Abstract
The immunosuppression that occurs after burn injury causes an increase in susceptibility to infection. The aim was to investigate time-related alterations in various cytokines following thermal injury and to modulate cytokines by use of an immunomodulant, cimetidine. Male Balb/c mice were anesthetized and given a 10% total body surface area full-thickness burn by submerging in 90°C water for 9 s. Time-dependent changes in delayed type hypersensitivity (DTH) and serum levels of the cytokines IL-2, IL-10, IL-12, IL-17 and TGFβ were then assessed at various post-burn day (PBD) timepoints. Effects of 10 mg cimetidine/kg on DTH responses and cytokine levels were evaluated up to PBD 14. In comparison to healthy non-burned control mice, levels of IL-2 and IL-17 significantly decreased at PBD 3, 5, 10, and 14, those of IL-10 at PBD 1, 3, 5, and 10, and those of IL-12 at PBD 1, 3, 5, 10, and 14. Administration of cimetidine significantly augmented the levels of IL-2 (at PBD 3, 5, and 10), IL-10 (at PBD 1 and 5), IL-12 (at PBD 3, 5, 10, and 14), and IL-17 (at PBD 3 and 14) as compared to those in burned counterparts who did not receive drug. In comparison to healthy mice, biphasic alterations were observed regarding TGFβ levels; values were significant decreased and increased at PBD 3 and PBD 14, respectively. Cimetidine significantly diminished the elevated TGFβ levels at PBD 14. Cimetidine also significantly augmented DTH responses at PBD 5, 10, and 14 as compared to responses in non-drug-treated burned hosts. Taken together, the results here showed significant time-dependent changes in serum cytokines levels after burn injury and that cimetidine was able to significantly augment IL-2, IL-10, IL-12, and IL-17 levels as well as DTH responses that are normally suppressed following thermal trauma.
Introduction
Severe thermal injury leads to a profound depression of humoral and cell-mediated immunities. It has been reported that the effector mechanisms employed in the immune system, such as phagocytosis, chemotaxis, lymphocyte proliferation, natural killer cell activity, and antibody production, were impaired following thermal trauma (Steinstraesser et al., Citation2004; Fayazov et al., Citation2009; Butler et al., Citation2010). The immunosuppression that occurs after burn injury also causes increased susceptibility to infection (Fayazov et al., Citation2009).
The immunodeficiency observed after burn injury has been attributed in part to T-lymphocyte dysfunction. It was demonstrated that T-lymphocyte unresponsiveness to infection is the consequence of the reduction of T-lymphocytes, suppression of cytokine synthesis, and an impaired activation of leukocytes (Wang et al., Citation2007; Tang et al., Citation2011). Helper T (TH) -lymphocyte cells divided into at least three distinct sub-sets, i.e., TH1, TH2, and TH17 cells that are characterized by distinct cytokine release profiles and cell/cytokine functions (Saito et al., Citation2010; Zhu and Paul, Citation2010). TH1 cells produce interferon (IFN)-γ and interleukin (IL)-2 cytokines that are involved in cell-mediated immune responses effective in defense against intracellular pathogens. In contrast, TH2 cells secrete IL-4, -10, -13, and IL-25 and have a key role in IgE production, eosinophilic inflammation, and immune responses against helminths. Lastly, TH17 cells express IL-17, -22, -17F, -21, and CCL20, and play a role in immune response against fungi and extracellular pathogens. Regulatory T-cells (Treg) produce transforming growth factor (TGF)-β and IL-10 that help regulate effector T-lymphocyte functions and so maintain peripheral tolerance. Clearly, any imbalance in these various CD4+ T-lymphocyte populations could result in adverse consequences for the affected host (Saito et al., Citation2010; Zhu and Paul, Citation2010).
Several studies about burn-induced immunosuppression have indicated there are reductions in percentages of T-lymphocytes (Dong et al., Citation2007), suppressed T-lymphocyte secretion of IL-2 and IFNγ (Schwacha et al., Citation2005), increased levels of soluble IL-2 receptors (Correia et al., Citation2002), an imbalance in TH1 and TH2 functions, and excessive production of TH2-type cytokines (including IL-4 and -10; Dong et al., Citation2007), higher production of macrophage inhibitory mediators (like prostaglandin E2; Schwacha, Citation2003), increased Treg cell activity (MacConmara et al., Citation2006), and increased generation of a number of immunosuppressive substances (Huang, Citation1992).
Some investigations have indicated that burn-induced immunosuppression could be overcome through administration of select cytokines (Kinoshita et al., Citation2006), hormones (Kovacs et al., Citation2004), antioxidants (Cetinkalee et al., 1999), CpG oligonucleotides (Yabuki et al., Citation2010), and/or immunomodulating drugs like indomethacin (Choudhry et al., Citation2002) and cimetidine (Gharegozloo et al., Citation2004), as well as by surgical excision of the burn wound (Kovacs et al., Citation2004). We recently demonstrated that cimetidine restores burn-induced suppression of delayed-type hypersensitivity (DTH) responses at 10 days post-thermal trauma (Jafarzadeh et al., Citation2010). It has also been shown that the burn blister fluid has an immunosuppressive activity that can be modulated with cimetidine (Gharegozloo et al., Citation2004).
The immunostimulatory effects of cimetidine have been reported in many investigations over the past 30 years (i.e., Ebtekar and Hassan, Citation1993; Li et al., Citation2011; Zhang et al., Citation2011). The study in an animal model presented here was conducted to evaluate time-dependent alterations in serum T-helper (TH)-1 type cytokines (i.e., IL-2 and -12), a TH2/Treg-type cytokine (IL-10), a TH17-type cytokine (IL-17), and a Treg-type cytokine (TGFβ at various timepoints over a post-burn period and to evaluate effects of the immunomodulator cimetidine on any burn-induced changes in these cytokine levels. Similarly, studies were also performed to ascertain the effects of thermal burns, and of cimetidine, on a cytokine-dependent immune response in these murine hosts, i.e., DTH.
Materials and methods
Animals
Male Balb/c mice (20–25 g, 8-weeks-old; Pasteur Institute, Tehran, Iran) were housed under standard conditions, i.e., a reverse daylight pattern maintained through artificial illumination and a constant temperature and relative humidity of 20–25°C and 70–80%, respectively. All mice were housed in a room where the testing procedure was performed so as to minimize any stress response potentially induced by novel environmental cues. Prior to any experiments, the mice were allowed ad libitum access to standard rodent chow and sterilized filtered water.
All experiments were conducted on-site at the Rafsanjan University of Medical Sciences and were performed in accordance with the recommendations of the Medical School Ethics Committee on Animal Experimentation. Throughout the entire study, the mice were monitored for health status and food intake to be certain that they were healthy. The mice were specific pathogen-free and sentinel mice were maintained/tested in the animal facility.
Induced thermal burn injury
For the experiments, mice were randomly assigned to several groups: one group as healthy non-burned controls and five burn groups for use in evaluation of immune parameters at PBD 1, 3, 5, 10, and 14. For each PBD timepoint, mice were sub-divided into two groups that contained mice with vs those without cimetidine treatment. Each sub-group was comprised of 5–7 mice per timepoint analyzed. Separate dedicated sets of burned/non-burned were used for the analyses of cytokines (see below) and for SRBC sensitization/use in DTH assessments (see below).
The burn injury protocol was performed according to the method described in Alexander et al. (Citation2005), and that was adapted from Walker and Mason (Citation1968). Briefly, the mice were anesthetized by intraperitoneal (IP) administration of pentobarbital (1 mg/mouse). The dorsal fur was then shaved and the animal held in a plastic mold such that 10% of the total body surface area (TBSA) at the dorsal surface was exposed. The TBSA along the dorsum was calculated based on a formula of A = K•W2/3, where A = area in cm2, K = 8.95 (a unit-less constant for Balb/c mice), and W = body weight in grams. The exposed dorsum was then submerged in 90°C water for 9 s; this procedure causes a burn injury histologically identical to one at full-thickness (i.e., third degree). Non-burned sham mice only had their backs shaved and then they were placed in the mold for the same amount of time as their burned counterparts. The mortality rate of this burn injury was < 5% (Alexander et al., Citation2005).
Cimetidine treatment protocol
Cimetidine (Tagamet) was purchased from the Chemidarou Company (Tehran). A dose of 10 mg/kg of cimetidine (in saline) was administrated daily in a total volume of 0.1 ml by IP injection from post-burn day (PBD) 0 up to PBD 10. The first administration was performed 2–3 h after the burn injury was delivered.
Detecting the level of cytokines in serum
Serum levels of selected cytokines were assessed at PBD 1, 3, 5, 10, and 14. At each timepoint, mice were bled by cardiac puncture and the sera was then isolated and stored at −20°C. Serum IL-2, -10, -12, and TGFβ levels were subsequently measured using commercial enzyme-linked immunosorbent assay (ELISA) kits from Bender Medsystems (Vienna, Austria); IL-17 was measured using a kit from IBL (Hamburg, Germany). The levels of sensitivity of the IL-2, IL-10, IL-12, IL-17, and TGFβ kits were 8.0, 3.5, 4.0, 1.6, and 48.0 pg/ml, respectively.
Measurements of delayed-type hypersensitivity (DTH) response
Sheep red blood cells (SRBC) were purchased from the Pasteur Institute (Tehran) and preserved in sterile Alsevers’ solution. After three washings, a suspension was prepared for use in sensitizing the mice. DTH responses were measured by first sensitizing mice with 108 SRBC injected subcutaneously (SC) in the back 3 days before the burn injury was delivered; controls were sensitized on the day of the sham injury. Sensitized burned hosts were then challenged with 108 SRBC injected SC into the left hind footpad on PBD 1, 3, 5, 10, or 14; for the non-burn controls, challenge was on Day 5 only. With each mouse, an equal volume of saline was injected into the right footpad. Foot thickness was then measured 24 h later using a dial vernier caliper (Mauser Prazisions Messmittel GmbH, Munich, Germany) and results expressed as percent increase in foot thickness relative to that in the saline-injected foot (Jafarzadeh et al., Citation2010).
Statistical analysis
Data were expressed as mean ± SEM. Differences in variables were analyzed using analysis of variance (ANOVA; for comparing means of > 2 groups) and t-tests (to compare 2 groups’ means), as appropriate; p-values of < 0.05 were considered significant. All data were analyzed by SPSS software (Chicago, IL).
Results
Effect of burn injury on DTH response
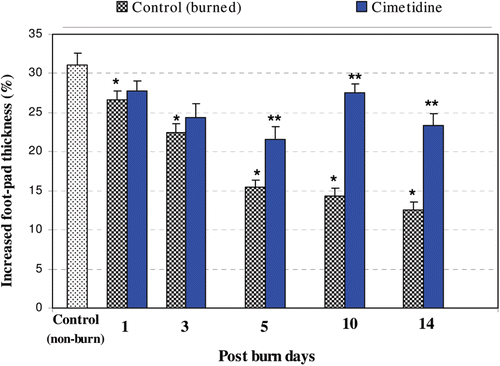
Alterations in host DTH responses over a period of 14 days post-thermal burn (i.e., PBD), as well as the effect of the immunomodulant cimetidine on these responses, are illustrated in . As indicated, DTH responses were significantly decreased at each timepoint analyzed (i.e., PBD 1, 3, 5, 10, and 14) and worsened with advancing post-burn time. Analyses at individual timepoints revealed that minimal responses occurred at PBD 14 (p < 0.0001). Administration of cimetidine significantly augmented the DTH responses (i.e., mitigated the effects from the burn injury itself) at PBD 5, 10, and 14 (relative to those in burned non-drug-treated mice).
Figure 1. DTH responses as a result of thermal burn injury and effects of pre-/co-treatment with cimetidine. Degrees of DTH responses were significantly decreased at PBD 1, 3, 5, 10, and 14, as compared with un-burned controls (p < 0.050, 0.001, 0.0001, 0.0001, and 0.0001, respectively). Administration of cimetidine significantly mitigated effects of the burn injury on the DTH responses at PBD 5, 10, and 14 (values compared to those in injured mice that did not receive the drug; using t-test = p < 0.01, 0.001, and 0.001, respectively). * Significant difference between burn and non-burn groups. ** Significant difference between cimetidine-/non-cimetidine-treated burn mice on a given day.
Effect of thermal burn injury on IL-2 levels
Alterations in serum IL-2 levels over a period of 14 days post-thermal burn and the effect of cimetidine on these levels are shown in . Serum IL-2 levels became significantly lower by PBD 3 and remained so thereafter as compared with values in non-burn controls. Cimetidine administration significantly mitigated the effects of the burn injury on IL-2 levels at PBD 3, 5, and 10 (relative to those in burned non-drug-treated mice). This effect was absent at PBD 14.
Figure 2. Effects of thermal burn injury and effects of pre-/co-treatment with cimetidine, on serum levels of select cytokines. (a) Serum IL-2 levels in burned hosts were significantly lower at PBD 3, 5, 10, and 14 compared with those in non-burned controls (p < 0.004, 0.002, 0.001, and 0.020, respectively). Cimetidine treatments significantly mitigated effects of injury on IL-2 at PBD 3, 5, and 10 (values compared to those in injured mice that did not receive drug; using t-test = p < 0.050, 0.003, and 0.050, respectively). (b) Serum levels of IL-10 were significantly lower at PBD 1, 3, 5, and 10 as compared with those in unburned controls (p < 0.020, 0.004, 0.001, and 0.010, respectively). Cimetidine administration significantly augmented serum IL-10 levels at PBD 1 and 5 (values compared to those in injured mice that did not receive drug; using t-test = p < 0.05 and 0.007, respectively). Serum levels of IL-10 returned to normal at PBD 14. (c) Serum IL-12 levels were significantly lower at PBD 1, 3, 5, 10, and 14 compared with values seen in non-burn control mice (p < 0.010, 0.020, 0.003, 0.001, and 0.030, respectively). Cimetidine treatments significantly mitigated the effects of injury on IL-12 at PBD 3, 5, 10, and 14 (values compared to those in injured mice that did not receive drug; using t-test = p < 0.020, 0.001, 0.030, and 0.020, respectively). (d) Serum IL-17 levels were significantly lower at PBD 1, 3, 5, 10, and 14 as compared with those in non-burned controls (p < 0.05, 0.03, 0.04, 0.04, and 0.05, respectively). Cimetidine treatments significantly mitigated the effects of injury on IL-17 at PBD 3 and 14 (values compared to those in injured mice that did not receive drug; using t-test = p < 0.04 and 0.05, respectively). (e) Serum TGFβ levels were significantly lower at PBD 3 (p < 0.01 vs unburned control values) but were significantly higher at PBD 14 as compared with the un-burned control group (0.01). Cimetidine treatments significantly diminished the serum levels of TGF-β at PBD 14 (values compared to those in injured mice that did not receive drug; using t-test = p < 0.04). * Significant difference between burn and non-burn groups. ** Significant difference between cimetidine-/non-cimetidine-treated burn mice on a given day.
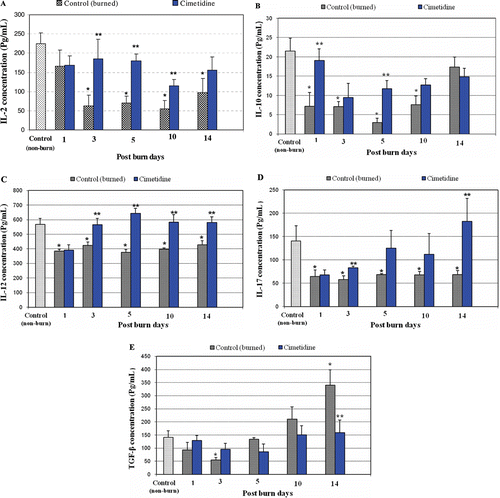
Effect of thermal burn injury on IL-10 levels
Serum levels of IL-10 were also significantly decreased with advancing time post-burn, up to PBD 10 (). In comparison to values in non-burn control hosts, IL-10 levels were significantly decreased at PBD 1, 3, 5, and 10 and returned to near-normal levels at PBD 14. Cimetidine treatment significantly enhanced IL-10 levels (i.e., mitigated burn-dependent effects) from their depressed values at PBD 1 and 5 relative to those in non-drug-treated injured mice.
Effect of thermal burn injury on IL-12 levels
Serum levels of IL-12 were also significantly decreased with advancing time post-burn (). In comparison to values in non-burn controls, serum IL-12 levels were significantly lower at PBD 1 and remained so thereafter. Administration of cimetidine significantly mitigated the effects of the burn injury on levels of IL-12 at PBD 3 and each timepoint thereafter.
Effect of thermal burn injury on IL-17 levels
Serum levels of IL-17 were significantly decreased with advancing time post-burn (). Compared to values in non-burn controls, serum IL-17 levels were significantly lower by PBD 1 and remained so thereafter. Cimetidine treatment resulted in significantly enhanced IL-17 levels (relative to those in non-drug-treated injured mice), but only at PBD 3 and 14.
Effect of thermal burn injury on TGFβ levels
Changes in serum TGFβ levels over a 14 day post-thermal burn period and the effect of cimetidine on these levels are shown in . TGFβ levels seemed to exhibit a biphasic pattern of alteration post-injury. In the early phase, serum TGFβ levels were significantly lower at PBD 3 relative to values in non-burned controls. In a latter phase, TGFβ levels at PBD 10 and thereafter were significantly elevated compared to values in those same counterparts. Cimetidine treatment significantly diminished these burn-induced elevations in TGFβ levels at PBD 14 (as compared to values seen in non-drug-treated burned control hosts).
Discussion
The results of the present study in mice demonstrated a marked decrease in DTH responses and in serum levels of IL-2, IL-12, and IL-17 after a burn trauma, with maximal reduction occurring at post-burn days (PBD) 10–14. Serum IL-10 levels were also significantly decreased, and reached a minimum at PBD 5. Moreover, biphasic alterations were seen in serum TGFβ levels (i.e., significant decreases and increases at PBD 3 and PBD 14, respectively). These results are not completely in keeping with those of Finnerty et al. (Citation2009), that showed that, following burn injury, serum levels of IL-6, IL-1β, IL-17, KC, G-CSF, tumor necrosis factor (TNF)-α, macrophage inflammatory protein (MIP)-1α, RANTES, and granulocyte-macrophage colony-stimulating factor (GM-CSF) were increased, while those of IL-2, -3, and -5 were decreased over a 14 day post-burn period. Varedi et al. (Citation2001) reported that plasma levels of TGFβ were elevated at PBD 6–8. Some investigators have also studied time-dependent changes in immunologic parameters in burn patients. In one study, it was reported that proliferation of T-lymphocytes, CD3+/CD4+ T-lymphocyte percentages, and IL-2 production were each significantly decreased in patients at PBD 1, 14, 21, and 28 (Dong et al., Citation2007).
The immunological basis of burned-induced immunosuppression and the cimetidine capacity for restoration of IL-2, -10, -12, and -17 levels seen here following burn injury could be partly explained by burn-related alterations in effector and regulatory T-lymphocytes (number and/or functions). Normally, upon antigenic stimulation, T-helper (TH) cells differentiate mainly into TH1, TH2, or TH17 sub-sets that are characterized by distinct cytokine release patterns. TH1 cells secrete cytokines like IFNγ and IL-2, TH2 cells primarily secrete IL-4, -10, and IL-13, and TH17 cells produce IL-17, -22, -17F, and IL-21 (along with CCL20) (Jager and Kuchroo, Citation2010; Saito et al., Citation2010; Zhu and Paul, Citation2010). Further, TH1 cells cytokines are responsible for the induction of cell-mediated immunity and DTH responses, while TH2 cytokines support antibody responses; TH17 cells play an important role in the clearing of fungal and pathogens that might not be adequately handled by TH1- or TH2-cell-driven responses (Jager and Kuchroo, Citation2010). In general, TH2-type cytokines (i.e., IL-4, IL-10) inhibit TH1 cell development/-mediated responses, whereas IFNγ (TH1-type cytokine) suppresses TH2 cell development/-mediated outcomes. Both TH1 and TH2 cells have inhibitory effects on TH17 cells (Harrington et al., Citation2005; Zhu and Paul, Citation2010). Lastly, CD4+ T-lymphocytes can differentiate into TH1, TH2, and TH17 cells, depending on the cytokine milieu. In the presence of IL-12, CD4+ T-lymphocytes differentiate into TH1 cells, whereas, in the presence of IL-4, the former differentiate into TH2 cells. TH17 cell differentiation specifically requires TGF-β and IL-6 (Awasthi and Kuchroo, Citation2009).
Our results regarding diminished production of IL-2 and IL-12 after thermal trauma are consistent with finding reported in other studies (Schwacha, Citation2003; Finnerty et al., Citation2009; Li et al., Citation2009). The results of the present study also showed that the serum levels of IL-17 were decreased after burn injury. Recently, it has been demonstrated that IL-17-producing CD4+ T-cells (TH17 cells) are not generated in burn patient PBMC cultures after antigenic stimulation (Inatsu et al., Citation2011). However, Oppeltz et al. (Citation2010) demonstrated that, at 3 h after thermal injury, IL-17 expression in cardiac tissues was significantly elevated. Accordingly, it seems that the expression of IL-17 after a burn injury may be a tissue-/time-dependent process.
The establishment of a balance among TH1, TH2, and TH17 cells plays an important role in regulation of the immune responses. It has been reported that TH1/TH2 imbalance occurs following a burn injury such that a down-regulation of TH1-type cytokines (such as IL-2, IFNγ, and IL-12) and an up-regulation of TH2-type cytokines (like IL-4 and IL-10) occurs after thermal trauma (Miller et al., Citation2007). It has also been shown that IL-4 was responsible for the inhibition of post-burn IL-12 production (Utsunomiya et al., Citation2001). Further, Mills (Citation2008) noted that TH2-type cytokines (including IL-4 and -10) suppress TH17 cell differentiation. Accordingly, diminished levels of IL-2, IL-12, and IL-17, as well as DTH responses over the post-burn period, are most likely to be (partly) attributable to a dominant TH2-mediated immune response.
It should be noted that TH cell effector functions are modulated by Treg cells. Treg cells exert a suppressive capacity via production of immunoregulatory cytokines like TGFα and IL-10, or by cell–cell interactions (Saito et al., Citation2010). The results of the present study also showed biphasic alterations in serum levels of TGF-β as significant decreasing and increasing at PBD 3 and PBD 14, respectively. Precise mechanisms responsible for these alterations remain to be determined. However, differing mechanisms may be responsible for the primary and secondary (biphasic) changes in serum TGFβ levels noted here. Enhanced CD4+CD25+ Treg activity has been reported to occur in burn-injured mice and in trauma patients (Ni Choileain et al., Citation2006; MacConmara et al., Citation2011; Yao and Huang, Citation2011). Thus, it seems that any increase in serum TGFβ levels may be attributed largely to enhanced Treg activity during progression of the post-burn period.
Our results demonstrated that serum levels of IL-10 were significantly decreased with advancing time post-burn (up to PBD 10) and returned to near-normal levels at PBD 14. In some studies, the association between thermal injury and IL-10 production has been evaluated, with controversial results. Some investigations noted that thermal injury resulted in suppressed IL-10 production while others noted higher levels (Schwacha et al., Citation2005; Gauglitz et al., Citation2008). The results of one study in C57BL/6 mice showed that triphasic alterations in serum IL-10 levels occurred (i.e., significant decrease at 9 h post-burn, significant elevation beginning at PBD 1, and a return to normal at PBD 10) (Finnerty et al., Citation2009). IL-10 was first thought to be secreted only by TH2 cells, but now is known to be produced by a variety of cell types—in particular, monocytes/macrophages and T-cell sub-types, including Tr1, Treg, and TH1 cells. IL-10 was first thought to be secreted only by TH2 cells, but now is known to be produced by a variety of cell types—in particular, monocytes/macrophages and T-cell sub-types, including Tr1, Treg, and TH1 cells (Sabat et al., Citation2010). It has been reported that the IL-10 production by macrophages is influenced by genetic background. Macrophages from a C57BL/6 strain produce increased IL-10 levels post-burn, whereas BALB/c cells display suppressed IL-10 production (Schwacha et al., Citation2005). Our current results regarding lower IL-10 levels up to PBD 10 are compatible with the animal strain used herein. In burn patients, associations between specific types of polymorphisms within the IL-10 promoter and decreased levels of IL-10 production have been reported (Huebinger et al., Citation2010). Lastly, it should also be noted here that the effects of thermal injury on IL-10-producing cells could also differ. That is, the injury may induce IL-10 secretions from some immune cells (such as TH2 and Treg) while simultaneously suppressing IL-10 production by other cells like TH1 and monocytes or macrophages. Accordingly, the final concentrations of IL-10 in vivo most probably reflects impacts of injury across various immune cells that produce IL-10 rather than upon any one cell type.
With regard to DTH, this response is a localized inflammatory reaction that consists of two (sensitization and effector) phases. During sensitization, antigen-presenting cells (APC) such as macrophages and dendritic cells (DC) obtain antigen and transport it to regional lymph nodes for presentation to TH cells that, in turn, become activated and differentiate into TH1 cells. During the effector phase, a subsequent exposure to the antigen (presented by APC) causes memory TH1 cells to be activated and secrete cytokines that recruit/activate macrophages (80–90% of infiltrating cells of the DTH response) (Black, Citation1999; Kobayashi et al., Citation2001). Several cytokines influence DTH responses, i.e., IL-1, IL-2, IL-3 TNFβ, IFNγ, TNFα, GM-CSF, monocyte chemotactic/activating factor (MCAF), and migration-inhibition factor (MIF) (Kobayashi et al., Citation2001).
We previously noted a marked suppression of DTH responses over a 30-day period after burn trauma, with maximal suppression at PBD 10–14 (Jafarzadeh et al., Citation2010). It has been suggested that burn-induced DTH suppression reported could be attributed to a reduction in antigen-presenting capacity by APC (Wang and Liang, Citation2007), activation and proliferation of regulatory T-cells (Hanschen et al., Citation2011), diminished production of IL-2, IFNγ, and IL-12 (Schwacha, Citation2003; Finnerty et al., Citation2009; Li et al., Citation2009), higher production of immunomodulatory cytokines such as TGFβ and IL-10 (Varedi et al., Citation2001; Finnerty et al., Citation2009), and/or overall reductions in TH1 responses (Miller et al., Citation2007). Moreover, several cells involved in propagating the DTH response have been seen to be affected by thermal burn. Hunt et al. (Citation1998) reported that cytotoxic T-lymphocyte (CTL) responses in burned rats were significantly decreased at PBD 3, but returned to baseline at PBD 7-10. Organ et al. (Citation1989) reported that, after thermal injury in a rat, significant peripheral, splenic, and thymic lymphopenia was observed at PBD 2 and 6; it took 60 days for tissue lymphocyte numbers to return to normal. It has been also reported that macrophage capacity to produce prostaglandin (PG)-E2 (a factor that suppresses T-lymphocyte proliferation and IL-2 production) was significantly increased after thermal injury and that the suppression of T-lymphocyte proliferation/IL-2 production at PBD 3 was PGE2-mediated (Schwacha, Citation2003). In the study here, the time-course of alterations in select cytokines and DTH responses approximate the appearance of post-burn immunosuppression observed by others. Divergences from those outcomes could be attributed, in part, to differences in animal strain used and/or the severity of burn injury.
The results here demonstrated that cimetidine (at 10 mg/kg) significantly increased serum levels of IL-2, -10, -12, and -17 following a thermal burn. How cimetidine might impart these protective effects is unclear at this point. Cimetidine is a histamine (H2) antagonist widely used for the treatment of duodenal ulcers and other gastric hyper-secretory situations (Khoshbaten et al., Citation2006). Cimetidine has been implicated in enhancement of cell-mediated cytotoxicity and in the impairment of suppressor T-lymphocyte function (Scheinfeld, Citation2003). Cimetidine has been effective in countering burn wound itch (Baker et al., Citation2001) and is used in protection against infection in experimental animals (Ishikura et al., Citation1999; Takahashi et al., Citation2002). This drug (at the same dose employed in our study) has also been used to abrogate burn blister fluid- and sulfur mustard-induced immunosuppresssion (Ebtekar and Hassan, Citation1993; Gharegozloo et al., Citation2004). It has also been recently shown that cimetidine enhances the protective effect of a DNA vaccine against Schistosoma japonicum viz: mice immunized with vaccine + cimetidine had elevated levels of interferon (IFN)-γ and IL-12 and low levels of IL-10 production by their splenocytes as compared to that by cells from vaccine-only-treated counterparts (Li et al., Citation2011).
It should be noted that higher plasma and tissue histamine levels occur during a post-burn period (Shimizu et al., Citation2002; Rantfors and Cassuto, Citation2003). Histamine, in addition to its potent effector functions during allergy and inflammation (mainly exerted via H1 receptors), may also have strong immunoregulatory activities mediated via H2 receptors on immune cells (Jutel et al., Citation2009). It has been shown that histamine inhibits IL-2 production by mouse splenocytes and that this effect is mediated via protein kinase A (Poluektova et al., Citation1999). Histamine also potently suppresses IL-12 by human peripheral blood mononuclear cells (PBMC) via H2 receptors (Elenkov et al., Citation1998). As would then be expected, cimetidine was able to reverse these effects of histamine on IL-2, -12, and -10 production by PBMC (Arad et al., Citation1996; Elenkov et al., Citation1998). Thus, the cimetidine capacity for restoration of IL-2, -12, and -17 production and of DTH responses in the post-burn period in the animals examined here might be a result, in part, of a reversal of the effects of histamine.
The results of this study also show that cimetidine treatment significantly enhanced IL-10 levels at PBD 1 and 5 (relative to those in non-drug-treated injured mice). Little is known about the effects of cimetidine on TH2-type immune responses. It was reported that inhibition of the interaction of histamine and histamine H2 receptor (H2R) by use of H2R antagonists such as ranitidine accelerate TH2 cell proliferation and TH2-type cytokines, thereby enhancing TH2 responses (Jutel et al., Citation2001). Moreover, intraperitoneal administration of cimetidine. In mice model, it has been shown that the intraperitoneal administration of cimetidine significantly enhances the production of antigen-specific IgE and TH2-type cytokine (Arae et al., Citation2011). On the other hand, the positive effects of cimetidine on the TH1-type cytokine have also been demonstrated (Mitsuishi et al., Citation2003; Takahashi et al., Citation2006). Collectively, these observations suggest that cimetidine can modulate TH1- as well as TH2-type immune responses. Interestingly, it was recently reported that cimetidine potentiates TH1/TH2 dual polarization (Zhang et al., Citation2011), an outcome consistent with our current results.
The results here also demonstrated that cimetidine significantly diminished the burn-induced elevated TGFβ (a Treg cytokine) levels at PBD 14. Cimetidine enhances immune responses, in part, via down-regulation of Treg cell function (Wang et al., Citation2008; Zhang et al., Citation2011). Those studies reported that cimetidine enhanced immune responses to hepatitis B DNA vaccination via inhibition of Treg cells. Thus, inhibition/reduction of CD4+CD25+ Treg cells by cimetidine could improve immune responses during a post-burn period. To test whether cimetidine effects on the immune responses are mediated partly through inhibition of Treg cells, the frequency and functions of Treg cells and other T-cell sub-sets (in absence/presence of the drug) need to be analyzed in future studies.
We also investigated the ability of cimetidine to affect cytokine levels and DTH response after thermal injury. In our previous study, the effects of cimetidine on DTH responses were evaluated only at PBD 10; here, effects were evaluated at PBD 1, 3, 5, 10, and 14. The results of the present study showed that cimetidine administration significantly mitigated burn effects on DTH responses at PBD 5, 10, and 14. The mitigating effects of cimetidine on these responses parallel those seen on IL-2 and IL-12 levels in the hosts. Cimetidine might cause such outcomes by influencing either/both sensitization and effector phases of the DTH response; this could occur via induced increases in APC function/secretion of IL-2, IFNγ, and IL-12, by causing decreases in production of immunomodulating cytokines and/or functions of Treg/TH2 cells, and/or by enhancing TH1-based responses.
In conclusion, burn-induced suppression of humoral and cell-mediated immunity makes burn patients susceptible to various infections (de Macedo and Santos, Citation2005; Stoilova, et al., Citation2007; Rezaei et al., Citation2011). As such, the cimetidine ability to ‘restore’ IL-2, -10, -12, and -17 production, as well as DTH responses, that was demonstrated in the present studies represents a noteworthy set of outcomes. Specifically, this information could be used to spur clinicians to employ this drug to combat any burn-induced immunosuppression and minimize the risk in these patients of any subsequent enhanced susceptibility to infections.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alexander, M., Daniel, T., Chaudry, I. H., Schwacha, M. G. 2005. Opiate analgesics contribute to the development of post-injury immunosuppression. J. Surg. Res. 129:161–168.
- Arad, G., Nussinovich, R., Na’amad, M., Kaempfer, R. 1996. Dual control of human IL-2 and IFNγ gene expression by histamine: Activation and suppression. Cell. Immunol. 170:149–155.
- Arae, K., Oboki, K., Ohno, T., Hirata, M., Nakae, S., Taguchi, H., Saito, H., Nakajima, T. 2011. Cimetidine enhances antigen-specific IgE and TH2 cytokine production. Allergol. Int. 60:339–344.
- Awasthi, A., Kuchroo, V. K. 2009. TH17 cells: From precursors to players in inflammation and infection. Int. Immunol. 21:489–498.
- Baker, R. A., Zeller, R. A., Klein, R. L., Thornton, R. J., Shuber, J. H., Marshall, R. E., Leibfarth, A. G., Latko, J. A. 2001. Burn wound itch control using H1 and H2 antagonists. J. Burn Care Rehab. 22:263–268.
- Black, C. A. 1999. Delayed-type hypersensitivity: Current theories with an historic perspective. Dermatol. Online J. 5:7.
- Butler, K. L., Ambravaneswaran, V., Agrawal, N., Bilodeau, M., Toner, M., Tompkins, R. G., Fagan, S., Irimia, D. 2010. Burn injury reduces neutrophil directional migration speed in microfluidic devices. PLoS One 5:e11921.
- Cetinkale, O., Senel, O., Bulan, R. 1999. The effect of antioxidant therapy on cell-mediated immunity following burn injury in an animal model. Burns 25:113–118.
- Choudhry, M. A., Mao, H., Haque, F., Khan, M., Fazal, N., Sayeed, M. M. 2002. Role of NFAT and AP-1 in PGE2-mediated T-cell suppression in burn injury. Shock 183:212–216.
- Correia, O., Delgado, L., Roujeau, J. C., Le Cleach, L., Fleming-Torrinha, J. A. 2002. Soluble IL-2 receptor and IL-1β in toxic epidermal necrolysis: A comparative analysis of serum and blister fluid samples. Arch. Dermatol. 138:29–32.
- de Macedo, J. L., Santos, J. B. 2005. Bacterial and fungal colonization of burn wounds. Mem. Inst. Oswaldo Cruz 100:535–539.
- Dong, N., Yao, Y. M., Cao, Y. J., He, L. X., Yu, Y., Chai, J. K., Sheng, Z. Y. 2007. The clinical significance of changes in immunological function of T-lymphocyte in severe burn patients with sepsis. Zhonghua Shao Shang Za Zhi 23:84–87.
- Ebtekar, M., Hassan, Z. M. 1993. Effect of immunomodulators pyrimethamine and cimetidine on immunosuppression induced by sulfur mustard in mice. Int. J. Immunopharmacol. 15:533–541.
- Elenkov, I. J., Webster, E., Papanicolaou, D. A., Fleisher, T. A., Chrousos, G. P., Wilder, R. L. 1998. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J. Immunol. 161:2586–2593.
- Fayazov, A. D., Shukurov, S. I., Shukurov, B. I., Sultanov, B. C., Namazov, A. N., Ruzimuratov, D. A. 2009. Disorders of the immune system in severely burned patients. Ann. Burns Fire Disasters 22:121–130.
- Finnerty, C. C., Przkora, R., Herndon, D. N., Jeschke, M. G. 2009. Cytokine expression profile over time in burned mice. Cytokine 45:20–25.
- Gauglitz, G. G., Song, J., Herndon, D. N., Finnerty, C. C., Boehning, D., Barral, J. M., Sand Jeschke, M. G., 2008. Characterization of the inflammatory response during acute and post-acute phases after severe burn. Shock 30:503–507.
- Gharegozloo, B., Hassan, Z. M., Ardestani, A., Tavassoli, N. 2004. Effect of immunomodulator pyrimethamine and cimetidine on immunosuppression induced by burn blister fluid. Iran J. Allergy Asthma Immunol. 3:139–143.
- Hanschen, M., Tajima, G., O’Leary, F., Ikeda, K., Lederer, J. A. 2011. Injury induces early activation of T-cell receptor signaling pathways in CD4+ regulatory T-cells. Shock 35:252–257.
- Harrington, L. E., Hatton, R. D., Mangan, P. R., Turner, H., Murphy, T. L., Murphy, K. M., Weaver, C. T. 2005. Interleukin-17-producing CD4+ effector T-cells develop via a lineage distinct from the T-helper type 1 and 2 lineages. Nat. Immunol. 6:1123–1132.
- Huang, W. H. 1992. Study of the abnormal plasma proteins after burn injury. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi 8:89–92.
- Huebinger, R. M., Rivera-Chavez, F., Chang, L. Y., Liu, M. M., Minei, J. P., Purdue, G. F., Hunt, J. L., Arnoldo, B. D., Barber, R. C. 2010. IL-10 polymorphism associated with decreased risk for mortality after burn injury. J. Surg. Res. 164:e141–145.
- Hunt, J. P., Hunter, C. T., Brownstein, M. R., Giannopoulos, A., Hultman, C. S., deSerres, S., Bracey, L., Frelinger, J., Meyer, A. A. 1998. The effector component of the cytotoxic T-lymphocyte response has a biphasic pattern after burn injury. J. Surg. Res. 80:243–251.
- Inatsu, A., Kogiso, M., Jeschke, M. G., Asai, A., Kobayashi, M., Herndon, D. N., Suzuki, F. 2011. Lack of TH17 cell generation in patients with severe burn injuries. J. Immunol. 187:2155–2161.
- Ishikura, H., Fukui, H., Takeyama, N., Tanaka, T. 1999. Cimetidine activates IL-12, which enhances cellular immunity. Blood 93:1782–1783.
- Jafarzadeh, A., Hassan, Z., Ebtekar, M., Mohaghegh-Hazrati, S., Altoriahi, T. 2010. Time-dependent changes of immunologic responses after burn injury and immunomodulation by cimetidine and pyrimethamine in an animal model. Pak. J. Pharm. Sci. 23:367–373.
- Jager, A., Kuchroo, V. K. 2010. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand. J. Immunol. 72:173–184.
- Jutel, M., Akdis, M., Akdis, C. A. 2009. Histamine, histamine receptors and their role in immune pathology. Clin. Exp. Allergy 39:1786–1800.
- Jutel, M., Watanabe, T., Klunker, S., Akdis, M., Thomet, O. A., Malolepszy, J., Zak-Nejmark, T., Koga, R., Kobayashi, T., Blaser, K., Akdis, C. A. 2001. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature 413:420–425.
- Khoshbaten, M., Fattahi, E., Naderi, N., Khaleghian, F., Rezailashkajani, M. 2006. A comparison of oral omeprazole and intravenous cimetidine in reducing complications of duodenal peptic ulcer. BMC Gastroenterol. 6:2.
- Kinoshita, M., Shinomiya, N., Ono, S., Tsujimoto, H., Kawabata, T., Matsumoto, A., Hiraide, H., Seki, S. 2006. Restoration of natural IgM production from liver B-cells by exogenous IL-18 improves the survival of burn-injured mice infected with Pseudomonas aeruginosa. J. Immunol. 177:4627–4635.
- Kobayashi, K., Kaneda, K., Kasama, T., 2001. Immunopathogenesis of delayed-type hypersensitivity. Microsc. Res. Tech. 53:241–245.
- Kovacs, E. J., Faunce, D. E., Messingham, K. A. 2004. Ethanol and burn injury: Estrogen modulation of immunity. Alcohol 33:209–216.
- Li, M. J., Lei, J. H., Wang, T., Lu, S. J., Guan, F., Liu, W. Q., Li, Y. L. 2011. Cimetidine enhances the protective effect of GST DNA vaccine against Schistosoma japonicum. Exp. Parasitol. 128:427–432.
- Li, X., Chaudry, I. H., Choudhry, M. A. 2009. ERK and not p38 pathway is required for IL-12 restoration of T-cell IL-2 and IFNγ in a rodent model of alcohol intoxication and burn injury. J. Immunol. 183:3955–3962.
- MacConmara, M. P., Maung, A. A., Fujimi, S., McKenna, A. M., Delisle, A., Lapchak, P. H., Rogers, S., Lederer, J. A., Mannick, J. A. 2006. Increased CD4+CD25+ T-regulatory cell activity in trauma patients depresses protective TH1 immunity. Ann. Surg. 244:514–523.
- MacConmara, M. P., Tajima, G., O’Leary, F., Delisle, A. J., McKenna, A. M., Stallwood, C. G., Mannick, J. A., Lederer, J. A. 2011. Regulatory T-cells suppress antigen-driven CD4 T-cell reactivity following injury. J. Leukocyte Biol. 89:137–147.
- Miller, A. C., Rashid, R. M., Elamin, E. M. 2007. The “T” in trauma: The helper T-cell response and the role of immunomodulation in trauma and burn patients. J. Trauma 63:1407–1417.
- Mills, K. H. 2008. Induction, function and regulation of IL-17-producing T-cells. Eur. J. Immunol. 38:2636–2649.
- Mitsuishi, T., Iida, K., Kawana, S., 2003. Cimetidine treatment for viral warts enhances IL-2 and IFNγ expression but not IL-18 expression in lesional skin. Eur. J. Dermatol. 13:445–448.
- Ni Choileain, N., MacConmara, M., Zang, Y., Murphy, T. J., Mannick, J. A., Lederer, J. A. 2006. Enhanced regulatory T-cell activity is an element of the host response to injury. J. Immunol. 176:225–236.
- Oppeltz, R. F., Zhang, Q., Rani, M., Sasaki, J. R., Schwacha, M. G. 2010. Increased expression of cardiac IL-17 after burn. J. Inflamm. (London) 7:38.
- Organ, B. C., Antonacci, A. C., Chiao, J., Chiao, J., Kumar, A., de Riesthal, H. F., Yuan, L., Black, D., Calvano, S. E. 1989. Changes in lymphocyte number and phenotype in seven lymphoid compartments after thermal injury. Ann. Surg. 210:78–89.
- Poluektova, L. Y., Huggler, G. K., Patterson, E. B., Khan, M. M. 1999. Involvement of protein kinase A in histamine-mediated inhibition of IL-2 mRNA expression in mouse splenocytes. Immunopharmacology 41:77–87.
- Rantfors, J., Cassuto, J. 2003. Role of histamine receptors in the regulation of edema and circulation post-burn. Burns 29:769–777.
- Rezaei, E., Safari, H., Naderinasab, M., Aliakbarian, H. 2011. Common pathogens in burn wound and changes in their drug sensitivity. Burns 37:805–807.
- Sabat, R., Grutz, G., Warszawska, K., Kirsch, S., Witte, E., Wolk, K., Geginat, J. 2010. Biology of IL-10. Cytokine Growth Factor Rev. 21:331–344.
- Saito, S., Nakashima, A., Shima, T., Ito, M. 2010. TH1/TH2/TH17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 63:601–610.
- Scheinfeld, N. 2003. Cimetidine: A review of the recent developments and reports in cutaneous medicine. Dermatol. Online J. 9:4.
- Schwacha, M. G. 2003. Macrophages and post-burn immune dysfunction. Burns 29:1–14.
- Schwacha, M. G., Holland, L. T., Chaudry, I. H., Messina, J. L. 2005. Genetic variability in the immune-inflammatory response after major burn injury. Shock 23:123–128.
- Shimizu, S., Tanaka, H., Sakaki, S., Yukioka, T., Matsuda, H., Shimazaki, S. 2002. Burn depth affects dermal interstitial fluid pressure, free radical production, and serum histamine levels in rats. J. Trauma 52:683–687.
- Steinstraesser, L., Oezdogan, Y., Wang, S. C., Steinau, H. U. 2004. Host defense peptides in burns. Burns 30:619–627.
- Stoilova, Y. D., Haidushkal, I. A., Murdjeval, M. A., Traikov, I. Z., Popova, T. A., Kevorkyan, A. K. 2007. Immunological and microbiological investigations of patients with burn injuries. Folia Med. (Plovdiv) 49:49–58.
- Takahashi, K., Tanaka, S., Furuta, K., Ichikawa, A. 2002. Histamine H2 receptor-mediated modulation of local cytokine expression in a mouse experimental tumor model. Biochem. Biophys. Res. Commun. 297:1205–1210.
- Takahashi, H. K., Watanabe, T., Yokoyama, A., Iwagaki, H., Yoshino, T., Tanaka, N., Nishibori, M. 2006. Cimetidine induces IL-18 production through H2-agonist activity in monocytes. Mol. Pharmacol. 70:450–453.
- Tang, Z., Yu, Y., Qiu, W., Zhang, J., Yang, X. 2011. Up-regulation of tim-3 expression contributes to development of burn-induced T-cell immune suppression in mice. J. Huazhong Univ. Sci. Tech. Med. Sci. 31:642–651.
- Utsunomiya, T., Kobayashi, M., Herndon, D. N., Pollard, R. B., Suzuki, F. 2001. A mechanism of IL-12 un-responsiveness associated with thermal injury. J. Surg. Res. 96:211–217.
- Varedi, M., Jeschke, M. G., Englander, E. W., Herndon, D. N., Barrow, R. E. 2001. Serum TGFβ in thermally-injured rats. Shock 16:380–382.
- Walker, H. L., Mason, A. D. Jr. 1968. A standard animal burn. J. Trauma 8:1049–1051.
- Wang, J., Su, B., Ding, Z., Du, X., Wang, B. 2008. Cimetidine enhances immune response of HBV DNA vaccination via impairment of the regulatory function of regulatory T-cells. Biochem. Biophys. Res. Commun. 372:491–496.
- Wang, K., Wang, D. C., Feng, Y. Q., Xiang-Feng, L. 2007. Changes in cytokine levels and CD4+/CD8+ T-cells ratio in draining lymph node of burn wound. J. Burn Care Res. 28:747–753.
- Wang, Z. P., Liang, H. P. 2007. Roles of dendritic cells in mediating decreased delayed-type hypersensitivity responses after trauma. Zhong. Yi Xue Ke Xue Yuan Xue Bao 29:501–505.
- Yabuki, T., Takeyama, N., Tsuda, M., Saitoh, F., Tanaka, T., Noguchi, H., Nakatani, T. 2010. CpG oligonucleotides activate the immune response in burned mice. J. Surg. Res. 161:111–118.
- Yao, Y. M., Huang, L. F. 2011. The potential role of regulatory T-cells in post-burn sepsis. Zhonghua Shao Shang Za Zhi 27:81–83.
- Zhang, W., Wang, J., Su, B., Li, R., Ding, Z., Kang, Y., Wang, B. 2011. Cimetidine augments TH1/TH2 dual polarized immune responses to recombinant HBV antigens. Vaccine 29:4862–4868.
- Zhu, J., Paul, W. E. 2010. Heterogeneity and plasticity of T-helper cells. Cell. Res. 20:4–12.