Abstract
Immune system development, particularly in the pre-natal and early post-natal periods, has far-reaching health consequences during childhood, as well as throughout life. Exposure to poly-chlorinated biphenyls (PCBs) during pre-natal and early life has been previously associated with changes in the incidence of infectious and allergic diseases in children, and humoral immunity alterations. Lymphocyte immunophenotyping is an important tool in the diagnosis of immunologic and hematologic disorders. This study used a lysed whole blood method for analysis of lymphocyte sub-populations in samples from children born and living in two districts: a highly-contaminated area (Michalovce) and one (Svidnik/Stropkov) with ≈ 2-fold lower environmental PCB levels. The percentages of B-lymphocytes (CD19+), activated HLADR+CD19+ cells, and CD8+ T-lymphocytes significantly increased at 6- and 16-months-of-age in both selected regions as compared to in cord blood values (p < 0.001). Levels of CD3+ cells increased significantly (from 61 to 65%) in samples from Michalovce (p < 0.01). Levels of CD4+ T-lymphocytes declined 10% among 16-month-olds in both regions (Michalovce at p < 0.001 and Svidnik/Stropkov at p < 0.01). Natural killer (NK) cell levels decreased 50% in Michalovce 6- and 16-month-old children and 42% among 6-month-olds in Svidnik/Stropkov (p < 0.001). Compared with the less-contaminated region, Michalovce samples showed significantly higher expression of CD3+ T-lymphocytes, B-lymphocytes, and activated B-lymphocytes, whereas NK cells were less expressed. Even after adjustment for selected covariates, e.g., maternal cigarette smoking, age, parity, ethnicity, birth weight, and gender of infant, the levels of CD19+, HLADR+CD19+, and CD3−CD(16 + 56)+ cells were seen to remain significantly different between the districts. These results showed that early-life environmental PCB exposure was associated with fluctuations in major lymphocyte subsets in children, suggesting that there is a post-natal immune system response to PCB exposures.
| Abbreviations: | ||
| CD, | = | cluster of differentiation; |
| PCB, | = | polychlorinated biphenyl; |
| SSC, | = | side scatter; |
| NK, | = | natural killer. |
Introduction
The immune system undergoes extensive changes in the first life stages. The development from neonate to adult massively influences the composition of peripheral blood leukocytes as well as that of the lymphocytes. Counts and subset distribution of lymphocytes in children are different from that in adults, with all lymphocyte subsets undergoing substantial dynamic change in the first year of life (Sack et al., Citation2007). Moreover it has been demonstrated that relative percentages of CD19+ B-lymphocytes increase during the first 5 months of life, and remain stable until ≈ age 5-years (followed by a gradual decrease to adulthood). The relative frequency of CD3+ T-lymphocytes remains within narrow median limits of 60–75%, and fluctuations in CD4+ and CD8+ lymphocyte subsets are limited. The percentages of NK cells show a dramatic 3-fold decline immediately after birth, followed by a slow 2-fold increase at adulthood (Comans-Bitter et al., Citation1997).
Development of the immune system, particularly in the pre-natal period, has far-reaching consequences for health during early childhood and throughout one’s whole life. Besides as a result of physiological changes, alteration of immune development may occur as a consequence of early life experience, including exposure to environmental chemicals. Dysregulation of the immune system or an aberrant trajectory or timing of events can result in atopy, asthma, a compromised ability to ward off infection, or autoimmune disease (Luster, Citation1996; Holladay and Smialowicz, Citation2000). Because the immune response plays a critical role in each of these diseases, it is of high importance to study the effects of toxicants on a developing immune system (Weisglas-Kuperus et al., Citation1995; Aoki, Citation2001; Garry, Citation2004; Holsapple et al., Citation2004; Duramad et al., Citation2007; Hertz-Picciotto et al., Citation2008).
In children < 5 years-of-age, > 33% of diseases are caused by environmental exposures (Smith et al., Citation1999; EPA, Citation2006; WHO, Citation2006). The main risk factors include pesticides, air and water pollution, lead, environmental tobacco smoke, infections, and inadequate diet. Environmental exposures to persistent xenobiotics like polychlorinated biphenyls (PCBs) during pre-natal and early post-natal development have been linked to a growing number of childhood diseases, including thyroid, metabolic, and immunologic disorders, neurodevelopmental toxicity, respiratory illness, leukemias, cancers and their metastases (Scheele et al., Citation1992; Park et al., Citation2009).
PCBs represent a group of 209 different chemicals, all of which are ubiquitous lipophiles. There are no known natural sources of PCBs in the environment. PCBs enter the air, water, and soil during their manufacture and use; however, because of toxicity and environmental persistence, most industrialized countries banned their production in the late 1970s. In Eastern Slovakia, PCBs were produced in the Michalovce region until 1985. A large quantity of PCB waste generated during their manufacture was released into the surrounding area by improper disposal, resulting in high PCB levels in various environmental matrices in the Michalovce district (Kocan et al., Citation2001). Concentrations of PCBs in human blood were seen to be far greater in Eastern Slovakia than in other Slovak areas (Pavuk et al., Citation2004; Hovander et al., Citation2006).
Human exposure to PCBs can occur transdermally, via inhalation, and orally mainly as the consequence of food chain contamination. PCBs accumulate in the body, especially in lipophilic tissues; they can cross the placenta and reach the fetus and they are excreted into breast milk (Kimbrough, Citation1987; Duarte-Davidson and Jones, Citation1994). Later in life, children can be exposed to PCBs in the same manner as adults. Because of their smaller weight, a child’s intake of PCB/kg body weight (for a given amount of contaminated product consumed) would be greater than in an adult (De Rosa et al., Citation1997; Barbalace, Citation2003).
The thymus is a primary lymphoid organ that reaches its maximum activity before puberty and continues to play an immunological role throughout life, even though its function declines with age. During the last stages of fetal life and the early neonatal period, the reticular structure of the thymus entraps lymphocyte precursors from the bone marrow, generates thymocytes, and subsequently mature T-lymphocytes. Once mature, T-lymphocytes emigrate from the thymus and constitute the peripheral T-lymphocyte repertoire responsible for directing many facets of the adaptive immune system (McCune et al., Citation1998). Park et al. (Citation2008) found an association between higher pre-natal PCB exposure and reduced thymus volume in newborns from the Michalovce region in Eastern Slovakia, where PCBs had been generated for many years. Pre-natal exposure to PCBs may adversely impact the immune responses to childhood vaccinations; there is suggestive evidence for dioxin-like compounds influencing the immune response by affecting lymphocyte population ratios and antibody production by B-lymphocytes (Dietert, Citation2006; Heilmann et al., Citation2006).
There are several reports of immunologic dysfunctions in patients with PCB exposure. These compounds are known immunosuppressants, affecting both humoral and cell-mediated components of the immune system. Previous studies reported evidence about the effects of PCBs on inflammatory mediators (e.g., chemokines, cytokines, growth factors) at both their levels of production and activity (Johnson et al., Citation1999; Noakes et al., Citation2005; Umannova et al., 2007; Diamond et al., Citation2008). A series of studies have shown an association between PCB exposure and changes in distributions of T- and B-lymphocytes and NK cells (Heinzow and Tinneberg, Citation1989; Svensson et al., Citation1994; Hagmar et al., Citation1995; Daniel et al., Citation2001; Hertz-Picciotto et al., Citation2008). Data indicated that early exposures to environmental toxicants could directly impact the development and function of children’s immunity. Many populations in polluted areas (such as Europe and North America) may be at risk of immunotoxicity, which could then manifest as diminished host resistance and increased incidence and severity of infectious diseases (Ross et al., Citation2004; Neale et al., Citation2005).
The objective of the present study was to study the dynamics of a cluster of differentiation (CD markers) in major lymphocyte subsets, namely CD3+, CD3+CD8+, CD3+CD4+, CD19+, CD3−CD(56 + 16)+, and HLADR+CD19+, in umbilical cord blood, and in samples of blood from 6- and 16-month-old children from two districts in Eastern Slovakia that have different levels of environmental PCB pollution. The goal was to explore the hypothesis of the induction of an immunomodulatory effect resulting from environmental exposure to PCBs during infancy. The effects of living environment, maternal cigarette smoking, age, parity, breastfeeding, ethnicity, birth weight, and gender of infant were considered as well.
Materials and methods
Study area
The specimens for CD markers assessment were delivered from two districts in Eastern Slovakia: Michalovce and Svidnik/Stropkov. The Michalovce district (population = 110,000) is a region with high levels of PCB contamination from a chemical manufacturing plant (Chemko Strazske) that produced PCBs from 1959 to 1984; the neighboring districts of Svidnik and Stropkov (70 km to the northwest, population = 55,000) each have lower environmental PCB levels. Kocan et al. (Citation2001) reported higher values of PCBs in ambient air (up to 1700 ng/m3), soil (from 0.4–53,000 mg/kg), surface water, sediment, and wildlife samples taken from the Michalovce district compared to in samples from Svidnik/Stropkov. In Michalovce, PCB levels in human adult serum were 2.3-times higher than in serum from individuals from the Svidnik and Stropkov districts (Jursa et al., Citation2006). A high concentration of sum of 15 PCBs congeners was found in the adult population of Michalovce (3105 and 1892 ng/g lipids, mean and median, respectively). Levels in Svidnik/Stropkov were remarkably lower (871 and 743 ng/g lipids, mean and median, respectively). Similarly, exposure of 8–10-year-old children from Michalovce was much higher if compared to among children from Svidnik/Stropkov (mean and median 766 and 502 ng/g of lipid in Michalovce and 372 and 274 ng/g of lipid in Svidnik/Stropkov, respectively) (Petrik et al., Citation2006).
Study population
During 2002–2004, women were recruited at delivery in hospitals in the Michalovce and Svidnik/Stropkov regions of Slovakia (Hertz-Picciotto et al., Citation2003). Blood specimens were obtained from mothers in each region to confirm PCB status/burden. PCB concentrations were determined in the National Reference Laboratory for Dioxins and Related Compounds for the Slovak Republic, at the Slovak Medical University in Bratislava. Descriptive data for PCB concentrations in maternal, cord, and infant serum were reported previously by Jusko et al. (Citation2010; Citation2011). The PCB levels in maternal blood in Michalovce were ≈ 2-fold higher than in mothers in Svidnik. The mean and median maternal serum levels of the sum of six PCBs (∑PCB) were 7.5 and 5.3 ng/ml (718.5 and 531.3 ng/g lipid) in Michalovce and 3.6 and 2.6 ng/ml (373.7 and 269.5 ng/g lipid) in Svidnik/Stropkov (Sonneborn et al., Citation2008). Similarly, the levels of hydroxylated-PCB (OH-PCB) metabolites were about twice as high in Michalovce mothers than those of Svidnik/Stropkov mothers. The median sum of OH-PCBs (∑OH-PCBs) was 0.55 ng/g wet weight in Michalovce mothers and 0.32 ng/g wet weight in Svidnik/Stropkov mothers (Park et al., Citation2007).
Each mother in this study was interviewed by a trained nurse to obtain information on: socio-demographic characteristics; past pregnancy; occupational history; medication history; living environment; and smoking habits of persons in the household. In these studies, individuals that were excluded were comprised of mothers: (a) with > 4 previous births; (b) < 18 years-of-age; (c) who resided < 5 years in their district; and (d) with a major illness during pregnancy, as well as (e) infants who had severe birth defects. Each mother gave signed informed consent.
For these studies, blood samples from umbilical vein at delivery (n = 362), and subsequently from children aged 6 (n = 349) and 16 months (n = 313) were collected. The characteristics of the study cohort (categorical variables) consisting of mother–infant pairs with delivery within 2002–2004 in two districts of Eastern Slovakia are reported in . Continuous variables, gestational age, birth weight, and maternal age are summarized in
Table 1 Characteristics of study cohort consisting of 362 mother-infant pairs in two districts, Michalovce (n = 301) and Svidnik/Stropkov (n = 61).
Table 2 The maternal and birth characteristics of the study group.
Immunophenotyping
The venous blood specimens from cord and infants were collected into EDTA vacutainer tubes. Whole blood specimens were treated with OptiLyse-C Lysing Solution (Beckman Coulter, Marseille, France) and washed with phosphate buffered saline (PBS, pH 7.4). Samples were then resuspended in PBS (containing 10% dimethyl sulfoxide [DMSO]) and then stored at −70°C until analyzed.
Each cell surface receptor of interest was analyzed by multi-color immunophenotyping using monoclonal antibodies CD19-PC7 (PE-Cy7; phycoerythrin-cyanine dye[7]), CD3-FITC (fluorescein isothiocyanate)/(CD56 + 16)-PE, CD4-PC7, CD8-PC5 (PE-Cy5), and HLADR-ECD (Electron Coupled Dye) (Beckman). Lymphocyte subsets were assayed after thawing, and propidium iodide was used as a vitality indicator. After incubation of 50 µl aliquots of blood specimens with 10 µl antibody for 20 min at room temperature, cell surface antigen analysis was performed using a Cytomics FC 500 flow cytometer and CXP software (Beckman). A minimum of 10,000 events per sample was acquired for analyses. Lymphocyte subset data were generated using a CD45/side-scatter (SSC) gate, and expressed as the percentage of all positive cells.
To assure validity of all data, all analyses were performed by the same flow cytometry laboratory (Slovak Medical University, Bratislava), using the same methods, monoclonal antibodies, and flow analyses software each time. This laboratory regularly participated in an external quality control (QC) scheme (Clinical Immunology, SEKK Comp., Division EQA, Immunophenotyping) and was accredited by the Slovak National Accreditation Service (SNAS). Fluorescent microspheres were used for cytometer alignment verification and fluorescence channel monitoring. Internal QC procedures were performed daily using appropriate reference biological controls (Immuno-Troll Cells, Beckman).
Statistical analyses
Database management and statistical analyses were performed using the SPSS statistical software package. Non-parametric Mann-Whitney and Wilcoxon tests were used for data evaluation. A p-value < 0.05 was used to indicate statistical significance. Bivariate correlation analysis was calculated using a Kendall’s test. The primary hypotheses were that the distribution of lymphocyte immunophenotype subsets would differ between samples of cord blood and blood from 6- and 16-months-of-age children when compared from among residents from Michalovce vs Stropkov/Svidnik. A multiple linear regression model was used to examine the effect of the potential confounders and important predictors that could influence lymphocyte surface receptor expressions (i.e., cells bearing these markers) in the cord, 6- and 16-month-olds’ samples.
Results
reports the descriptive characteristics (i.e., mean, median, range) of the total lymphocyte sub-populations in cord blood samples, and samples obtained from the same children at 6- and 16-months-of-age in the Michalovce and Svidnik/Stropkov regions. In , it can be seen that surface markers (i.e., cells bearing the markers for) CD19+, HLADR+CD19+, and CD3+CD8+ increased significantly at 6- and 16-months-of-age (relative to values seen in cord blood) among children residing in both regions. In comparison, levels of CD3+ expression increased in children from Michalovce only. In further contrast, the relative levels of CD4+ T-lymphocytes showed a 10% decline among 16-month-olds in both regions (p < 0.001 and p < 0.01 for Michalovce and Svidnik/Stropkov, respectively). Compared to values in cord blood samples, natural killer (NK) cell levels decreased ≈ 50% in samples from both 6- and 16-month-old children from Michalovce (p < 0.001) and 42% among 6-month-olds from Svidnik/Stropkov (p < 0.001). The impact of age on the dynamics of lymphocyte surface receptors expression over the period from birth through infancy was determined in the total study group (Michalovce and Svidnik/Stropkov), regardless of PCB contamination. Significant positive correlations for the analyzed CD markers have been found between cord, 6- and 16-month-old children’s samples in the total study cohort ().
Table 3 Total main lymphocyte subpopulations in cord, 6- and 16-months blood samples from Michalovce and Svidnik/Stropkov districts.
Table 4 CD marker correlation between samples of blood from children in the total study cohort.
Figure 1 Dynamics of the lymphocyte subsets in children in the Svidnik/Stropkov and Michalovce regions. The immunophenotyping was performed by multi-color analysis of blood samples collected form children at birth (cord blood), and again at 6- and 16-months-of-age. Populations of CD3+, CD3+CD4+, and CD3+CD8+ T-lymphocytes, CD19+ B-lymphocytes, HLADR+CD19+ activated B-lymphocytes, and CD3−CD(56 + 16)+ NK cells in each sample were analyzed. Flow cytometric analysis was performed on a Cytomics FC 500 (Beckman Coulter). Statistical analysis was performed using an SPSS System. p-Values reflect comparisons made to cord blood levels. Data shown are as mean of percentage (± SEM).
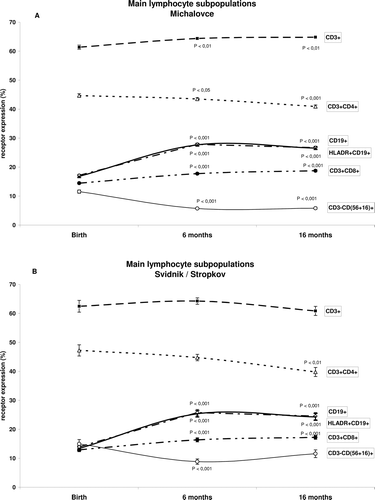
The other main findings are as follows: in samples from 16-month-old children from the Michalovce region, levels of CD3+ T-lymphocytes were significantly more expressed (64.98 ± 0.46%) as compared with in blood from age-matched children from the less polluted region (60.86 ± 1.51%, p < 0.01). Similarly, in Michalovce, the proportions of B-lymphocytes and activated B-lymphocytes at birth (17.19 ± 0.38% and 16.83 ± 0.38%, respectively) were greater than in the samples from newborns from the cleaner region (13.81 ± 0.76%, and 13.27 ± 0.76%, respectively, p < 0.001 both comparisons). In contrast, compared to children from the less polluted sites, NK cells were less expressed in children from Michalovce at all timepoints examined (11.51 ± 0.56% vs 14.9 ± 1.48% at birth, p < 0.05; 5.69 ± 0.24% vs 8.85 ± 0.94% at 6-months-of-age, p < 0.01; and 5.79 ± 0.34% vs 11.37 ± 1.29% at 16 months-of-age, p < 0.001).
Maternal cigarette smoking was associated with expression of major lymphocyte subsets in children. Maternal smoking increased the percentages of CD8+ T–lymphocytes in offspring at all timepoints assessed (smoker vs non-smoker: 16.53 ± 0.76% vs 13.72 ± 0.33% at birth; 19.53 ± 0.69% vs 17.00 ± 0.32% at 6-months-of-age; 20.87 ± 0.80% vs 18.19 ± 0.35% at 16-months-of-age, all p < 0.01). In contrast, CD3+ and CD4+ T-lymphocyte levels in children declined if their mothers smoked (smoker vs non-smoker: 62.27 ± 0.92% vs 65.17 ± 0.45% at birth, p < 0.05; 39.59 ± 1.10% vs 44.99 ± 0.46% at 6-months-of-age, p < 0.001; 36.98 ± 1.10% vs 41.55 ± 0.53% at 16-months-of-age, p < 0.01). Positive association was found between maternal age and the cell surface markers (i.e., for CD19+ and HLADR+CD19+) in cord samples (r = 0.1; p < 0.05).
Mother age, parity, ethnicity, birth weight, and gender were also found to be associated with children’s lymphocyte subset levels. The children of multiparous women had higher relative numbers of B-lymphocytes (17.40 ± 0.45%) and HLADR+CD19+ activated cells (17.05 ± 0.45%) as compared to among those of primiparous women (15.7 ± 0.55%, 15.2 ± 0.55%, respectively; p < 0.05). Levels of CD8+ cells were elevated in children of Romani ethnicity in comparison to those who were non-Romani (20.03 ± 0.70% vs 16.89 ± 0.32% at 6-months-of-age, p < 0.001; 20.68 ± 0.92% vs 18.19 ± 0.32% at 16-months of age, p < 0.01); CD3+ and CD4+ T-lymphocyte levels were diminished in this ethnicity (p < 0.01 and p < 0.001). The birth weight of Romani children was lower in comparison to non-Romanis (2966.82 ± 419.74 g vs 3390.00 ± 492.13 g; p < 0.001). Weak negative associations between birth weight in 6-month-old children and levels of B-lymphocytes and NK cells, as well as a positive correlation between birth weight and levels of CD3+ and CD4+ T-lymphocytes were noted (p < 0.05). Girls had higher numbers of CD8+ T-lymphocytes (15.0 ± 0.45%) at birth compared to boys (13.5 ± 0.39%; p < 0.05). These analyses did not reveal any associations between CD markers and breastfeeding status.
Results from multiple linear regressions for immunophenotyping parameters are shown in . After adjustment for covariates, the levels of CD19+, HLADR+CD19+, CD3−CD(16 + 56)+, and CD8+ T-lymphocytes in cord blood samples remained significantly different between the two districts studied. While levels of CD8+ T-lymphocytes, as well as of B- and activated B-lymphocytes were raised in the blood of children from the Michalovce area, the levels of NK cells in these same samples were depressed. Samples taken again at 6- and 16-months-of-age revealed (in multivariate models) the effect of district on CD19+, HLADR+CD19+, and CD3−CD(16 + 56)+ expression. Expression of CD3+ was significantly different between the two districts only in the 16-month-old children (i.e., levels were elevated in the children of Michalovce). When caesarean sections were excluded from the analysis, due to the possible effect on CD expression in cells in cord blood, the findings remained essentially unchanged. Likewise, when Romani ethnicity was excluded from the model in samples from 6- and 16-month-old children, the findings remained essentially unchanged.
Table 5 Coefficients and standard errors in models predicting immunophenotyping markers in samples of blood from children from Michalovce and Svidnik/Stropkov.
Discussion
Before discussing particular results of our study, some general statements are appropriate. The children face potentially elevated risk from the toxic environmentally persistent compounds also due to age-specific behaviors (distinct dietary patterns and enhanced exposure via hand-to-mouth and object-to-mouth transfer in children) (Faustman et al., Citation2000). Several reports have shown the immunosuppressant effects of PCBs, and their influence on allergic and autoimmune morbidity, infections, and cancer (Daniel et al., Citation2001; De et al., Citation2004). We observed temporal changes in levels of T- and B-lymphocytes and NK cells from birth to 6- to 16-months-of-age in children living in PCB-contaminated environments. Bivariate analyses showed an association between CD markers and relevant confounders, such as maternal cigarette smoking, age, parity, birth weight, and gender of infant. After adjustment for covariates in multiple linear regression models, the immunophenotyping parameters used here, i.e., CD19+, HLADR+CD19+, and CD3−CD(16 + 56)+, remained significantly different between the districts in each timepoint. Specifically, B- and activated B-lymphocytes were more expressed, whereas NK cells were less expressed, in samples obtained from neonates/infants from the Michalovce region.
The heavy water-borne and airborne industrial pollution by PCB and other persistent organochlorinated pollutants (POP) in the East Slovakian district of Michalovce resulted in very high environmental and blood levels of these toxic substances and their metabolites (Kocan et al., Citation1994; Hovander et al., Citation2006; Jursa et al., Citation2006). Because of a high correlation that was found to exist between the measured levels of several major POP (i.e., PCB, DDE, and HCB), PCB levels were considered here to be useful as a marker for all POP components (Langer et al., Citation2009). POP exposure can cause death and illnesses, including disruption of the endocrine, reproductive, and immune systems as well as neurobehavioral disorders and cancers. The immune system is particularly vulnerable to POP toxicity, with observations of thymus atrophy and reduced T-lymphocyte functions. POP are associated with changes in lymphocyte proliferation that could result in increased susceptibility to infections (Ritter et al., Citation1995; Jones and de Voogt, Citation1999; Ross and Birnbaum, Citation2001; Levin et al., Citation2005; Langer, Citation2010). The children living in these two East Slovakian regions are also exposed to other non-PCB pollutants that could be contributing to the observed effects. As such, additional studies will be needed to determine the impact of other non-POP pollutants exposure on cell surface marker expression in children born and living in the East Slovakia districts. Such future assessments of non-POP substances (e.g., heavy metals) may confirm or refute their potential confounding effects on the lymphocyte subsets in children from heavily PCB-polluted areas.
It is important to note that these studies covered events that occurred during the first year of life, when the immune system encounters many new antigens that, in turn, induce massive activation, proliferation, and maturation processes of many cell types and the child’s immune system in general. Immunologic immaturity in the young may be responsible for their observed increased susceptibility to infections during the first years of life. On the other hand, immunophenotyping data from early childhood should be interpreted with caution, as during that period the immune system undergoes much expansion and maturation and is characterized by significant variations in lymphocyte subpopulations (Wilson, Citation1986). Comans-Bitter et al. (Citation1997), in reviewing the immunophenotyping of blood lymphocytes in children, showed that the levels of CD3−CD(16 + 56)+ lymphocytes dramatically declined immediately after birth and those of B-lymphocytes increased during the first 5 months post-partum. In accordance with this, we noted that in children from both regions there were lower numbers of NK cells and increased numbers of B-lymphocytes at 6-/16-months-of-age compared with in their cord samples.
In contrast to the less polluted area, Michalovce samples demonstrated significantly higher levels of CD3+ T-lymphocytes, B-lymphocytes, and activated B-lymphocytes; however, NK cells were less evident. Reduced numbers of NK cells may be an indication of depressed innate immunity (van den Heuvel et al., Citation2002). Hagmar et al. (Citation1995) found a positive correlation between consumption of PCB-contaminated fish from the Baltic Sea and the proportion of B-lymphocytes in the general population. Here, we demonstrate the influence of PCB exposure on B-lymphocyte subsets in children. A more evident trend of increases in the relative levels of CD19+ cells after birth was observed in blood samples from children from the highly-contaminated area in comparison to among those from the less-PCB-contaminated environments; taken in conjunction with an increase in expression of HLADR+CD19+ cells, this may indicate B-lymphocyte proliferation in these subjects as well as activation/differentiation of their B-lymphocytes into antibody-producing plasma or memory cells. In line with these findings is a report that PCB exposure may lead to an increased adaptive immune response, augmentation of allergic disease, or an onset of autoimmune reactions (Krzystyniak et al., Citation1995). Alterations in lymphocyte sub-population dynamics after PCB exposure may also be related to a potentially increased susceptibility of children to inflammatory diseases, autoimmune diseases, allergy, immune deficiencies, and cancers; the clinical relevance of particular cell subset distributions has been widely described (Strelkauskas et al., Citation1976; McFarlane et al., Citation1977; Bullen and Losowsky, Citation1978; Matsumoto et al., Citation1980; Victorino and Hodgson, Citation1980; Balch et al., Citation1983; Crockard et al., Citation1990; Raes et al., Citation1997; Michalkova et al., Citation2000; Yssel and Groux, Citation2000).
Our current study suggests that other factors, such as maternal cigarette smoking, age, parity, ethnicity, birth weight, and gender of infants were all associated with alterations in major lymphocyte subsets. We noted that maternal age and parity were in a positive association with CD19+ and HLADR+CD19+ lymphocyte numbers in their offspring. Hertz-Picciotto et al. (Citation2002) have assessed the association between air pollution and lymphocyte immunophenotype distributions in two districts in the Czech Republic; that study found that maternal age was positively associated with the percentage of B-lymphocytes—and that parity was associated with CD3+ T-lymphocytes percentages—in cord blood samples. Our results did not support the previously reported inverse relationship between umbilical cord blood total nucleated cell levels and either mother’s age or obstetric history (McGuckin et al., Citation2007). We have shown that CD8+ T-lymphocytes were significantly higher in children from smoking women compared to non-smokers.
Cytotoxic T-lymphocytes represent a major defense against pathogens by both their ability to produce interferon (IFN)-γ and their cytolytic activity. Smoking lowered the ratio between CD4+ and CD8+ cells in the peripheral blood and lung (Byron et al., Citation1994; Mattoli et al., Citation1997). CD8+ T-lymphocyte accumulation in inflammatory processes could be related to an autogenic stimulus and it seems that smoking alone is a sufficient activating factor (Chrysofakis et al., Citation2004; Koch et al., Citation2007). In addition, the CD8+ expression was elevated in children with Romani ethnicity compared to those who were non-Romani; we hypothesize that this finding was mainly due to a higher frequency of smoking among the Roma populations. The cigarette smoking was almost 2-times greater in Romani than in non-Romani individuals, and 60% of Romani and ≈ 7% of non-Romani mothers had a history of smoking (data not shown). In this paper, we have not considered indirect smoke exposure during pregnancy and post-natal development as potential confounders to the observed effects of PCB on the immune system. A partial indication could be assumed from the completed questionnaires that non-smokers and mothers avoiding smoking during pregnancy have protected their newborns from indirect smoke exposure at home; nevertheless, future scientific evidence for the effects of sidestream smoke on the measured parameters will be required to fully account for this issue. Lastly, in agreement with the findings of Jiang et al. (Citation2004), we also demonstrated there were CD8+ subset variations between genders, with a higher percentage of CD8+ cells being identified in females. Such an association between gender and lymphocyte levels (i.e., CD4+, but not CD8+, cell counts) was previously reported by Bunders et al. (Citation2005). In contrast to our results, Cairo et al. (2004) suggested that there were increased levels of CD3+CD8+ lymphocyte subsets in males.
We found that the association between birth weight and lymphocyte sub-population counts, specifically for CD19+ and CD3−CD(16 + 56)+ cells, was negative, but that for CD3+ and CD4+ cells the association was positive. Several studies have reported that birth weight was significantly associated with higher B-lymphocyte counts (see Bartha and Comino-Delgado, Citation1999; Duijts et al., Citation2009). However, there are other studies that declared there was no effect on total leukocyte counts and lymphocyte sub-populations (Collinson et al., Citation2008). Ballow et al. (Citation2006) suggested that the numbers of CD8+ T-lymphocytes were significantly lower in the first month of life in premature infants with very low birth weight. Post-natal T-lymphocyte phenotypic changes may parallel the T-lymphocyte ontogenetic process that occurs during the last trimester of pregnancy in full-term infants. The prevalence of newborns with low birth weight (≤ 2500 g) in our cohort was < 6.0%. We found that low birth weight was associated with a lower percentage of CD3+ T-lymphocytes, but not with those with a CD8+ marker. Decreased levels of CD3+ cells in the low birth weight children born at full-term could be a consequence of the limit of division having been met early in life through accelerated apoptosis of lymphocytes (Barg et al., Citation2004; Raqib et al. Citation2007). An association between breastfeeding and lymphocyte subsets was not observed in our study; this finding is consistent with those from a previous study (Kaneko, Citation2006).
In the present paper, we reported changes in several immune system parameters (e.g., percentages of CD3+ T-lymphocytes, activated B-lymphocytes, and NK cells) in association with both pre-natal and early-life exposures to PCBs. The fetus is probably continuously exposed to PCBs while in their mothers during the process of development, and exposure to these toxic compounds continues post-natally, mainly via breastfeeding and ingestion of PCB-containing foods (Guvenius et al., Citation2003; U.S. EPA and TEACH Database, 2009). Dioxin-like-PCBs induce gene expression via a ligand-dependent trans-activating factor, the aryl hydrocarbon receptor (AhR). The other category of PCBs, with two or more ortho-chlorines, can act through non-AhR-mediated mechanisms and have different toxic potentials. The cellular mechanisms of PCBs toxic action(s) remain elusive, but there are several hypotheses. Several of these converge on the modification of cellular Ca2+ homeostasis, inducing changes in mitochondrial membrane integrity, oxygen radical generation, or protein kinase C translocation (Harper et al., Citation1993; BEST, Citation2001; Pocar et al., Citation2006; Ferrante et al., Citation2011). It has been speculated that the PCBs interact with the sheep red blood cell receptor or specific membrane functions of human T-lymphocytes (Heinzow and Tinneberg Tinnerberg in refs., Citation1989).
In conclusion, this paper summarizes effects of environmental PCB contamination and other co-factors on immunophenotypic modulation of lymphocytes. Multivariate linear regression models showed that besides the effect of PCB contamination levels in the two districts, a mother’s age was associated with higher percentages of HLADR+CD19+ cells, and that higher parity was associated with increased CD3-CD(16 + 56)+ and decreased CD3+, CD4+, and CD8+ expression in cord samples. Birth weight seemed to be associated with the levels of B-lymphocytes, HLADR+CD19+ cells, as well as of CD3+ and CD4+ T-lymphocytes in 6-month-old children.
Further, offspring gender and mother education status were other predictive variables; this was specifically the case for male children having lower amounts of CD4+ and higher percentages of CD8+ T-lymphocytes at 6- and 16-months-of-age, respectively. The status of a mother that had secondary/higher education could be used to predict lower levels of CD3+ and CD4+ T-lymphocytes in children (6- and 16-month-olds). Lastly, maternal cigarette smoking was associated with a decreased number of B-lymphocytes (as seen in 16-month-old children).
With respect to two regions with different environmental PCB contamination levels, we also observed variations in levels of T- and B-lymphocytes and NK cells in newborns and infants. Long-term low-level environmental exposure to PCBs affects immune functions in hosts, in part, by suppression of NK cells and induction/activation of T- and B-lymphocytes. This negative impact may be even more pronounced if PCB exposure starts before birth and continues post-partum, as the developing fetus/infant represents one of the most sensitive human sub-populations when it comes to environmental insults.
Acknowledgments
This work was supported by the US NIH grant # R01-CA096525, Grant of the Ministry of Health of the Slovak Republic # 2005/31-SZU-09, and the 6th FP EU research projects “HEIMTSA” (no. GOCE-CT-2006-036913-2) and “INTARESE” (no. GOCE 018385). We wish to thank Mikulas Krnac and Olga Liskova for their technical assistance. We are grateful to our regional cooperators from the Hospitals in Michalovce and in Svidnik for their responsible work within the project and we wish to thank all mothers with children who participated in the project.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aoki Y. 2001. Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans as endocrine disrupters - what we have learned from Yusho disease. Environ. Res. 86:2–11.
- Balch C. M., Tilden A. B., Dougherty P., Cloud G. A., and Abo T. 1983. Depressed levels of granular lymphocytes with natural killer (NK) cell function in 247 cancer patients. Ann. Surg. 198:192–199.
- Ballow M., Cates K. L., Rowe J. C., Goetz C., and Pantschenko A. G. 2006. Peripheral blood T-cell sub-populations in the very low birth weight (< 1500 g) infant. Am. J. Hematol. 24:85–92.
- Barbalace R. C. 2003. The chemistry of polychlorinated biphenyls PCB, the manmade chemicals that won’t go away. Available online at: http://environmentalchemistry.com/yogi/chemistry/pcb.html.
- Barg E., Gasiorowski K., Brokos B., Swierdrych A., and Skorkowska K. 2004. A high frequency of apoptosis was found in cultures of lymphocytes isolated from the venous blood of children born with a low birth weight. Cell. Mol. Biol. Lett. 9:135–143.
- Bartha J.L., and Comino-Delgado R. 1999. Lymphocyte sub-populations in intrauterine growth retardation in women with or without previous pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 82:23–27.
- BEST, Board on Environmental Studies and Toxicology. 2001. A risk management strategy for PCB-contaminated sediments. Appendix G; Toxicity of PCBs, pp. 363–427.
- Bullen A. W., and Losowsky M. S. 1978. Lymphocyte sub-populations in adult celiac disease. Gut 19:892–897.
- Bunders M., Thorne C., and Newell M. L. 2005. Matrenal and infant factors and lymphocyte, CD4 and CD8 cell counts in uninfected children of HIV-1-infected mothers. AIDS 19:1071–1079.
- Byron K. A., Varigos G. A., and Wootton A. M. 1994. IL-4 production is increased in cigarette smokers. Clin. Exp. Immunol. 95:333–336.
- Cairo M. S., Wagner E.L., Fraser J., Cohen G., van de Ven C., Carter S. L., Kernan N. A., and Kurtzberg J. 2005. Characterization of banked umbilical cord blood hematopoietic progenitor cells and lymphocyte subsets and correlation with ethnicity, birth weight, sex, and type of delivery: A cord blood transplantation (COBLT) study report. Transfusion 45:856–866.
- Chrysofakis G., Tzanakis N., Kyriakoy D., Tsoumakidou M., Tsiligianni I., Klimathianaki M., and Siafakas N. M. 2004. Perforin expression and cytotoxic activity of sputum CD8+ lymphocytes in patients with COPD. Chest 125:71–76.
- Collinson A. C., Ngom P. T., Moore S. E., Morgan G., and Prentice A. M. 2008. Birth season and environmental influences on blood leukocyte and lymphocyte sub-populations in rural Gambian infants. BMC Immunol. 9:18–28.
- Comans-Bitter W. M., de Groot R., van den Beemd R., Neijens H. J., Hop W. C., Groeneveld K., Hooijkaas H., and van Dongen J. J. 1997. Immunophenotyping of blood lymphocytes in childhood. J. Pediatr. 130:388–393.
- Crockard A. D., Alexander H. D., Stephenson C. F., McCrea P., Desai Z. R., Morris T. C. M., and McNeill T. A. 1990. An analysis of circulating CD4 lymphocyte sub-populations in B-cell chronic lymphocytic leukemia. Leukemia Lymph. 3:127–133.
- Daniel V., Huber W., Bauer K., Suesal C., Conradt Ch., and Opelz G. 2001. Association of blood levels of PCB, HCHs, and HCB with numbers of lymphocyte sub-populations, in vitro lymphocyte response, plasma cytokine levels, and immunoglobulin autoantibodies. Environ. Health Perspect. 109:173–178.
- De Rosa C. T., Johnson M. F., Hansen H., and Mumtaz M. M. 1997. Public health implications of hazardous waste sites: Findings, assessment and research. Food Chem. Toxicol. 34:11–12.
- De S., Pramanik S. K., Williams A. L., andDutta S. K. 2004. Toxicity of polychlorobiphenyls and its bioremediation. Int. J. Hum. Genet. 4:281–290.
- Diamond M. P., Wirth J. J., and Saed G. M. 2008. PCBs enhance collagen I expression from human peritoneal fibroblasts. Fertil. Steril. 90:1372–1375.
- Dietert R. R. 2006. Developmental immunotoxicity testing and protection of children’s health. PloS Med. 3:1216–1217.
- Duarte-Davidson R., and Jones K. C. 1994. Polychlorinated biphenyls (PCBs) in the population: Estimated intake, exposure and body burden. Sci. Total Environ. 151:131–152.
- Duijts L., Bakker Jonges L. E., Labout J. A., Jaddoe V. W., Hofman A., Steegers E. A., van Dongen J. J., Hooijkaas H., and Moll H. A. 2009. Fetal growth influences lymphocyte subset counts at birth: Generation R study. Neonatology 95:149–156.
- Duramad P., Tager I. B., andHolland N. T. 2007. Cytokines and other immunological biomarkers in children’s environmental health studies. Toxicol. Lett. 172:48–59.
- EPA. 2006. Children’s Environmental Health: 2006 Report. Washington DC: Environmental Protection Agency and the United States (EPA).
- Faustman E. M., Silbernagel S. M., Fenske R. A., Burbacher T. M., and Ponce R. A. 2000. Mechanisms underlying children’s susceptibility to environmental toxicants. Environ. Health Perspect. 108(S1):13–21.
- Ferrante M. C., Mattace Raso G., Esposito E., Bianco G., Iacono A., Clausi M.T., and Amero P. 2011. Effects of non-dioxin-like polychlorinated biphenyl congeners (PCB 101, PCB 153 and PCB 180) alone or mixed on J774A.1 macrophage cell line: Modification of apoptotic pathway. Toxicol. Lett. doi: 10.1016/j.toxlet.2011.01.023
- Garry V. F. 2004. Pesticides and children. Toxicol. Appl. Pharmacol. 198:152–163.
- Guvenius D. M., Aronsson A., Ekman-Ordeberg G., Bergmann A., and Noren K. 2003. Human prenatal and postnatal exposure to polychlorinated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ. Health Perspect. 111:1235–1241.
- Hagmar L., Hallberg T., Leja M., Nilsson A., and Schutz A. 1995. High consumption of fatty fish from the Baltic Sea is associated with changes in human lymphocyte subset levels. Toxicol. Lett. 77:335–342.
- Harper N., Connor K., and Safe S. 1993. Immunotoxic potencies of polychlorinated biphenyl (PCB), dibenzofuran (PCDF), and dibenzo-p-dioxin (PCDD) congeners in C57BL/6 and DBA/2 mice. Toxicology 80:217–227.
- Heilmann C., Grandjean P., Weihe P., Nielsen F., and Budtz-Jorgensen E. 2006. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PloS Med. 3:1352–1259.
- Heinzow B. G., and Tinnerberg H. R. 1989. Effects of polychlorinated biphenyls on T-lymphocytes using a modified erythrocyte rosette inhibition test. Chemosphere 19:823–828.
- Hertz-Picciotto I., Dostál M., Dejmek J., Sherry G., Selevan S. G., Wegienka G., Gomez-Caminero A., and Šrám R. J. 2002. Air pollution and distribution of lymphocyte immunophenotypes in cord and maternal blood at delivery. Epidemiology 13:172–183.
- Hertz-Picciotto I., Park H. Y., Dostal M., Kocan A., Trnovec T., and Sram R. 2008. Prenatal exposures to persistent and non-persistent organic compounds and effects on immune system development. Basic Clin. Pharmacol. Toxicol. 102:146–154.
- Hertz-Picciotto I., Tmovec T., Kocan A., Charles M. J., Ciznar P., Langer P., Sovcikova E., and James R. 2003. PCBs and early childhood development in Slovakia: Study design and background. Fresenius Environ. Bull. 12:208–214.
- Holladay S. D., and Smialowicz R. J. 2000. Development of the murine and human immune system: Differential effects of immunotoxicants depend on time of exposure. Environ. Health Persepect. 108(S):463–473.
- Holsapple M. P., Paustenbach D. J., Charnley G., West L. J., Luster M. I., Dietert R. R., and Burns Naas L. A. 2004. Symposium summary: children’s helath risk - What’s so special about the development immune system? Toxicol. Appl. Pharmacol. 199:61–70.
- Hovander L., Linderholm L., Athanasiadou M., Athanassiadis I., Bignert A., Fängström B., Kocan A., Petrik J., Trnovec T., and Bergman A. 2006. Levels of PCBs and their metabolites in the serum of residents of a highly contaminated area in Eastern Slovakia. Environ. Sci. Technol. 40:3696–3703.
- Jiang W., Kang L., Lu H. Z., Pan X., Lin Q., Pan Q., Xue Y., Wenig X., and Tang Y. W. 2004. Normal values for CD4 and CD8 lymphocyte subsets in healthy Chinese adults from Shanghai. Clin. Diagn. Lab. Immunol. 2004:811–813.
- Johnson B. L., Hicks H. E., Cibulas W., Faroon O., Ashizawa A. E., and De Rosa C. T. 1999. Public Health Implications of Exposure to Polychlorinated Biphenyls (PCBs). Atlanta, GA: Agency for Toxic Substances and Disease Registry (ATSDR)/U.S. Department of Public Health and Human Services.
- Jones K. C., and de Voogt R. 1999. Persistent organic pollutants (POPs): State of the science. Environ. Poll. 100:209–221.
- Jursa S., Chovancova J., Petrik J., and Loksa J. 2006. Dioxin-like and non-dioxin-like PCBs in human serum of Slovak population. Chemosphere 64:686–691.
- Jusko T. A., De Roos A. J., Schwartz S. M., Lawrence B. P., Palkovicova L., Nemessanyi T., Drobna B., Fabisikova A., Kocan A., Sonneborn D., Jahnova E., Kavanagh T. J., Trnovec T., and Hertz-Picciotto I. 2010. A cohort study of developmental polychlorinated biphenyl (PCB) exposure in relation to post-vaccination antibody response at 6-months-of-age. Environ. Res. 110:388–395.
- Jusko T. A., De Roos A. J., Schwartz S. M., Lawrence B. P., Palkovicova L., Nemessanyi T., Drobna B., Fabisikova A., Kocan A., Sonneborn D., Jahnova E., Kavanagh T. J., Trnovec T., and Hertz-Picciotto I. 2011. Maternal and early postnatal polychlorinated biphenyl exposure in relation to total serum immunoglobulin concentrations in 6-month-old infants. J. Immunotoxicol. 8:95–100.
- Kaneko H. 2006. Effects of dioxins on the quantitative levels of immune components in infants. Toxicol. Ind. Health 22:131–136.
- Kimbrough R. D. 1987. Human health effects of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs). Ann. Rev. Pharmacol. Toxicol. 27:87–111.
- Kocan A., Petrik J., Drobna B., and Chovancova J. 1994. Levels of PCB and some organochlorine pesticides in the human population of selected areas of the Slovak Republic. Chemosphere 29:2315–2325.
- Kocan A., Petrik J., Jursa S., Chovancova J., and Drobna B. 2001. Environmental contamination with polychlorinated biphenyls in the area of their former manufacture in Slovakia. Chemosphere 43:595–600.
- Koch A., Gaczkowski M., Sturton G., Staib P., Schinkothe T., Klein E., Rubbert A., Bacon K., Wassermann K., and Erdmann E. 2007. Modification of surface antigens in blood CD8+ T-lymphocytes in COPD: Effects of smoking. Eur. Respir. J. 29:42–50.
- Krzystyniak K., Tryphonas H., and Fournier M. 1995. Approaches to the evaluation of chemical-induced immunotoxicity. Environ. Health Perspect. 103(S9):17–22.
- Langer P. 2010. The impacts of organochlorines and other persistent pollutants on thyroid and metabolic health. Front. Neuroendocrinol. 31:497–518.
- Langer P., Kočan A., Tajtáková M., Sušienková K., Rádiková Ž., Koška J., Kšinantová L., Imrich R., Hučková M., Drobná B., Gašperíková D., Trnovec T., and Klimeš I 2009. Multiple adverse thyroid and metabolic health signs in the population from the area heavily polluted by organochlorine cocktail (PCB, DDE, HCB, dioxin). Thyroid Res. 2:3–9.
- Levin M., De Guise S., and Ross P. S. 2005.Association between lymphocyte proliferation and polychlorinated biphenyls in free-ranging harbor seal (Phoca vitulina) pups from British Columbia, Canada. Environ. Toxicol. Chem. 24:1247–1252.
- Luster M. I. 1996. Immunotoxicology: Clinical consequences. Toxicol. Ind. Health 12:533–535.
- Matsumoto K., Osakabe K., Ohi H., Yoshizawa N., Harada M., and Hatano M. 1980. Alteration of T-lymphocyte sub-populations in patients with primary renal diseases and systemic lupus erythematosus. Scan. J. Immunol. 11:187–193.
- Mattoli S., Kleimberg J., Stacey M. A., Bellini A., Sun G., and Marini M., and Hatano M., and Hatano M. 1997. The role of CD8+ TH2 lymphocytes in the development of smoking-related lung damage. Biochem. Biophys. Res. Commun. 239:146–149.
- McCune J. M., Loftus R., Schmidt D. K., Caroll P., Webster D., Swor Yim L. B., Francis I. R., Gross B. H., and Grant R. M. 1998. High prevalence of thymic tissue in adults with human immunodeficiency virus-1 infection. J. Clin. Invest. 101:2301–2308.
- McFarlane I. G., Eddleston A. L., and Williams R. 1977. Lymphocyte sub-populations in chronic liver disease. Clin. Exp. Immunol. 30:1–3.
- McGuckin C. P., Basford C., Hanger K., Habibollah S., and Forraz N. 2007. Cord blood revelations: The importance of being a first-born girl, big, on time, and to a young mother! Early Hum. Dev. 83:733–741.
- Michalkova D., Mikulecky M., and Tibenska E. 2000. Alterations in lymphocyte sub-populations in peripheral blood at manifestation of Type 1 diabetes mellitus in childhood. Bratisl. Med. J. 101:365–370.
- Neale J. C.,Kenny T. P., Tjeerdema R. S., andGershwin M. E. 2005. PAH- and PCB-induced alterations of protein tyrosine kinase and cytokine gene transcription in harbor seal (Phoca vitulina) PBMC. Clin. Dev. Immunol. 12:91–97.
- Noakes P. S., Taylor P., Wilkinson S., and Prescott S. L. 2005. The relationship between persistent organic pollutants in maternal and neonatal tissues and immune responses to allergens: A novel exploratory study. Chemosphere 63:1304–1311.
- Park H. Y., Hertz Picciotto I., Petrik J., Palkovicova L., Kocan A., and Trnovec T. 2008. Prenatal PCB exposure and thymus size at birth in neonates in Eastern Slovakia. Environ. Health Perspect. 116:104–109.
- Park H. Y., Park J. S., Sovcikova E., Kocan A., Linderholm L., Bergman A., Trnovec T., and Hertz Picciotto I. 2009. Exposure to hydroxylated polychlorinated biphenyls (OH-PCBs) in the prenatal period and subsequent neurodevelopment in Eastern Slovakia. Environ. Health Perspect. 117:1600–1606.
- Park J. S., Linderholm L, Charles M. J., Athanasiadou M., Petrik J., Kocan A., Drobna B., Trnovec T., Bergman A., and Hertz-Picciotto I. 2007. Polychlorinated biphenyls and their hydroxylated metabolites (OH-PCBs) in pregnant women from Eastern Slovakia. Environ. Health Perspect. 114:20–27.
- Pavuk M., Cerhan J. R., Lynch C. F., Schecter A., Petrik J., Chovancova J.,, and Kocan A. 2004. Environmental exposure to PCBs and cancer incidence in Eastern Slovakia. Chemosphere 54:1509–1520.
- Petrik J., Drobna B., Pavuk M., Jursa S., Wimmerova S., and Chovancova J. 2006. Serum PCB and organochlorine pesticides in Slovakia: Age, gender, and residence as determinants of organochlorine concentrations. Chemosphere 65:410–418.
- Pocar P., Brevini T. A., Antonini S., and Gandolfi F. 2006. Cellular and molecular mechanisms mediating the effcet of polychlorinated biphenyls on oocyte in vitro maturation. Reprod. Toxicol. 22:242–249.
- Raes M., Peeters V., Alliet P., Gillis P., Kortleven J., Magerman K., and Rummens J. L. 1997. Peripheral blood T- and B-lymphocyte sub-populations in infants with acute respiratory syncytila virus brochiolitis. Pediatr. Allergy Immunol. 8:97–102.
- Raqib R., Alam D. S., Sarker P., Ahmad S. M., Ara G., and Yunus M. 2007. Low birth weight is associated with altered immune function in rural Bangladeshi children : A birth cohort study. Am. J. Clin. Nutr. 85:845–852.
- Ritter L., Solomon K. R., and Forget J. 1995. Persistent organic pollutants. An Assessment Report for the International Programme on Chemical Safety (IPCS). pp. 1–43.
- Ross P. S., and Birnbaum L. S. 2001. III. Case Studies. A. Persistent organic pollutants (POPs) in humans and wildlife. pp. 1–26.
- Ross P. S., Jeffries S. J., Yunker M. B., Addison R. F., Ikonomou M. G., and Calambokidis J. C. 2004. Harbor seals (Phoca vitulina) in British Columbia, Canada, and Washington State, USA, reveal a combination of local and global polychlorinated biphenyl, dioxin, and furan signals. Environ. Toxicol. Chem. 23:157–165.
- Sack U., Gerling F., and Tárnok A. 2007. Age-related lymphocyte subset changes in the peripheral blood of healthy children - a meta-study. Transfus. Med. Hemother. 34:176–181.
- Scheele J., Teufel M., and Niessen K. H. 1992. Chlorinated hydrocarbons in the bone marrow of children: Studies on their association with leukemia. Eur. J. Pediatr. 151:802–805.
- Smith K. R., Corvalán C. F., and Kjellstrom T. 1999. How much global ill health is attributable to environmental factors? Epidemiology 10:573–584.
- Sonneborn D., Park H. Y, Babinska K., Palkovicova L., Trnovec T., Kocan A., Nguyen D. V, and Hertz-Picciotto, I. 2008. Serum PCB concentrations in relation to locally produced food items in Eastern Slovakia. J. Expo. Sci. Environ. Epidemiol. 18:581–587.
- Strelkauskas A. J., Schauf V., and Dray S. 1976. Alterations in T-, B-, and null cells in children with autoimmune diseases. Clin. Immunol. Immunopathol. 6:359–368.
- Svensson B. G., Hallberg T., Nilsson A., Schultz A., and Hagmar L. 1994. Parameters of immunological competence in subjects with high consumption of fish contaminated with persistent organochlorine compounds. Int. Arch. Occup. Environ. Health 65:351–358.
- U.S. EPA Toxicity and Exposure Assessment for children’s Health. 2009. Polychlorinated Biphenyls (PCBs). TEACH Chemical Summary. Available online at: http://www.epa.gov/teach/,
- Umannová L., Zatloukalová J., Machala M., Krčmár P., Májková Z., Hennig B., Kozubík A., andVondráček J.. Tumor necrosis factor-α modulates effects of aryl hydrocarbon receptor ligands on cell proliferation and expression of cytochrome P450 enzymes in rat liver “stem-like” cells. Toxicol. Sci. 99:79–89.
- van den Heuvel R. L., Koopen G., Staessen J. A., Hond E. D., Verheyen G., Nawrot T. S., Roels H. A., Vlietinck R., and Schoeters G. E. 2002. Immunologic biomarkers in relation to exposure markers of PCBs and dioxins in Flemish adolescents (Belgium). Environ. Health Perspect. 110:595–600.
- Victorino R. M., and Hodgson H. J. 1980. Alteration in T-lymphocyte sub-populations in inflammatory bowel disease. Clin. Exp. Immunol. 41:156–165.
- Weisglas-Kuperus N., Sas T. C., Koopman-Esseboom C., van der Zwan C. W., De Ridder M. A., Beishuizen A., Hooijkaas H., and Sauer P. J. 1995. Immunologic effects of background prenatal and postnatal exposure to dioxins and polychlorinated biphenyls in Dutch infants. Pediatr. Res. 38:404–410.
- WHO, Geneva. 2006. Almost a Quarter of All Disease Caused by Environmental Exposure. Geneva: World Health Organization.
- Wilson C. B. 1986. Immunologic basis for increased susceptibility of the neonate to infection. J. Pediatr. 108:1–12.
- Yssel H., and Groux H. 2000. Characterization of T-cell sub-populations involved in the pathogenesis of asthma and allergic diseases. Int. Arch. Allergy Immunol. 121:10–18.