Abstract
Sudanonautes africanus is a freshwater crab local to Nigeria and West Africa that has no documentation of its innate immunity reactions. The objective of this study was to assess the effect of endotoxin (lipopolysaccharide [LPS]) on coagulation and on phenoloxidase (PO) activity in the hemolymph fractions of S. africanus. The hemolymph from each of 10 live crabs was obtained by carapace puncture and then fractionated into plasma and hemocytes. The hemocytes were then processed and then fractionated into hemocyte lysate (HL), hemocyte lysate supernatant (HLS), and hemocyte lysate debris (HLD). In one study, each fraction was then incubated with a fixed level of LPS in the presence or absence of exogenous calcium (Ca2+) ion. In another study, the LPS concentration was varied in order to study its effect on protein coagulation when an optimal ratio mixture of plasma:HLS was present as well as on PO activity in the plasma and HLS fractions. The results of the first set of studies demonstrated that a presence of Ca2+ in the LPS-induced clotting reactions was essential. The next set of studies showed that a 7:1 plasma:HLS mixture yielded a higher level of coagulation than any other ratio tested in the presence of 1 EU LPS/ml. When this same plasma:HLS mixture ratio was used to ascertain the effect of varying LPS level on coagulation, the response trended higher up to a dose of 3.0 EU/ml., and decreased thereafter until 7 EU/ml. As expected based on the effect of LPS on PO activation, an increasing presence of LPS led to a general trend increase in activity of the enzyme in the plasma fraction; however, the effect was moreover inhibitory in the HLS fraction. From the results here, we conclude that protein coagulation is an important response, along with increased PO activity, that could manifest in Sudanonautes africanus after exposure to ‘free’ LPS or select LPS-bearing organisms in their environment.
Introduction
Sudanonautes africanus, an African freshwater crab, is a common brachyuran freshwater crab widely distributed in Nigeria and Central Africa (Cumberlidge, Citation1999). The common strains, Sudanonautes floweri and S. africanus, are adapted to the freshwater by having lecitothrophic eggs, direct development (without larvae), and brood care. They are edible and provide a means of livelihood to people who hunt them for sale.
It has been established that invertebrates, which lack adaptive systems, have defense systems that respond to pathogen-associated molecular patterns on the surface of potential pathogens. The systems employed include melanization, hemolymph coagulation, cell agglutination, active oxygen formation, anti-microbial action, and phagocytic action. The presence of lipopolysaccharide (LPS) and β-(1-3)-d-glucan-sensitive serine protease zymogens in Limulus hemocytes, both of which trigger the coagulation cascade, has exemplified how invertebrate like Limulus detect and respond to foreign materials (Muta et al., Citation1995).
Blood cells (hemocytes) within the hemolymph detect trace amounts of LPS molecules on invading microorganisms and respond quickly to release granular components into the external milieu (Muta et al., Citation1995). Ghidalia et al (Citation1987) established that freshwater crabs have fibrinogen-like proteins in their plasma that require triggering factors found in hemocytes to effect their clotting. Martin and colleagues (Citation1991), in evaluating shrimp (Sicyonia ingentis), sheep crab (Loxorhynchus grandis), and crayfish/spiny lobsters (Procambarus clarkii) noted that the sheep crabs tended to have the smallest proportion of circulating hyaline hemocytes and exhibited type A coagulation - one not necessarily characterized by massive clotting of the hemolymph proteins but instead by aggregation of hemocytes.
In the Atlantic Horseshoe crab (Limulus polyphemus), the coagulation system is composed of three serine protease zymogens, i.e., factor C, factor B, and pro-clotting enzyme, and a clottable protein - coagulogen that is activated by LPS to form insoluble coagulin gel (reviewed in Kawabata, Citation2010). The coagulation system also responds to β-(1,3) glucan through the activation of a unique hetero-dimeric serine protease zymogen, factor G. As a result, pathogens are engulfed in a gel and subsequently killed by substances (with various specificities) that are also released from the hemocytes. Limulus has also developed two kinds of serine protease zymogens as biological sensors, i.e., factor C and factor G, that are responsive to LPS on Gram-negative bacteria and select fungi, respectively. There is a potential that these LPS and β-(1, 3)-D-glucan-sensitive factors could be utilized as a unique tool to analyze other biological reactions caused by LPS or glucan (Opal and Esmon, Citation2003; Kawabata, Citation2010).
Stimulation of the crab innate immune system by LPS activates a network of responses to ensure host defense against invading pathogens. Granular hemocytes selectively respond to LPS via a G-protein-dependent exocytic pathway that depends on the proteolytic activity of LPS-responsive coagulation factor G (Morita et al., Citation1981; Seki et al., Citation1994; Muta et al., Citation1995). In addition, the hemocyte secretes transglutaminase (TGase) and several other defense molecules, e.g., coagulation factors, lectins, anti-microbial peptides, and substrate for TGase (Osaki et al., Citation2002; Matsuda et al., Citation2007). This LPS-induced exocytosis is enhanced (via feedback mechanisms) by the anti-microbial tachyplesin; this protein also plays a key role in the functional conversion of hemocyanin to phenoloxidase (PO) (Nagai et al., Citation2001). Ultimately, the coagulation cascade triggered by LPS (or β-glucan) also results in a formation of coagulin fibrils (that are, in turn, stabilized by TGase-dependent cross-linking; Osaki et al., Citation2002). Thus, it can be readily seen that LPS-induced hemocyte exocytosis leads not only to coagulation but also the induction of a response network that can coordinately affect both pathogen recognition/clearance and wound healing.
The major goal of this study was to investigate the extent to which LPS could potentially impact on coagulation and PO activities in a locally derived freshwater crab (S. africanus). This is the first report of its kind on LPS-induced immune responses in S. africanus.
Materials and methods
Animals and reagents
This work was carried out with approval of the Committee for Post-graduate Studies in the Department of Biochemistry, University of Ilorin, Nigeria. Ten live, healthy, S. africanus crabs (both sexes, each weighing 60 ± 5 g) were purchased from the Obo road market in Unity, Ilorin. All specimens were positively identified at the University of Ilorin, Zoology Department; a sample was deposited for reference and an identification voucher issued. Crabs were acclimatized for 24 hr with free access to water and food in a facility that provided a natural cycle of light/dark hours, before their hemolymph was collected.
Crab health was determined over the entire study by repeated evaluation of their characteristic active, aggressive, and evasive natures. Healthy crabs are agile, ready to protect themselves with their claws, and try to evade being handled. All the crabs here exhibited these characteristics at purchase, during acclimatization, and when their hemolymph was collected. As the studies here were all performed in vitro, and conditions were uniform for all crabs, sample-to-sample variability in hemolymph was deemed to not be a potential problem. All chemicals and reagents used here were from the University of Ilorin, Biochemistry Department.
Extraction of hemolymph from S. africanus
Hemolymph from each crab was obtained after placing the animal in a bowl (preloaded with 4.5 ml of anti-coagulant [phosphate buffer containing 0.01 M EDTA]) and inducing euthanization via dorsi-ventral carapace puncture. Total specimen collected from the puncture site was ≈ 4.5 ml/crab. Hemolymph samples from each of five crabs were used for fractionation studies; samples from each of the remaining five were used for of metal ion level measures.
Preparation of S. africanus hemolymph fractions
Each hemolymph sample was diluted to twice its volume with EDTA buffer (pH 7.4) and centrifuged at 800 × g for 20 min at room temperature (RT). The supernatant plasma was recovered and the hemocyte (pellet) was washed twice (each time) with 1 ml phosphate buffer containing 0.1 M calcium chloride (CaCl2) to restore Ca2+ ions that might have been lost due to the anti-coagulant. The pellet was homogenized in 10 mM phosphate buffer containing 10 mM EDTA (pH 7.4) using an ice-cold mortar and pestle. The homogenate was suspended in the same buffer to the starting diluted hemolymph volume; this material was deemed the hemocyte lysate (HL). The HL was then centrifuged at 800 × g for 20 min and the hemocyte lysate debris (HLD) in the pellet was separated from the hemocyte lysate supernatant (HLS). The HLD was washed with phosphate buffer to remove any trace of HLS; phosphate buffer was then added to the HLD-containing tube to the volume of the starting diluted hemolymph.
Once isolated, the total protein concentrations in the plasma, HL, HLS, and HLD were estimated using the method of Gornall et al. (Citation1949). The corresponding protein contents of the fractions were 134 (± 6.5), 27 (± 1.4), 20 (± 0.55), and 15 (± 0.25) mg/ml, respectively.
Preparation of Escherichia coli endotoxin
Endotoxin (LPS) from Escherichia coli was prepared as reported in Salawu et al. (Citation2010). Briefly, a 5% (w/v) dextrose solution was prepared and then inoculated with E. coli. The solution was then divided into two portions. Portion A was sampled and plated onto SDA, EMB, and NA agar plates that were then incubated for 24 hr at 37°C to determine initial microbial load. Portion A was sterilized immediately after inoculation by autoclaving for 30 min. Portion B was similarly sampled for microbial load by plating on the media and then the sample was not sterilized but, rather, incubated at 37°C for 48 hr before sampling for final load. The Portion B material was then sterilized by autoclaving (30 min). The LPS concentration in the sample was then determined using the rabbit test method and Limulus amoebocyte lysate (LAL) kits. The stock solution was later used to prepare LPS solutions of various strengths for use in the studies.
Determination of concentration of select metal ions in the plasma
The concentrations of select metals, e.g., magnesium, cadmium, lead, copper, nickel, and iron (Mg, Cd, Pb, Cu, Ni, and Fe) in the plasma recovered from the S. africanus crabs (n = 5) were determined using flame spectrometry, according to the methods of Arthur et al. (2009).
Coagulation reactions in S. africanus hemolymph fractions
To assess coagulation, 0.8 ml of each isolated hemolymph fraction was combined with 0.1 ml of 0.1 M CaCl2 and 0.1 ml of LPS (1 EU/ml final concentration) in de-pyrogenated glass test tubes. Controls were prepared in parallel without CaCl2 or lacking LPS. All test and control samples were incubated at 37°C for 1 hr and then centrifuged at 800 × g for 5 min. The supernatants were discarded and the pellets (coagulates) in each tube dissolved in 0.9% (w/v) saline solution. Protein content in each pellet was estimated by adding 9 ml Biuret reagent to each test tube, allowing each mixture to stand at room temperature for 20 min, and then measuring absorbance at 550 nm in an M105 spectrophotometer (Spectronic Camspec, UK) (Gornall et al., Citation1949).
When compared against the designated corresponding control tubes (i.e., sample with LPS [± Ca] or with Ca [± LPS]), the absorbance values obtained for samples with three components (i.e., fraction, endotoxin, and CaCl2) reflected the increase in amount of protein in the fraction that had coagulated in the 60 min incubation. Within each given fraction, since the protein content in the test and control tubes is essentially the same, any relative difference in the total amount of coagulation that occurred (i.e., total measurable protein in each pellet [OD550]) would depend solely on the experimental variable (i.e., LPS concentration or presence/absence of Ca2+). Such data could then be expressed in terms of “% change in total protein that coagulated” within each fraction; these values, in turn, could be then be compared across the fractions.
Four sets of studies assessing coagulation as the key endpoint were performed. In one, each fraction and the plasma were tested for dependence on the presence of Ca2+ ion. In a second study, each fraction and plasma was tested for responsiveness to LPS (1 EU/ml) in the presence of Ca2+. Next, mixtures of plasma and HLS (at various ratios) were exposed to a fixed LPS level (1 EU/ml, in presence of Ca2+) to determine an optimal ratio that would produce the highest difference in amount of protein coagulated (when compared to unstimulated control samples). Last, a plasma:HLS mixture (at the now-determined optimal ratio) was exposed to varying LPS concentrations to determine the extent of LPS-dependence in the coagulation reactions.
Measures of phenoloxidase activity
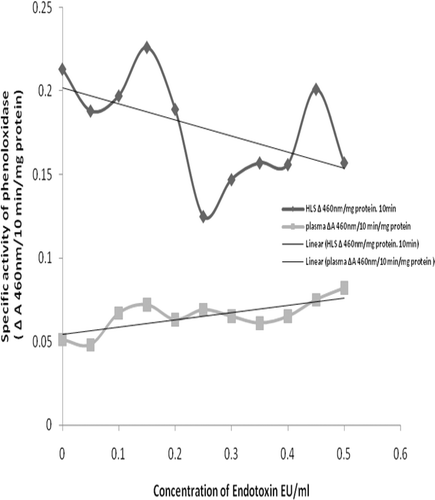
To analyze the relationship between LPS exposure and PO activity in the hemolymph materials isolated from the S. africanus hosts, a method used by Sung et al. (Citation1998) and Tanner et al. (Citation2006)-themselves derivatives of the original method by Mason (Citation1947)-that is based on the conversion of l-3, 4-dihydoxyphenyl alanine (l-DOPA) to dopachrome (with peak absorbance at ≈460 nm at pH 8.0) was employed. Briefly, aliquots (0.1 ml) of plasma and HLS were added 0.2 ml of 5 mM l-DOPA, 1.7 ml bicarbonate buffer (pH 8.0), and 0.1 ml LPS solution (0–5.0 EU/ml). Blanks were prepared in parallel using LPS-free water in place of LPS. All samples were prepared in quadruplicate and incubated at 37°C. Absorbance (at 460 nm) of each mixture was measured at time 0 (immediately upon LPS addition) and after 10 min; this timeframe allowed for measures of the conversion of l-DOPA to dopachrome (dark product). From these values, the △A460 nm/10 min was determined and used to reflect general PO activity in each sample. Based on the protein content in the aliquots of each fractions used in the assay (i.e., HLS = 2.0 mg and plasma = 13.4 mg), the changes in PO specific activity were calculated (i.e., in terms of △A460 nm/10 min•mg protein).
Statistical analyses
For the coagulation reactions, the results were analyzed using a one-way analysis of variance (ANOVA) followed by a paired t-test. Values were considered significantly different at p < 0.05.
Results
Measures of some metal ions in the plasma revealed that Mg, Cd, Pb, and Cu were present in relatively high concentrations, while Ni was present at relatively low levels ().
Table 1. Concentrations of select metal cations in the plasma of S. africanus.
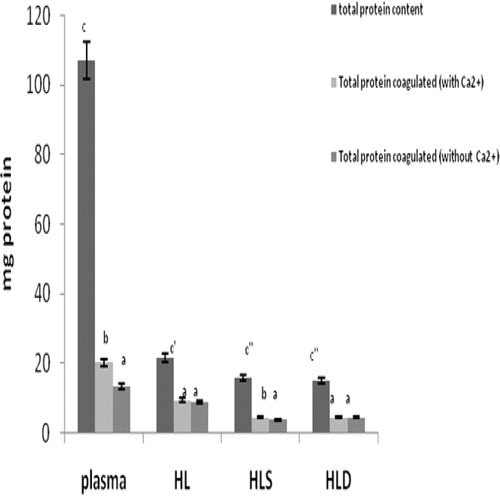
Examination of the effect of the presence of Ca2+ on clotting properties of the plasma and hemolymph fractions showed that coagulation was significantly elevated by its presence only with the plasma and HLS (HLS; however, only marginally so) (). Added presence of the ion had no apparent effect in the HL or HLD fractions. Specifically, the relative changes above control (i.e., no Ca2+ value; when expressed as % change in total protein that coagulated) in the plasma, HL, HLS, and HLD fractions were ≈ 85, 10, 5, and < 1%, respectively.
Figure 1. The effect of calcium (Ca2+) ions on observable coagulation in the HL, plasma, HLD, and HLS fractions of the hemolymph obtained from S. africanus. All data are expressed as mean mg (± SD) of four determinations. The left-most bar in each set represents total protein in reaction at start; other columns indicate amount in precipitate after reaction. Within each specific fraction (i.e., comparing with vs. without Ca2+), bars with differing letter designations are significantly different at p < 0.05.
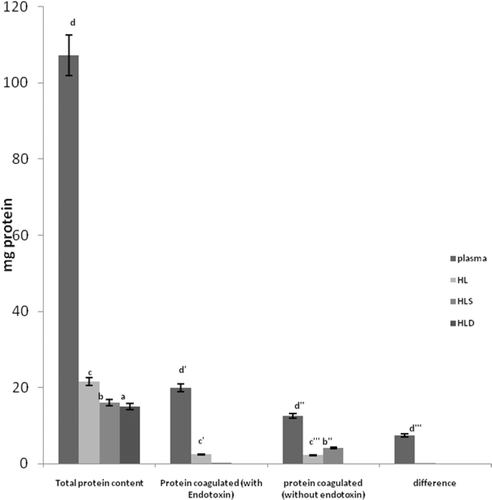
The noted effects of LPS on clotting properties of the fractions of hemolymph () indicated that the degree of protein coagulation induced by LPS (when Ca2+ was present) was not significantly increased in any of the fractions except plasma. Specifically, for the plasma, the level of coagulation (% change) associated with a presence of LPS was ≈ 96.5% of the total protein coagulated from all of the fractions (when combined).
Figure 2. The effect of LPS on clotting within the hemolymph fractions from the S. africanus crabs. Data are expressed as mean mg (± SD) of four determinations. Within each specific fraction (i.e., comparing with vs. without LPS), bars with differing letter designations are significantly different at p < 0.05.
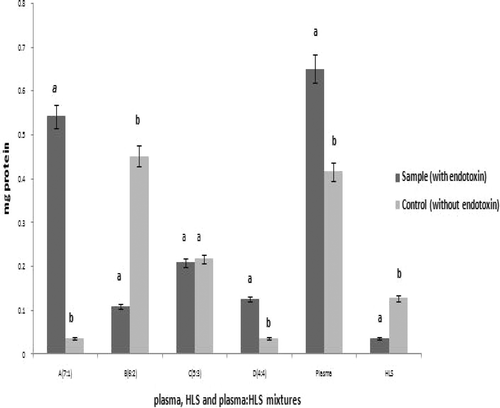
An investigation was carried out to determine if mixtures of plasma and HLS produced differential protein coagulation reactions when present at varying ratios (but at a fixed amount of LPS). In the absence of LPS, the greatest response appeared to occur at a ratio of 6:2 (). When LPS was present, a 7:1 ratio yielded the highest coagulation value. The net change due to the presence of LPS was ≈ 1733%; in comparison, for the plasma alone, the net change in coagulated protein was significant as well, but only on the order of ≈ 53%. With respect to comparisons between the 6:2 and 7:1 experimental systems, in both cases, values observed for the samples with vs. those without LPS were significantly different; however, the results were completely opposite. It is not presently clear as to why at a 7:1 ratio, the extent of coagulation appeared to collapse after increasing in a somewhat ratio-related (i.e., increasing percentage of plasma in the mixture) manner in the LPS-free samples.
Figure 3. Effect of varying plasma:HLS mixture ratios on LPS-induced protein coagulation. Within each fraction indicated, bars with differing letter designations are significantly different at p < 0.05.
Based on the finding of the maximal response to LPS occurring with the 7:1 mixture, the extent of LPS-dependence in the coagulation reactions was tested. The results indicate that LPS-induced protein coagulation in the HLS/plasma (optimal ratio) mixture increased steadily over a test range of 0.5 to 3.0 EU/ml. Subsequent increases in LPS levels caused coagulation to decline. Somewhat unexpectedly, at 8.0 EU/ml, the degree of coagulation increased; however, even further increases in LPS concentration (i.e., ≥ 8.0 EU/ml) did not produce a peak as high as that noted at 3.0 EU/ml ().
Figure 4. Effect of varying concentrations of LPS on protein coagulation within a plasma:HLS mixture (7:1 ratio).
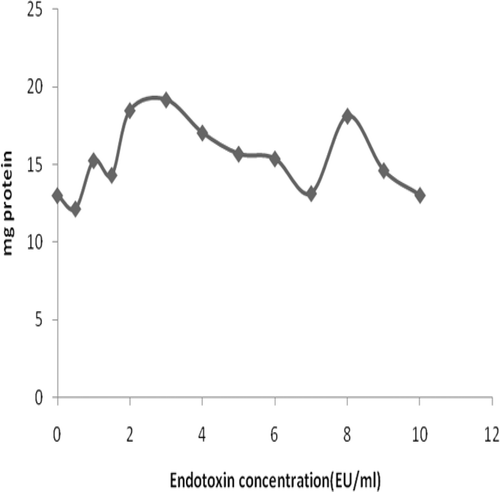
As expected based on the effect of LPS on phenoloxidase (PO) activation, an increasing presence of LPS led to a general (trending) increase in PO activity in the plasma fraction (). In contrast, although PO activity appeared to initially be slightly increased by addition of LPS to the HLS fraction, the effect was moreover inhibitory over the range of LPS doses tested. Even though the two fractions displayed opposing trends in PO activity in response to LPS, in general, the specific activities in the plasma were consistently lower than those in the HLS.
Discussion
The focus of this work was to investigate coagulation reactions mediated by endotoxin (LPS) in S. africanus. In these studies, we attempted to study the effect of LPS on phenoloxidase activity in the plasma and HLS fraction of these crabs. This was intended to obtain the first record of an innate immune response of these crabs to LPS and, hence, Gram-negative organisms.
The crabs used here were collected from the banks of Asa River (Ilorin, Nigeria) that runs through an industrial estate in the city. The presence of cadmium (Cd) and lead in the plasma of these animals was an indication of ongoing pollution of their habitat. It also suggested that the poor waste disposal that takes place in the municipality could predispose aquatic life in the river and its banks to risks, including increased susceptibility to bacterial, viral, or fungal pathogens.
Heavy metals have been noted in crustaceans obtained from the Ojo Market in Lagos, Nigeria (Olowu et al, Citation2010), several hundred miles SW of Ilorin. Those authors reported that Cd was present in crabs and prawns at levels of 1.66 (± 1.48) and 0.07 (± 0.08) ppm (µg/g), respectively. These values are far lower than those here in the hemolymph of the S. africanus crabs (i.e., 26.53 [± 6.67] mg/l (ppm), ≈ 15 times higher than crab value and 377 times that for prawn). Cd levels in Blue crab and crayfish sampled in Delta State, Nigeria (several hundred miles SE of Ilorin) were between, 1.62–12.88 ppm and 0.038–18.13 ppm, respectively (Olowoyo, Citation2010). The concentrations of metals in the tissues of these various crustaceans appeared to vary with their environments. Such variations could be as a result of different levels of pollution in these sites. It is also possible that the differences between the S. africanus crab results and those of the other investigations was due to analyses of a single portion of the crabs (i.e., the plasma) as opposed to the whole organism. Specifically, there could be concentrating effects of the metals by plasma proteins as often occurs with fish/mammalian species after ingestion/exposure to metal contaminants in the diet or environment. At this time, it is not certain what effects the presence of the various metals assessed might have for the S. africanus crabs in general, or with respect to the various endpoints measured herein specifically.
In this study, the degree of change in coagulation (i.e., % change in total protein that coagulated) in the presence of Ca2+ ion was found to be significantly highest with the plasma as compared to that in any other fraction. With each other fraction, with Ca2+ present, the degree of coagulation was relatively the same, albeit that the change noted with the HLS was significant. Therefore, it is reasonable to conclude that Ca2+ is required for coagulation in S. africanus hemolymph and apparently maximal in the plasma environ once cellular components are released from hemocytes. This is also suggestive of a potential role for a Ca2+-dependent coagulation process mediated by a TGase, possible akin to that used for the clotting in Pacifastacus leniusculus (Söderhäll and Cerenius, Citation1992).
LPS-induced coagulation was significantly higher in plasma than in its non-stimulated counterpart; for the plasma alone, the net change in coagulated protein was significant as well, but only on the order of ≈ 53%. There were no significant changes induced by LPS in the HLS, HLD, and HL fractions. When mixtures of plasma and HLS (in plasma:HLS ratios of 1:1 up to 7:1) were exposed to equal amounts of LPS (1 EU/ml), the amount of protein coagulated was maximal at a 7:1 ratio. In this case, the net change due to the presence of LPS was ≈1733% (i.e., shift from 0.03 mg to 0.55 mg protein in coagulate). When viewed in the context of the relative change in the total coagulated protein seen with the plasma alone, this outcome suggested that optimal hemolymph clotting in the crab was likely comprised of both cellular and plasma factors, a finding similar to that reported in other crustaceans (Durliat, Citation1985). This data (see ) also suggested that simple increases in amounts of HLS fraction (i.e., with no presence of LPS) were not alone sufficient to increase coagulation potential. In contrast, continuing increases in the relative amount of plasma (apart from inexplicable anomalous result with 6:2 ratio) showed a corresponding trend toward increased coagulation outcomes.
The reasonable conclusion we draw from these findings is that S. africanus plasma likely contained a key coagulogen substrate whereas HLS contained little to no substrate but sufficient amounts of coagulation enzyme (TGase) that required Ca2+ ion to function and was activated in a presence of LPS. In the case of the separate lysate fractions, the enzyme and substrate were not apparently brought together in sufficient amounts to yield any appreciable coagulation. In contrast, at a minimum, trace amounts of TGase were likely to have been present in the plasma so that coagulation responses could readily be induced and significant changes monitored (see and ). In fact, a presence of TGase in the plasma of several aquatic species has been reported, with enzyme concentrations varying greatly from species-to-species (lobster - Lorand and Conrad, Citation1984; ridgeback prawns - Martin et al., Citation1991; freshwater crayfish - Wang et al., Citation2001). However, for the most part, the amounts of TGase in each case were consistently low relative to the amounts found in host hemocytes.
When tested in a system here bearing increasing amounts of LPS, the plasma:HLS mixture-derived coagulation displayed a steady increase between 0.5–3.0 EU LPS/ml, then trended lower. This confirmed that LPS-induced coagulation was an important process for clearing pathogens out of the general circulation in the hemolymph of S. africanus. However, the latter results also indicate that in the presence of high amounts of LPS, the protective response could be inhibited. This could reasonably occur during an overwhelming infection or in an immunocom-promised host. The latter is a significant possibility in light of the presence of several immuno-suppressive metals in the Ilorin market crabs. Further study of this LPS-induced inhibition (i.e., determination of mechanism of inhibition in situ and ex vivo) is clearly warranted.
PO is a copper (Cu)- dependent enzyme that catalyzes the synthesis of O-diphenols (from mono-phenols) that, in turn, are converted to O-quinones (Kong et al., 1997). As these quinone products can give rise to cytotoxic interactions with iron (Fe) and Cu ions, these provide the hosts an additional mechanism for killing invading pathogens. In our study, the level of PO activity in the plasma overall was lower than that in the HLS fraction, irrespective of the observed trend toward increased activity with increases in LPS levels. The relative difference in activity in these two hemolymph components would be in keeping with the fact that PO has been identified in crab hemocytes (i.e., Atlantic blue crab, Callinectes sapidus; Tanner et al., Citation2006); however, information as to presence of PO in the plasma of these hosts remains unclear.
Even if there is no inherent PO in the S. africanus plasma, the data indicate LPS-responsive conversions reflective of PO activity. It has been reported that diphenoloxidase (diPO, catechol oxidase; PO family member) is present in Pacific oyster (Crassostrea giga) hemolymph plasma (Söderhäll and Cerenius, Citation1992); this might also be the case here. It is also plausible that S. africanus plasma contained prophenoloxidase (PPO); PPO conversion to active enzyme can be brought about by miniscule amounts of LPS (Söderhäll and Cerenius, Citation1998). In the horseshoe crab (Carcinoscorpius rotundicauda), there are agents (i.e., serine proteases) that transiently exist in the plasma (Jiang et al., Citation2009) that are key players in PPO conversion to PO. Whether or not there is diPO or there is active PPO conversion in S. africanus plasma remains to be seen. However, the study here clearly suggests that PO activity, either mediated by some nascent PO or by PO derived from PPO, seems to be present in the S. africanus plasma. Lastly, we wish to note that the choice to use l-DOPA as the substrate here may have tied our hands in establishing a clear picture of PO activities in each hemolymph component, i.e., l-DOPA will not allow for differentiation between PO and diPO activity. Our future evaluations should select a PO-specific substrate or utilize concurrent assays with catechol to distinguish contributions from diPO to help us more clearly define component-related differences in PO activity.
Irrespective of the problem with the use of l-DOPA substrate, the question that remains to be resolved from the findings here is why there were differential PO response-trends to LPS by the plasma and HLS. As noted above, PO activity tended to decrease with increasing LPS level for the HLS fraction; in contrast, with plasma, increasing LPS levels generally led to increased PO activity (albeit that even when stimulated, the levels were lower compared to those found with HLS). In C. giga, PO activity was found to be LPS dose-independent (Söderhäll and Cerenius, Citation1992). If this is so for S. africanus as well, then some other factor(s) inherent to each component are acting to modulate the responses. As noted above, a potential means for an increase in measured PO activity due to LPS-at least in the plasma-is PPO conversion to active enzyme. However, this would suggest that there had to have been little to no PPO in the HLS to propagate such a reaction. This would be contrary to the findings of Terwilliger and Ryan (Citation2006) who noted that in assessments of PO and hemocyanin (a PPO phylogenetically related to PO) isolated from hemolymph of the brachyuran crab Cancer magister (Terwillinger et al., 2006), both the PO and hemocyanin found in hemocytes could convert diphenols to O-quinones and that the hemocyanin appeared to be present ‘in [a] much higher concentration’. Similarly, PO activity in spiny lobster (Panulirus argus) hemolymph was due to a presence of PPO in hemocytes and hemocyanin in the plasma (Perdomo-Morales et al., Citation2007). Thus, if the LPS were acting to bring about PPO conversion to PO, then the HLS here should have contained ample material (assuming horseshoe crab and S. africanus in this context are the same) and so yielded an increase in measurable enzyme activity as LPS levels increased.
If the presence/amount of the key enzyme is not the issue, an alternative explanation for the differential responses might be there was: (A) a difference in component content of some rate-limiting co-factor; (B) the presence of exogenous inhibitors (like some select metal ions); or, (C) formation of some product that led to backfeed inhibition (at least in the HLS) or to removal of substrate/end-product. In the case of (A), had the HLS been lacking/limited in the amount of some co-factor, then with increasing amounts of LPS, the reaction should have plateaued once all the cofactors had been consumed, i.e., there would be no continual down-trend in PO activity. In the case of (B), if there is a presence of metal ions that are known to inhibit PO activity in the HLS of tiger shrimp (Peneaus monodon) and giant freshwater prawns (Macrobrachium rosenbergii) (Sung et al., Citation1998), then as in (A), the measured outcomes should have stayed constant (if not abrograted from start, depending on relative amounts of the ions in each hemolymph component). Therefore, it seems most likely (C) was governing our observations.
Other products apart from dopaquinone (and eventually, dopachrome) are formed in the PO-mediated reaction with l-DOPA. Nappi and Vass (Citation2001) as well as Komarov et al. (Citation2005) demonstrated that superoxide anion and hydrogen peroxide (i.e., reactive oxygen species, ROS) are readily formed and that each could interact with (consume) l-DOPA, converting it to dopa-semiquinone. Though the latter can be converted to dopaquinone via another oxidation reaction, it also has can react with other constituents, i.e., thiol-containing compounds (Kalyanaraman et al., Citation1984; Miura et al., Citation1999; Sugumaran et al., Citation2000). If in fact there is sufficient PO/PPO/hemocyanin in the HLS to mediate reactions with exogenous l-DOPA, then with increasing LPS more and more ROS would be formed. This, in turn, would lead to more dopa-semiquinone/complexation of thiol-bearing compounds-in effect, reducing potential dopachrome generation by ‘removing’ the l-DOPA needed for its formation. In the case of plasma, the aforementioned limitations on PO/PPO/hemocyanin availability would suggest that these inhibitory events might occur, but not at sufficient levels to negatively impact LPS-induced outcomes. Of course, (C) is speculative by its nature; it will be of great interest to measure PO activities in each hemolymph component in the presence of superoxide dismutase/horseradish peroxidase to mitigate potential effects from ROS by-product formation and obtain true measures of PO activity (accepting the earlier caveat about use of a more PO-selective substrate as well).
Conclusion
Coagulation involves both the cellular and humoral components. These components help in the defense system of S. africanus by responding to the entry of endotoxin (LPS) causing their immobilization, and subsequently leading to their death. Each of the hemolymph fractions of S. africanus appeared to have an ability to undergo some measure of coagulation; however, the plasma component was uniformly more responsive to stimulation by LPS and more sensitive to the presence/absence of calcium ion for the reaction to take place. Phenoloxidase activity, which eventually leads to the formation of melanin that is also utilized in host resistance mechanisms, was seen as differentially expressed in the plasma and HLS of hemolymph of S. africanus. These studies also showed that phenoloxidase activity in response to LPS appeared to be dose-related in the plasma but inversely-so in the HLS.
Acknowledgements
Special thanks to the following individuals; Modupe O., Alagbe B.G., Olabiyi T.B., Okedare B.J., Adeniyi O.S., Agbana O.J., Olupo A., Oginni T., Adeniyi S. of the Department of Biochemistry, University of Ilorin, Ilorin, Nigeria for their contributions to this work.
Declaration of interest:.The authors report no conflicts of interest. The authors are alone responsible for the content and writing of the paper.
References
- Cumberlidge, N. (Eds.)1999. The Freshwater Crabs of West Africa. Family Potamonautidae. Paris: Faune et Flore Tropicales 35, Institut de recherche pour le développement IRD ( ex-ORSTOM)), pp. 1–382.
- Durliat, M. 1985. Clotting processes in Crustacea Decapoda Biol Rev. 60:473–498.
- Ghidalia, W., Vendrely, R., Montmory, C., Coirault, Y., and Brouard, M. O. 1987. Coagulation in decapod crustacea. J Comp Physiol B. 142:473–478.
- Gornall, A. G., Bardawill, C. J., and David, M. M. 1949. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 177:751–766.
- Iwanaga, S., and Kawabata, S. 1998. Evolution and phylogeny of defense molecules associated with innate immunity in horseshoe crab. Front Biosci. 3:D973–D984.
- Jiang, N., Thangamani, S., Chor, C. F., Wang, S. Y., Winarsih, I., Du, R. J., Sivaraman, J., Ho, B., and Ding, J. L. 2009. A novel serine protease inhibitor acts as an immunomodulatory switch while maintaining homeostasis. J Innate Immun. 1:465–479.
- Kalyanaraman, B., Felix, C. C., and Sealy, R. C. 1984. Peroxidatic oxidation of catecholamines. A kinetic electron spin resonance investigation using the spin stabilization approach. J Biol Chem. 259:7584–7589.
- Kawabata, S. 2010. Immunocompetent molecules and their response network in horseshoe crabs. Adv Exp Med Biol. 708:122–136.
- Komarov, D. A., Slepneva, I. A., Glupov, V. V., and Khramtsov, V. V. 2005. Superoxide and hydrogen peroxide formation during enzymatic oxidation of DOPA by phenoloxidase. Free Radic Res. 39:853–858.
- Lorand, L., and Conrad, S. M. 1984. Transglutaminases. Mol Cell Biochem. 58:9–35.
- Martin, G. G., Hose Sidne Omori, J. E., Chong, C., Hoodbhoy, T., and McKrell, N. 1991. Localization and roles of coagulogen and transglutaminase in hemolymph coagulation in decapod crustaceans. Comp Biochem Physiol B: Comp Biochem. 100:517–522.
- Mason, H. S. 1947. The chemistry of melanin: The oxidation of di hydroxyphenylalanine by mammalian dopa oxidase. J Biol Chem. 168:433–438.
- Matsuda, Y., Koshiba, T., Osaki, T., Suyama, H., Arisaka, F., Toh, Y., and Kawabata, S. 2007. An arthropod cuticular chitin-binding protein endows injured sites with transglutaminase-dependent mesh. J Biol Chem. 282:37316–37324.
- Miura, T., Muraoka, S., and Fujimoto, Y. 1999. Inactivation of creatine kinase induced by dopa and dopamine in the presence of ferrylmyoglobin. Chem Biol Interact. 123:51–61.
- Morita T., Tanaka, S., Nakamura, T., and Iwanaga, S. 1981. A new (1,3)-β-D-glucan-mediated coagulation pathway found in limulus amebocytes. FEBS Lett. 129:318–321.
- Muta, T., Seki, N., Takaki, Y., Hashimoto, R., Oda, T., Iwanaga, A., Tokunaga, F., and Iwanaga, S. 1995. Purified horseshoe crab factor G. Reconstitution and characterization of the (1–>3)-β-D-glucan-sensitive serine protease cascade. J Biol Chem. 270:892–897.
- Nagai, T., Osaki, T., and Kawabata, S. 2001. Functional conversion of hemocyanin to phenoloxidase by horseshoe crab antimicrobial peptides. J Biol Chem. 276:27166–27170.
- Nappi, A. J., and Vass, E. 2001. The effects of nitric oxide on the oxidations of L-DOPA and dopamine mediated by tyrosinase and peroxidase. J Biol Chem. 276:11214–11222.
- Olowoyo D. N., Ajayi, O. O., Amoo, I. A., and Ayeisanmi, A. F. 2010. Seasonal variation of metal concentrations in catfish, blue crab, and crayfish from Warri coastal water of Delta State, Nigeria. Pak J Nutr. 9:1118–1121.
- Olowu R. A., Ayejuyo O. O, Adejoroi A., Adewuyi G. O., Osundiya M. O., Onwordi C.T., Yusuf K A., and Owolabi, M.. 2010. Determination of heavy metals in crab and prawn in Ojo Rivers, Lagos, Nigeria. E J Chem. 7:526–530.
- Opal, S. M. and Esmon, C. T. 2003. Bench-to-bedside review: Functional relationships between coagulation and the innate immune response and their respective roles in the pathogenesis of sepsis. Crit Care. 7:23–38.
- Osaki, T., Okino, N., Tokunaga, F., Iwanaga, S., and Kawabata, S. 2002. Proline-rich cell surface antigens of horseshoe crab hemocytes are substrates for protein cross-linking with a clotting protein coagulin. J Biol Chem. 277:40084–40090.
- Perdomo-Morales, R., Montero-Alejo, V., Perera, E., Pardo-Ruiz, Z., and Alonso-Jiménez, E. 2007. Phenoloxidase activity in the hemolymph of the spiny lobster Panulirus argus. Fish Shellfish Immunol. 23:1187–1195.
- Salawu, M. O., Oloyede, O. B., Oladiji, A. T., Yakubu, M. T., and Atata, R. F. 2010. Effect of delayed sterilization on the production of intravenuous infusion (parenterals). Afr J Biotech. 9:6948–6951.
- Seki, N., Muta, T., Oda, T., Iwaki, D., Kuma, K., Miyata, T., and Iwanaga, S. 1994. Horseshoe crab (1,3)-β-D-glucan-sensitive coagulation factor G. A serine protease zymogen heterodimer with similarities to β-glucan-binding proteins. J Biol Chem. 269:1370–1374.
- Söderhäll, K., and Cerenius, L. 1992. Crustacean immunity. Ann Rev Fish Dis. 2:3–23.
- Söderhäll, K., and Cerenius, L. 1998. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr Opin Immunol. 10:23–28.
- Sugumaran, M., Nellaiappan, K., and Valivittan, K. 2000. A new mechanism for the control of phenoloxidase activity: Inhibition and complex formation with quinone isomerase. Arch Biochem Biophys. 379:252–260.
- Sung, H. H., Chang, H. J., Her, C. H., Chang, J. C., and Song, Y. L. 1998. Phenoloxidase activity of hemocytes derived from Penaeus monodon and Macrobrachium rosenbergii. J Invertebr Pathol. 71:26–33.
- Tanner, C. A., Burnett, L. E., and Burnett, K. G. 2006. The effects of hypoxia and pH on phenoloxidase activity in the Atlantic blue crab, Callinectes sapidus. Comp Biochem Physiol, Part A Mol Integr Physiol. 144:218–223.
- Terwilliger, N. B., and Ryan, M. C. 2006. Functional and phylogenetic analyses of phenoloxidases from brachyuran (Cancer magister) and branchiopod (Artemia franciscana, Triops longicaudatus) crustaceans. Biol Bull. 210:38–50.
- Wang, R., Liang, Z., Hal, M., and Sö,derhall, K. 2001. A transglutaminase involved in the coagulation system of the freshwater crayfish, Pacifastacus leniusculus. Tissue localisation and cDNA cloning. Fish Shellfish Immunol. 11:623–637.