls; Park31 n = 133 COPD patients, n = 1082 controls; Lam30 n = 496 COPD patients, n = 6861 controls; Funakoshi28 n = 645 COPD patients, n = 6544 controls; Chung27 n = 1039 COPD patients, n = 6077 controls. An asterisk (*) indicates significant difference in prevalence of metabolic syndrome between COPD patients and controls.
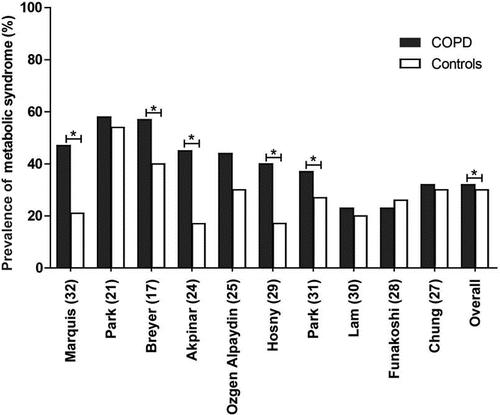
Figure 3. Pooled prevalence of metabolic syndrome components in COPD and controls. Prevalence of components of metabolic syndrome was based on 1725 COPD patients and 18380 controls. Abbreviations: BG, blood glucose; BP, blood pressure; COPD, Chronic obstructive pulmonary disease; HDL, high density lipoprotein; TG, triglycerides; WC, waist circumference.
Contributing factors of MetS in COPD
Smoking status in MetS and non-MetS patients was reported in 5 studies Citation(24, 28–30, 35). Four studies found no significant difference Citation(24, 28, 30, 35) and one study showed significantly less smoking in the MetS group expressed by pack-years Citation(29). Physical activity level was assessed in three studies Citation(24, 25, 31) by measuring the physical activity level with an accelerometer or by measuring the sedentary time. One study found no significant difference in sedentary time between the MetS group and non-MetS group Citation(25), however, the other studies showed significantly reduced physical activity levels and lower activity intensity in the COPD patients with MetS compared to the patients without MetS Citation(24, 31). Medication use between MetS and non-MetS patients with COPD was reported in three studies Citation(28, 30, 35). These studies showed more inhaled or oral steroid use, more statin use, more beta-blockers use and more antihypertensives in the MetS group compared to the non-MetS group. No differences were found in use of anti-diabetics, insulin and long term oxygen therapy Citation(28, 30).
Discussion
This systematic review of current literature shows that the prevalence of MetS in COPD is significantly higher in COPD patients compared to controls. MetS is more prevalent in overweight and obese patients with less advanced airflow obstruction and seems to occur more frequently in female patients. The three most prevalent MetS components in both COPD patients and controls are hypertension, abdominal obesity and hyperglycemia. Smoking does not seem to be a discriminative factor between patients with and without MetS, while further studies are needed to assess the impact of other lifestyle factors and medications on MetS prevalence.
To our knowledge, this is the first systematic review on the prevalence of MetS and its components in COPD patients. Our findings are strengthened by the large number of included subjects in both the patient and control groups and the review provides some new insights about the risk profile for MetS in COPD.
A high MetS prevalence implies a significant risk for development of T2DM with or without CVD. Previous research has indeed shown that both CVD and T2DM are frequent co-morbidities in COPD Citation(4, 5). Furthermore, COPD patients with CVD, hypertension and T2DM were shown to be at increased risk for hospitalizations and all-cause mortality Citation(4). The high MetS prevalence in lower disease stages coincides with the reported high cardiovascular-related mortality in mild to moderate disease Citation(5). Furthermore, recent studies have identified a so called “co-morbidity predominant subtype” of COPD patients, which is characterized by a cluster of metabolic co-morbidities, including obesity, CVD and T2DM Citation(43, 44). This seems to coincide with the most prevalent MetS components found in our review.
COPD patients with MetS were more frequently females, had higher BMI and higher FEV1 compared to COPD patients without MetS. The later was also shown by many studies reporting the highest MetS prevalence in patients with GOLD stage II compared to higher GOLD stages Citation(28, 29, 32, 34, 36). This observation could be due to a relatively higher influence of lifestyle on body composition and metabolic health in less advanced disease compared to COPD induced triggers on the wasting process in advanced disease Citation(45). Secondly, if we assume that MetS in COPD is also related to higher CVD risk, these patients might die earlier of CVD mortality, not reaching end-stage COPD.
Smoking is an established risk factor for COPD and has been associated with increased MetS prevalence and increased CVD risk Citation(11). It would thus be expected that the prevalence of smokers is higher in COPD patients with MetS compared to patients without MetS. Furthermore, physical inactivity and sedentary activity are also associated with MetS Citation(12, 13) and typical for COPD patients Citation(3). However, limited studies reported smoking prevalence and physical activity level and future studies addressing MetS in COPD should include detailed assessment of lifestyle factors including smoking behavior and physical activity level as well as dietary quality which was recently shown to be poor in these patients Citation(46).
Medications can directly influence the prevalence of MetS. Accordingly, the NCEP ATP III and IDF definitions of MetS include medication use for dyslipidemia, hypertension or diabetes as fulfillment of the selected criteria (). However, not all studies clearly reported use of medications as a positive criterion for MetS. This inconsistency may explain the vast differences in the prevalence of hyperglycemia and dyslipidemia among studies. Furthermore, oral glucocorticoids can increase blood glucose levels, HDL levels and appetite, and cause muscle atrophy and abdominal obesity Citation(47). Indeed, of the four studies in COPD reporting medication use, two found a significantly higher use of steroids in the group with MetS Citation(28, 31). Other common medications in COPD, such as anti-depressants can cause impaired glucose tolerance Citation(48), further contributing to MetS. Medications can thus influence MetS prevalence in COPD and need to be considered in future studies.
MetS and components were prevalent in COPD patients, but the prevalence varied greatly among studies, which could be due to differences in study design and setting. As MetS is a predictor for CVD and DM, existing co-morbidities can greatly affect its' prevalence. Minas et al., which excluded COPD patients with DM and CVD with the exception of hypertension, reported the lowest MetS prevalence (22%), whereas Diez-Manglano et al. and Breyer et al. found the highest MetS prevalence in COPD patients with more co-morbidities Citation(28, 30). Furthermore, included studies used different MetS definitions (), which are similar, but apply slightly different criteria for diagnosing MetS. Moreover, the average prevalence of MetS differed in different regions, with a lower MetS prevalence in the Asian studies (28%) compared to European (41%) and American studies (53%). This is consistent with findings from population studies, which found lower prevalence in Asia (21% in China Citation(6) and 31% in Korea Citation(7)) compared to USA (34%) Citation(8). Furthermore, the difference in MetS prevalence between COPD patients and controls was small. This could be explained by the fact that the control group included subjects without COPD, but with other co-morbidities. Altogether, the study diversity has probably contributed to the broad range of reported MetS prevalence and components prevalence observed in our review and should be considered when interpreting the results.
The concept of MetS has received criticism on its applicability in scientific research and it has been questioned whether it better predicts cardiometabolic risk compared to its individual components Citation(49). Studies assessing individual MetS components or insulin resistance as the cornerstone of MetS might help unveil the background of increased cardiometabolic risk in COPD patients and allow us to specifically target these factors. While the relative influence of lifestyle versus disease specific determinants and medication is still unclear, we know that exercise training may improve MetS in other risk populations Citation(50). Pulmonary rehabilitation is an established intervention in COPD focusing on exercise training but the effects on modification of MetS is surprisingly not yet investigated in detail. Studies assessing the effect of such interventions on the cardiometabolic risk in COPD patients would contribute importantly to the understanding of MetS and to reducing disease burden for both patients and the healthcare system.
Conclusion
The prevalence of MetS is higher in COPD patients compared to controls. Its most prevalent components are abdominal obesity, hypertension and hyperglycemia. MetS is more prevalent in female patients, patients with less severe COPD and high BMI. Smoking does not seem to be discriminative for MetS in COPD. Data is lacking on the contribution of physical activity and medications to MetS prevalence in COPD. Future longitudinal and interventional studies are needed to unveil the relation of lifestyle and disease to MetS prevalence as well as the best management possibilities.
Acknowledgments
Authors Lipovec and Beijers contributed equally to this work.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Funding
This research was supported by the Lung Foundation Netherlands (grant number 3.4.12.023).
Supplementary_information_1140732.docx
Download MS Word (20.7 KB)References
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3(11):e442.
- Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010; 11:122.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4):347–65.
- Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008; 32(4):962–9.
- Patel AR, Hurst JR. Extrapulmonary comorbidities in chronic obstructive pulmonary disease: state of the art. Expert Rev Respir Med 2011; 5(5):647–62.
- Xi B, He D, Hu Y, Zhou D. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: the China Health and Nutrition Survey in 2009. Prev Med 2013; 57(6):867–71.
- Suh S, Lee MK. Metabolic syndrome and cardiovascular diseases in Korea. J Atheroscler Thromb 2014; 21 Suppl 1:S31–5.
- Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Rept 2009; (13):1–7.
- Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med 2011; 9:48.
- Slagter SN, van Vliet-Ostaptchouk JV, Vonk JM, Boezen HM, Dullaart RP, Kobold AC, et al. Associations between smoking, components of metabolic syndrome and lipoprotein particle size. BMC Med 2013; 11:195.
- Sun K, Liu J, Ning G. Active smoking and risk of metabolic syndrome: a meta-analysis of prospective studies. PLoS One 2012; 7(10):e47791.
- Bankoski A, Harris TB, McClain JJ, Brychta RJ, Caserotti P, Chen KY, et al. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care 2011; 34(2):497–503.
- Ford ES, Kohl HW, 3rd, Mokdad AH, Ajani UA. Sedentary behavior, physical activity, and the metabolic syndrome among U.S. adults. Obes Res 2005; 13(3):608–14.
- Wells CE, Baker, E.H. Metabolic syndrome and diabetes mellitus in COPD. European Respiratory Monograph. 2013; 59:117–34.
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15(7):539–53.
- Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med 1999;16(5):442–3.
- Expert Panel Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001; 285(19):2486–97.
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112(17):2735–52.
- Einhorn D, Reaven GM, Cobin RH, Ford E, Ganda OP, Handelsman Y, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract 2003; 9(3):237–52.
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006; 23(5):469–80.
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120(16):1640–5.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7):e1000097.
- Marquis K, Maltais F, Duguay V, Bezeau AM, LeBlanc P, Jobin J, et al. The metabolic syndrome in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2005; 25(4):226–32.
- Park SK, Larson JL. The relationship between physical activity and metabolic syndrome in people with chronic obstructive pulmonary disease. J Cardiovasc Nurs 2014; 29(6):499–507.
- Park SK, Larson JL. Metabolic syndrome and associated factors in people with chronic obstructive pulmonary disease. West J Nurs Res. 2014; 36(5):620–42.
- Poulain M, Doucet M, Drapeau V, Fournier G, Tremblay A, Poirier P, et al. Metabolic and inflammatory profile in obese patients with chronic obstructive pulmonary disease. Chron Respir Dis 2008; 5(1):35–41.
- Tanni SE, Zamuner ATS, Coelho LS, Vale SA, Godoy I, Paiva SAR. Are metabolic syndrome and its components associated with 5-year mortality in chronic obstructive pupmonary disease patients? Metab Syndr Relat Disord 2015; 13(1):52–4.
- Diez-Manglano J, Barquero-Romero J, Almagro P, Cabrera FJ, Lopez Garcia F, Montero L, et al. COPD patients with and without metabolic syndrome: clinical and functional differences. Intern Emerg Med 2014; 9(4):419–25.
- Minas M, Kostikas K, Papaioannou AI, Mystridou P, Karetsi E, Georgoulias P, et al. The association of metabolic syndrome with adipose tissue hormones and insulin resistance in patients with COPD without co-morbidities. COPD 2011; 8(6):414–20.
- Breyer MK, Spruit MA, Hanson CK, Franssen FM, Vanfleteren LE, Groenen MT, et al. Prevalence of metabolic syndrome in COPD patients and its consequences. PLoS One 2014; 9(6):e98013.
- Watz H, Waschki B, Kirsten A, Muller KC, Kretschmar G, Meyer T, et al. The metabolic syndrome in patients with chronic bronchitis and COPD: frequency and associated consequences for systemic inflammation and physical inactivity. Chest 2009; 136(4):1039–46.
- Fumagalli G, Fabiani F, Forte S, Napolitano M, Marinelli P, Palange P, et al. INDACO project: a pilot study on incidence of comorbidities in COPD patients referred to pneumology units. Multidiscip Respir Med 2013; 8(1):28.
- Skyba P, Ukropec J, Pobeha P, Ukropcova B, Joppa P, Kurdiova T, et al. Metabolic phenotype and adipose tissue inflammation in patients with chronic obstructive pulmonary disease. Mediators Inflamm 2010; 2010:173498.
- Akpinar EE, Akpinar S, Ertek S, Sayin E, Gulhan M. Systemic inflammation and metabolic syndrome in stable COPD patients. Tuberk Toraks 2012; 60(3):230–7.
- Kupeli E, Ulubay G, Ulasli SS, Sahin T, Erayman Z, Gursoy A. Metabolic Syndrome is associated with increased risk of acute exacerbation of Chronic Obstructive Pulmonary Disease: A preliminary study. Chest 2010; 38(1):76–82.
- Ozgen Alpaydin A, Konyar Arslan I, Serter S, Sakar Coskun A, Celik P, Taneli F, et al. Metabolic syndrome and carotid intima-media thickness in chronic obstructive pulmonary disease. Multidiscip Respir Med 2013; 8(1):61.
- Hosny H, Abdel-Hafiz H, Moussa H, Soliman A. Metabolic syndrome and systemic inflammation in patients with chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc 2013; 62(1):85–9.
- Park BH, Park MS, Chang J, Kim SK, Kang YA, Jung JY, et al. Chronic obstructive pulmonary disease and metabolic syndrome: a nationwide survey in Korea. Int J Tuberc Lung Dis 2012; 16(5):694–700.
- Lam KB, Jordan RE, Jiang CQ, Thomas GN, Miller MR, Zhang WS, et al. Airflow obstruction and metabolic syndrome: the Guangzhou Biobank Cohort Study. Eur Respir J 2010; 35(2):317–23.
- Funakoshi Y, Omori H, Mihara S, Marubayashi T, Katoh T. Association between airflow obstruction and the metabolic syndrome or its components in Japanese men. Intern Med 2010; 49(19):2093–9.
- Chung JH, Hwang HJ, Han CH, Son BS, Kim DH, Park MS. Association between sarcopenia and metabolic syndrome in chronic obstructive pulmonary disease: The Korea National Health and Nutrition Examination Survey (KNHANES) from 2008 to 2011. COPD 2015; 12(1):82–9.
- Diez-Manglano J, Barquero-Romero J, Almagro P, Cabrera FJ, Lopez Garcia F, Montero L, et al. COPD patients with and without metabolic syndrome: clinical and functional differences. Intern Emerg Med 2014; 9(4):419–25.
- Burgel PR, Paillasseur JL, Peene B, Dusser D, Roche N, Coolen J, et al. Two distinct chronic obstructive pulmonary disease (COPD) phenotypes are associated with high risk of mortality. PLoS One 2012; 7(12):e51048.
- Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, Bruijnzeel PL, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187(7):728–35.
- Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 2005; 82(1):53–9.
- van de Bool C, Mattijssen-Verdonschot C, van Melick PP, Spruit MA, Franssen FM, Wouters EF, et al. Quality of dietary intake in relation to body composition in patients with chronic obstructive pulmonary disease eligible for pulmonary rehabilitation. Eur J Clin Nutr 2014; 68(2):159–65.
- Saag KG, Furst DE. 29 July 2014. Major side effects of systemic glucocorticoids. Available from: http://www.uptodate.com (accessed: 03 July, 2015).
- Khoza S, Barner JC. Glucose dysregulation associated with antidepressant agents: an analysis of 17 published case reports. Int J Clin Pharm 2011; 33(3):484–92.
- Kahn R, Buse J, Ferrannini E, Stern M, American Diabetes, European Association for the Study of Diabetes. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2005; 28(9):2289–304.
- Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol 2013; 3(1):1–58.