Abstract
Leukotrienes, divided into cysteinyl leukotrienes (CysLTs), which are important mediators of asthmatic responses, and leukotriene B4 (LTB4), a chemotactic and chemokinetic agent for leukocytes, are potent lipid mediators generated from arachidonic acid by 5-lipoxygenase (5-LO). Leukotrienes are also considered to have immunoregulatory and pro-inflammatory actions. Propofol is an intravenous anesthetic widely used for anesthesia and sedation that is alleged to possess anti-inflammatory properties. The present study examined the effect of propofol on leukotriene production by dendritic cells (DC). In murine bone marrow-derived DC, propofol significantly suppressed CysLT and LTB4 production after short-term stimulation with zymosan. The protein levels of cytosolic phospholipase A2 and 5-LO, or arachidonic acid release from plasma membranes, were not affected by the presence of propofol. Although zymosan treatment induced or enhanced the phosphorylation of ERK1/2, p-38 MAPK, and JNK, which presumably up-regulates the activity of 5-LO, the presence of propofol had no additional effect on the phosphorylation status of any of these MAPKs. Similarly, zymosan significantly increased the concentration of intracellular calcium, which is the most crucial activator of 5-LO, but no additional concentration changes were observed with the addition of propofol. Lastly, in an in-vitro cell-free ferrous oxidation-xylenol orange assay, propofol significantly inhibited the 5-LO activity of purified human recombinant 5-LO enzyme with an IC50 of ~7.5 µM. Thus, propofol’s inhibition of 5-LO is not likely restricted to the circumstances surrounding the production of leukotrienes from DC, but applicable to other types of immune and non-immune cells that produce leukotrienes. The 5-LO-inhibiting activity of propofol may, at least in part, contribute to the well-known anti-inflammatory activity of propofol.
Introduction
Leukotrienes are lipid mediators generated when arachidonic acid (AA) is metabolized by 5-lipoxygenase (5-LO) (Needleman et al., Citation1986; Lewis et al., Citation1990; Henderson, Citation1994; Byrum et al., Citation1999). Generally, leukotrienes are divided into cysteinyl leukotrienes (CysLTs) and leukotriene B4 (LTB4). CysLTs, which consist of LTC4, LTD4, and LTE4, are important mediators of asthmatic responses and induce smooth muscle contraction, increase microvascular permeability, stimulate mucus secretion, and promote inflammatory cell migration, particularly eosinophils and neutrophils, into the airways (Lewis et al., Citation1990; Henderson, Citation1994; Byrum et al., Citation1999). CysLTs also mediate dendritic cell migration and maturation (Alvarez et al., Citation2011; Dannull et al., Citation2012). However, LTB4 is a potent chemotactic and chemokinetic agent for leukocytes (Shin et al., Citation2006; Del Prete et al., Citation2007; Miyahara et al., Citation2008), enhances the phagocytic and antimicrobial activity of neutrophils and macrophages, and stimulates the secretion of immunoglobulins by lymphocytes (Lewis et al., Citation1990; Henderson, Citation1994; Byrum et al., Citation1999). LTB4 is reportedly related to regulation of the immune response, as well as the pathogenesis of inflammatory diseases, such as arthritis, asthma, and atherosclerosis (Lewis et al., Citation1990).
Leukotrienes are rapidly generated at the site of inflammation by a series of reactions initiated initially by cytosolic phospholipase A2 (cPLA2), which releases AA from membrane phospholipids (Needleman et al., Citation1986; Jiang et al., Citation2003). The enzyme 5-LO then acts on the AA to form an unstable intermediate, LTA4 (Radmark et al., Citation1984; Needleman et al., Citation1986; Lewis et al., Citation1990; Henderson, Citation1994; Byrum et al., Citation1999). LTA4 is converted to LTB4 by LTA4 hydrolase. Some cells, such as eosinophils, basophils, mast cells, alveolar macrophages, and dendritic cells, possess LTC4 synthase, which conjugates LTA4 with reduced glutathione to form LTC4 (Yoshimoto et al., Citation1988). LTC4 is transferred to the extracellular space, followed by rapid metabolism to LTD4 and LTE4. In addition to the transcriptional levels of the above-mentioned key enzymes, intracellular calcium concentrations and phosphorylation by the MAPK family may also influence the levels of leukotriene production through the modulation of cPLA2 and 5-LO activity (Rehfeldt et al., Citation1993; Werz et al., Citation2002; Jiang et al., Citation2003; Radmark et al., Citation2007).
Dendritic cells (DC) are probably some of the most crucial cells in immunity (Sompayrac, Citation2003; Mak and Saunders, Citation2006). Immature DC capture and process antigens in situ and migrate to the draining lymph node, where they present the processed antigen peptides to lymphocytes, leading to the establishment of adaptive immunity (Sompayrac, Citation2003; Mak and Saunders, Citation2006). Both human DC and murine bone marrow-derived DC express 5-LO and are capable of producing leukotrienes (Spanbroek et al., Citation2000; Jozefowski et al., Citation2005). Several lines of evidence indicate that DC are not only an important source of leukotrienes, but also targets of leukotrienes (Spanbroek et al., Citation2000; Jozefowski et al., Citation2005). Because leukotrienes are considered to have dramatic effects on inflammation and immune functions, they could affect DC in an autocrine/paracrine manner, potentially affecting the establishment and maintenance of inflammatory and/or allergic diseases (Hedi and Norbert, Citation2004; Thivierge et al., Citation2009; Alvarez et al., Citation2011).
Propofol (2,6-di-isopropylphenol) is an intravenous anesthetic widely used for surgery and for sedation (Vanlersberghe and Camu, Citation2008). Surgery suppresses the immune system due to its inevitable tissue damage-induced inflammation and stress, whose modification with anesthesia could affect the post-operative immunosuppression (Slade et al., Citation1975; Hensler et al., Citation1997; Bauer et al., Citation1998; Ogawa et al., Citation2000). Propofol is unique in its anti-inflammatory properties that could potentially modulate the surgery-induced inflammation, leading to modification of immune suppression after surgery. Although some of propofol’s anti-inflammatory effects could be attributable to its anti-oxidative and anti-cyclooxygenase properties (Vasileiou et al., Citation2009; Inada et al., Citation2011a), we hypothesized that propofol also possesses anti-leukotriene production properties, which might contribute to its anti-inflammatory properties. In the present study, using murine bone marrow-derived DC, we examined the effect of propofol on leukotriene synthesis. Elucidating the effect of propofol on DC is important because DC play a central role in immunity (especially tumor immunity as well as transplantation immunity), which may determine the fate of patients undergoing surgery.
Materials and methods
Reagents
The following chemical reagents were purchased from Sigma Aldrich (St. Louis, MO): propofol (2,6-di-isopropylphenol), indomethacin, U0126, SB203580, SP600125, and ethylene glycol-bis(2-aminoethylether)-N,N,N’,N’-tetraacetic acid (EGTA). Zymosan was purchased from InvoivoGen (San Diego, CA), and arachidonic acid was obtained from Cayman Chemical (Ann Arbor, MI). Recombinant murine granulocyte macrophage colony stimulating factor (GM-CSF) was obtained from Peprotech (London, England), and recombinant human 5-LO was obtained from Cayman Chemical. Tritiated arachidonic acid ([3H]-AA) was purchased from PerkinElmer (Yokohama, Japan).
Animals
Male C57BL/6 mice (8–9 weeks old) were purchased from CLEA (Osaka, Japan) and housed in an animal facility maintained at 20–26°C, with 30–70% relative humidity and 12-h light/dark cycles. All animals were handled according to institutional guidelines. The mice were provided access to standard rodent chow and autoclaved water ad libitum. This study was approved by the Institutional Animal Care and Use Committee of the Kansai Medical University.
Generation and culture of bone marrow-derived DC
Bone marrow-derived DC were generated as described previously by Lutz et al. (Citation1999). Briefly, bone marrow cells were collected from the femurs and tibiae of male C57BL/6 mice. These cells (2 × 105 cells/ml, 10 ml per dish) were cultured in 100-mm bacteriological Petri dishes (Falcon, No. 1029, Beckton Dickinson, Heidelberg, Germany) in RPMI complete medium [RPMI 1640 medium (Sigma) supplemented with 10% fetal calf serum (FCS; endotoxin <10.0 EU/ml) (HyClone, Logan, UT), penicillin (50 U/ml), streptomycin (50 µg/ml), and 2-mercaptoethanol (55 µM; Gibco, Grand Island, NY)] containing 20 ng/ml GM-CSF.
On day 3 of culture, another 10 ml of RPMI complete medium containing GM-CSF was added. On days 6 and 8, one-half of the culture medium was collected and centrifuged; the cell pellet was re-suspended in 10 ml fresh RPMI complete medium containing GM-CSF and added back to the culture. On day 10, floating cells were collected and CD11c+ DC purified by magnetic sorting using anti-CD11c antibody-coated magnetic beads (Milteny Biotec, Bergisch Gladbach, Germany). The purity of the sorted CD11c+ cells was >97%, based on flow cytometry.
In all experiments, DC were stimulated with zymosan (10 µg/ml) at a cell concentration of 5 × 105 cells/ml. Zymosan, a ligand for the Toll-like receptor (TLR)-2 and dectin-1 (Dillon et al., Citation2006), was used for DC stimulation because it is a strong stimulant for leukotriene synthesis in murine DC (Jozefowski et al. Citation2005) and because the far more widely-used TLR-4 receptor agonist lipopolysaccharide did not significantly up-regulate leukotriene production by DC in our previous experiments (Inada et al. Citation2011b) and in others (Jozefowski et al. Citation2005). For leukotriene production measurements, the cells (5 × 105 cells/ml) were cultured in RPMI complete medium in round-bottom 96-well plates (200 µl/well) with or without zymosan (10 µg/ml), in the presence or absence of various concentrations of propofol (0–30 µM), for 30 min at 37°C in a humidified chamber containing 5% CO2. DMSO (final concentration < 0.1%) was used when propofol was absent. Cell-free supernatants were collected and stored at −80°C before analysis. Propofol concentrations of 7.5, 15, and 30 µM, as assessed by electroencephalography, correspond to human plasma concentrations achieved during moderate sedation, deep sedation, and anesthesia, respectively (Rigouzzo et al., Citation2008).
Cysteinyl leukotrienes (CysLTs) and leukotriene B4 (LTB4) measurements
CysLTs and LTB4 concentrations were measured using an enzyme immunoassay (Cayman Chemical) based on the competition between CysLTs (or LTB4) and a CysLT (or LTB4)-acetyl-cholinesterase conjugate for a limited amount of anti-CysLT (or -LTB4) antibody.
Arachidonic acid (AA) release
DC (5 × 105 cells/well in 24-well plates) were seeded in serum-free RPMI containing [3H]-AA (0.25 µCi/ml) and incubated for 1.5 h at 37°C. The cells were washed twice with serum-free RPMI and stimulated with zymosan (10 µg/ml) for 30 min or 4 h in RPMI/0.2% fatty acid-free bovine serum albumin (Sigma) in round-bottom 96-well plates (5 × 105 cells/ml, 200 µl/well), and then 100 µl of each supernatant was transferred to 1 ml OPTI FLUOR (Perkin Elmer) and the radioactivity of the released AA measured. After removing the culture medium, cells were lysed with 200 µl of 3% Triton X-100, and the radioactivity in 100 µl of the lysate (in 1 ml OPTI FLUOR) was measured. The amount of AA released was expressed as a percentage and calculated as follows: 100 × (released radioactivity)/(released radioactivity + radioactivity of lysed cells).
Western blot
DC [stimulated with zymosan (10 µg/ml) at a concentration of 2 × 106 cells/4 ml/well in 6-well plates] were washed with PBS containing Phosphatase Inhibitor Cocktail (Active Motif, Carlsbad, CA) and lysed in Complete Lysis Buffer (Active Motif) containing a protease inhibitor cocktail and dithiothreitol. Cell lysates (15 µg/lane) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride membrane (Bio-Rad Laboratories, Hercules, CA). The membranes were incubated with p44/42 MAPK rabbit mAb, phospho-p44/42 MAPK rabbit mAb, p38 MAPK Ab, phospho-p38 MAPK rabbit mAb, JNK rabbit mAb, phospho-JNK rabbit mAb, cPLA2 rabbit mAb (1:1,000; all Cell Signaling), anti-5-LO polyclonal antibody (1:5,000; Cayman Chemical), or anti-α-tubulin antibody (1:1,000; Santa Cruz). Proteins were visualized using horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse antibody, as appropriate (1:10,000; Zymed Laboratories, South San Francisco, CA) and ECL-plus Western Blotting Detection Reagent (Amersham Biosciences, Buckingham, UK).
Intracellular calcium concentration
DC were loaded with 2.5 µM Fura2-AM (Dojindo, Kumamoto, Japan) in Hanks’ HEPES buffer (Dojindo) containing 0.04% pluronic F-127 and 1.25 mM probenecid for 1 h. Cells were washed and cultured for 30 min in the presence or absence of zymosan (10 µg/ml)/propofol (30 µM). The intensity of fluorescence at 500 nm elicited by 340 nm and 380 nm excitation was measured using CAF-110 intracellular calcium monitoring (JASCO, Tokyo, Japan). At the end of each recording, Triton X-100 was added (final concentration 0.2%) to the cells to determine the maximal fluorescence ratio. Next, EGTA (final concentration 10 mM) was added to determine the minimal fluorescence ratio. Intracellular calcium levels were calculated according to Grynkiewicz et al. (Citation1985).
Assessment of 5-LO activity
The activity of 5-LO enzyme was assessed using the ferrous oxidation-xylenol orange (FOX) assay as described previously (Cho et al., Citation2006). Briefly, human recombinant 5-LO (5 U) was pre-incubated with propofol for 4 min at room temperature in 50 mM Tris-HCl buffer (pH 7.4) containing 0.4 mM CaCl2. Reactions were initiated by the addition of AA (final concentration 10 µM). Four minutes later, the reaction was terminated by the addition of FOX reagent containing 25 mM sulfuric acid, 100 µM xylenol orange, and 100 µM ferrous sulfate dissolved in methanol/water (9:1). After 30 min, the absorbance was measured at 575 nm. The background absorbance without 5-LO enzyme was subtracted from each measurement and the 5-LO activity (%) expressed as the ratio of absorbance in the presence of propofol to absorbance in the absence of propofol.
Statistical analysis
One-way analysis of variance (ANOVA) with the Bonferroni post hoc test was performed using Graph Pad Prism 4 (GraphPad, San Diego, CA). Data were expressed as mean ± SEM. A p-value < 0.05 was considered significant.
Results
Propofol suppresses CysLTs and LTB4 production by DC without affecting cPLA2 and 5-LO protein levels
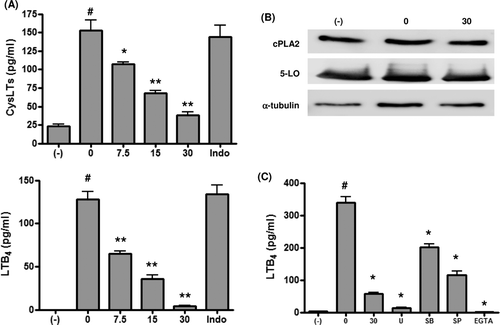
After short-term zymosan stimulation, CysLT and LTB4 production increased significantly, but it was suppressed by propofol in a dose-dependent manner (). As previous studies indicated a link between prostaglandin E2 and leukotrienes (Harizi et al., Citation2003), DC were cultured with zymosan in the presence of indomethacin in some experiments. No suppression of production was observed with indomethacin treatment, indicating a lack of association between these eicosanoids in our experimental system. Western blotting showed that cPLA2 and 5-LO protein expression was not affected by either zymosan or zymosan plus propofol (). Therefore, zymosan-induced increases in leukotriene production may be due to the increased activities of cPLA2 and/or 5-LO, and the propofol-induced suppression of leukotriene production is caused by direct inhibition of cPLA2/5-LO activity, or indirect inhibition via MAPK inhibition and/or a decrease in intracellular calcium levels (Rehfeldt et al., Citation1993; Werz et al., Citation2002; Jiang et al., Citation2003; Radmark et al., Citation2007). MAPK inhibition and calcium depletion resulted in the suppression of both LTB4 () and CysLT (data not shown) production.
Figure 1. (a) Cysteinyl leukotriene (CysLT) and leukotriene B4 (LTB4) production. Dendritic cells (DC) were stimulated with zymosan (10 µg/ml) in the presence of propofol for 30 min and the concentrations of CysLTs and LTB2 in the culture supernatants measured. Indomethacin was used at 10 µM (n = 6). #p < 0.001 vs no zymosan. *p < 0.05, **p < 0.001 vs zymosan without propofol. (b) Cytosolic phospholipase A2 (cPLA2) and 5-lipoxygenase (5-LO) protein expression. DC were stimulated with zymosan (10 µg/ml) for 30 min in the presence of 30 µM propofol and then cPLA2, 5-LO, and α-tubulin protein expression determined by Western blotting. Representative blots from at least three independent experiments are shown. (c) MAPK inhibition, calcium depletion, and LTB4 production. DC were stimulated with zymosan (10 µg/ml) in the presence of propofol (30 µM), U0126 (U; an MEK inhibitor; 10 µM), SB203580 (SB; a p38 MAPK inhibitor; 10 µM), SP600125 (SP; a JNK inhibitor; 10 µM), or EGTA (a calcium chelator; 5 mM) for 30 min and LTB2 concentrations measured in the culture supernatants (n = 6). #p < 0.001 vs no zymosan. *p < 0.001 vs zymosan with vehicle.
Propofol does not affect AA release from DC
One possible reason for the suppression of leukotriene production by propofol may be the suppression of cPLA2 activity, resulting in decreased AA release from the plasma membrane. To examine this possibility, we measured the AA release from DC after zymosan stimulation in the presence of propofol. As shown in , propofol did not significantly affect the zymosan-induced increase in AA release. Therefore, alteration of cPLA2 activity by propofol is not likely the cause, but the inhibition of 5-LO activity could be responsible for the suppression.
Figure 2. Arachidonic acid (AA) release. DC were labeled with 0.25 µCi/ml [3H]-AA for 1.5 h and then stimulated with or without zymosan (ZYN) (10 µg/ml) in the presence or absence of propofol for 30 min or 4 h. The amount of released AA was measured (n = 6). * p < 0.01, ** p < 0.001 vs no zymosan. n.s. = not significant.
![Figure 2. Arachidonic acid (AA) release. DC were labeled with 0.25 µCi/ml [3H]-AA for 1.5 h and then stimulated with or without zymosan (ZYN) (10 µg/ml) in the presence or absence of propofol for 30 min or 4 h. The amount of released AA was measured (n = 6). * p < 0.01, ** p < 0.001 vs no zymosan. n.s. = not significant.](/cms/asset/60fa6a77-deca-481a-9b92-0ef0a5ea0a7a/iimt_a_712066_f0002_b.gif)
MAPK phosphorylation is not affected by propofol
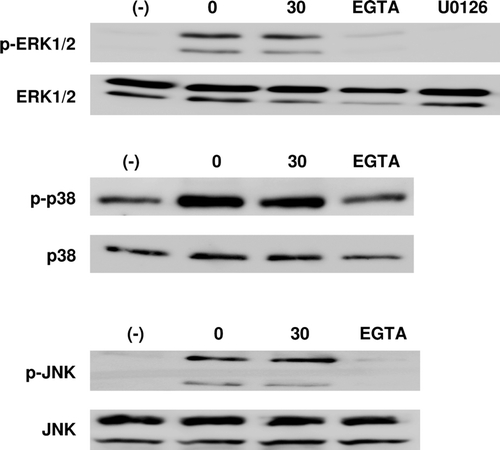
Another possible reason for the inhibition of leukotriene synthesis by propofol may be the inhibition of 5-LO activity by the modification of MAPK activity (Werz et al., Citation2002; Radmark et al., Citation2007). Among MAPKs, ERK1/2, p-38 MAPK, and JNK may be particularly pertinent for the activation of 5-LO (Werz et al., Citation2002; Radmark et al., Citation2007). Western blot showed that zymosan treatment consistently induced or up-regulated the phosphorylation of these MAPKs and that propofol had no further effect on phosphorylation status (). Thus, these kinases are not relevant to the suppression of leukotriene synthesis observed after propofol treatment.
Figure 3. Western blotting to assess the phosphorylation of ERK1/2, p38 MAPK, and JNK in DC. DC were stimulated with or without zymosan (10 µg/ml), with propofol (30 µM), U0126 (an MEK inhibitor; 10 µM), or EGTA (a calcium chelator; 5 mM) for 30 min and the cells lysed for analysis. Representative blots from at least three independent experiments are shown.
Intracellular calcium levels are not affected by propofol
Increased intracellular calcium may be a potent activator of 5-LO and cPLA2 (Rehfeldt et al., Citation1993; Radmark et al., Citation2007). Previous studies indicated that propofol suppresses intracellular calcium levels in certain cell types (Barhoumi et al., Citation2007). Zymosan increased intracellular calcium concentrations slowly and steadily until at least 30 min; thus, we compared the intracellular calcium values at 30 min after zymosan treatment. As shown in , zymosan significantly increased the intracellular calcium levels, which were not affected further by propofol.
Figure 4. Intracellular calcium concentration. DC were loaded with Fura2-AM, washed, and cultured for 30 min in the presence or absence of zymosan (10 µg/ml) with or without propofol (30 µM). The intensity of fluorescence at 500 nm elicited by 340 nm and 380 nm excitation was measured to determine the intracellular calcium concentration ([Ca2+]i) (n = 6). *p < 0.05 vs no zymosan. n.s. = not significant.
![Figure 4. Intracellular calcium concentration. DC were loaded with Fura2-AM, washed, and cultured for 30 min in the presence or absence of zymosan (10 µg/ml) with or without propofol (30 µM). The intensity of fluorescence at 500 nm elicited by 340 nm and 380 nm excitation was measured to determine the intracellular calcium concentration ([Ca2+]i) (n = 6). *p < 0.05 vs no zymosan. n.s. = not significant.](/cms/asset/ce0d66da-a710-4127-b822-4707472de6f6/iimt_a_712066_f0004_b.gif)
Propofol inhibits the 5-LO activity of purified recombinant human 5-LO enzyme
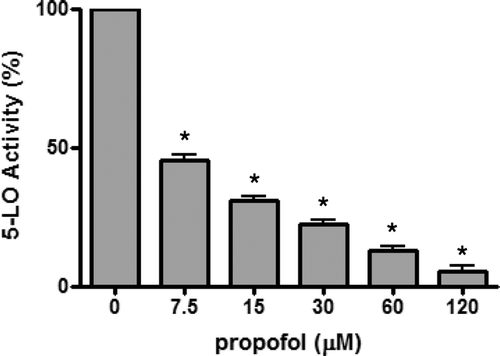
We hypothesized that propofol may have inhibitory activity towards purified 5-LO enzyme in a cell-free system. We examined this possibility using the ferrous oxidation-xylenol orange (FOX) assay. Human recombinant 5-LO enzyme was used because an appropriate murine version was not commercially available. As shown in , propofol dose-dependently suppressed the 5-LO enzyme activity with an IC50 of ~7.5 µM.
Figure 5. 5-LO activity of purified human recombinant 5-LO enzyme. The activity of 5-LO was assessed using the ferrous oxidation-xylenol orange (FOX) assay (n = 6). The 5-LO activity (%) was expressed as the ratio of absorbance in the presence of propofol to absorbance in the absence of propofol (n = 8). *p < 0.001 vs without propofol (i.e. with DMSO).
Discussion
The present study showed that propofol suppresses the production of CysLTs and LTB4 by murine DC. The expression of cPLA2 and 5-LO protein was not altered by propofol. Furthermore, AA release, intracellular calcium concentrations, and the phosphorylation status of ERK1/2, p38 MAPK, and JNK were unaffected by propofol. However, the activity of purified 5-LO enzyme was inhibited by propofol. Thus, propofol most likely suppresses DC leukotriene production by inhibiting 5-LO enzyme activity directly.
Suppression of leukotriene production by DC with propofol could affect the DC inflammatory microenvironment and, thus, subsequent immune function. Given this, what is the implication of propofol-suppressed leukotriene synthesis by DC? Several implications relevant to DC are plausible when one considers that leukotrienes produced by DC have autocrine/paracrine effects on the DC themselves (Rola-Pleszczynski and Stankova, Citation1992; Harizi et al., Citation2003). Leukotrienes have been reported to act on DC to modulate their migration, maturation, and activation (Robbiani et al., Citation2000; Machida et al., Citation2004; Jozefowski et al., Citation2005; Del Prete et al., Citation2007; Dannull et al., Citation2012). Among these roles of leukotrienes, the promotion of DC migration has been well studied (Del Prete et al., Citation2007; Dannull et al., Citation2012). LTC4 induces the migration of human monocyte-derived DC without a loss of immunostimulatory function (Dannull et al., Citation2012). LTB4 is required for the migration of DC to regional lymph nodes, as well as for the development of airway hyper-responsiveness and airway inflammation (Del Prete et al., Citation2007; Miyahara et al., Citation2008). LTB4 also stimulates human monocyte-derived DC chemotaxis (Shin et al., Citation2006). Furthermore, in 5-LO deficient mice, the activation-dependent migration of Langerhans cells and dermal DC to skin-draining lymph nodes is markedly impaired (Doepping et al., Citation2007). Therefore, leukotrienes are involved in DC migration, and the suppression of leukotriene production by propofol could interfere with the induction of adaptive immune responses by interfering with DC trafficking to regional lymph nodes.
Although far less is known about the role of leukotrienes in DC maturation and function, some data are available. For example, CysLTs have been shown to promote DC activation in antigen-induced immune responses in the lung (Okunishi et al., Citation2004). In addition, LTB4 and LTD4 can affect calcium mobilization from endoplasmic reticulum stores in DC (Itagaki et al., Citation2010) and LTB4 may induce interleukin-6 production (Rola-Pleszczynski and Stankova, Citation1992), both of which have important implications in the regulation of DC maturation. Thus, via leukotriene deprivation, propofol could potentially affect DC maturation and function.
Propofol inhibited the activity of purified human 5-LO. This observation indicates that propofol’s suppressive activity is not restricted to 5-LO in DC. Thus, propofol could potentially suppress leukotriene production by immune, as well as non-immune, cells. Propofol is a dual inhibitor of cyclooxygenase (Inada et al. Citation2011a) and 5-LO. The mechanism of the direct inhibition is not known, but might be related to the phenol-based structure of propofol. In light of this, it has recently been reported that a Vitamin E metabolite, 13′-carboxychromanol, which possesses a phenol-based structure, directly inhibits both the cyclooxygenase (Jiang et al., Citation2008) and 5-LO (Jiang et al., Citation2011) enzyme activities.
Leukotrienes also affect cells other than DC. Acting on bronchial smooth muscle cells and endothelial cells, CysLTs are potent mediators of asthma and hypersensitivity (Henderson, Citation1994; Byrum et al., Citation1999), inducing bronchoconstriction and increased microvascular permeability (Henderson, Citation1994; Byrum et al., Citation1999). In contrast, acting on neutrophils, LTB4 stimulates their aggregation, reactive oxygen species production, and chemotaxis into inflammatory lesions (Henderson, Citation1994; Byrum et al., Citation1999). Thus, propofol likely exerts its anti-inflammatory properties by inhibiting leukotriene production by various kinds of inflammatory cells. Also, propofol may be beneficial in alleviating asthma symptoms by inhibiting leukotriene production by mast cells, basophils, and infiltrating eosinophils. Besides the possible benefit in asthma patients, there are plenty of theoretical clinical benefits related to the suppression of leukotriene production with propofol. Examples include the reduction of the multiple organ injury and dysfunction in endotoxemia (Collin et al., Citation2004; Rossi et al., Citation2009) and the reduction of metastasis after tumor extirpation surgery (Sethi et al., Citation2012). These implications are very intriguing, but are obviously too speculative and further study is definitely needed.
Conclusions
Intravenous anesthetic propofol suppresses leukotriene production by DC, and possibly other immune and non-immune cells, by directly inhibiting 5-LO enzyme activity. This finding should be clinically pertinent, as leukotrienes have dramatic effects on inflammation and immune function. However, the specific effects this suppression may have remain to be determined.
Declaration of interest: This work was supported by Grant-in-AID for Scientific Research (C)-22591721 from the Japan Society for the Promotion of Science. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alvarez, C., Amaral, M. M., Langellotti, C., Vermeulen, M. 2011. Leukotriene-C4 prevents the complete maturation of murine dendritic cells and modifies IL-12/IL-23 balance. Immunology 134:185–197.
- Barhoumi, R., Burghardt, R. C., Qian, Y., Tiffany-Castiglioni, E. 2007. Effects of propofol on intracellular Ca2+ homeostasis in human astrocytoma cells. Brain Res. 1145:11–18.
- Bauer, M., Rensing, H., Ziegenfuss, T. 1998. Anesthesia and perioperative immune function. Anaesthesist 47:538–556.
- Byrum, R. S., Goulet, J. L., Snouwaert, J. N., Griffiths, R. J., Koller, B. H. 1999. Determination of contribution of cysteinyl leukotrienes and leukotriene B4 in acute inflammatory responses using 5-lipoxygenase- and leukotriene A4 hydrolase-deficient mice. J. Immunol. 163:6810–6819.
- Cho, Y. S., Kim, H. S., Kim, C. H., Cheon, H. G. 2006. Application of the ferrous oxidation-xylenol orange assay for the screening of 5-lipoxygenase inhibitors. Anal. Biochem. 351:62–68.
- Collin, M., Rossi, A., Cuzzocrea, S., Patel, N. S., Di Paola, R., Hadley, J., Collino, M., Sautebin, L., Thiemermann, C. 2004. Reduction of the multiple organ injury and dysfunction caused by endotoxemia in 5-lipoxygenase knockout mice and by the 5-lipoxygenase inhibitor zileuton. J. Leukocyte Biol. 76:961–970.
- Dannull, J., Schneider, T., Lee, W. T., de Rosa, N., Tyler, D. S., Pruitt, S. K. 2012. Leukotriene C4 induces migration of human monocyte-derived dendritic cells without loss of immunostimulatory function. Blood 119:3113–3122.
- Del Prete, A., Shao, W. H., Mitola, S., Santoro, G., Sozzani, S., Haribabu, B. 2007. Regulation of dendritic cell migration and adaptive immune response by leukotriene B4 receptors: a role for LTB4 in up-regulation of CCR7 expression and function. Blood 109:626–631.
- Dillon, S., Agrawal, S., Banerjee, K., Letterio, J., Denning, T. L., Oswald-Richter, K., Kasprowicz, D.J., Kellar, K., Pare, J., van Dyke, T., Ziegler, S., Unutmaz, D., Pulendran, B. 2006. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Invest. 116:916–928.
- Doepping, S., Funk, C. D., Habenicht, A. J., Spanbroek, R. 2007. Selective 5-lipoxygenase expression in Langerhans cells and impaired dendritic cell migration in 5-LO-deficient mice reveal leukotriene action in skin. J. Invest. Dermatol. 127:1692–1700.
- Grynkiewicz, G., Poenie, M., Tsien, R. Y. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440–3450.
- Harizi, H., Juzan, M., Moreau, J. F., Gualde, N. 2003. Prostaglandins inhibit 5-lipoxygenase-activating protein expression and leukotriene B4 production from dendritic cells via an IL-10-dependent mechanism. J. Immunol. 170:139–146.
- Hedi, H., Norbert, G. 2004. 5-Lipoxygenase pathway, dendritic cells, and adaptive immunity. J. Biomed. Biotechnol. 2004:99–105.
- Henderson, W. R. Jr. 1994. The role of leukotrienes in inflammation. Ann. Intern. Med. 121:684–697.
- Hensler, T., Hecker, H., Heeg, K., Heidecke, C. D., Bartels, H., Barthlen, W., Wagner, H., Siewert, J. R., Holzmann, B. 1997. Distinct mechanisms of immunosuppression as a consequence of major surgery. Infect. Immun. 65:2283–2291.
- Inada, T., Kubo, K., Shingu, K. 2011a. Possible link between cyclooxygenase-inhibiting and anti-tumor properties of propofol. J. Anesth. 25:569–675.
- Inada T., Kubo K., Ueshima H., Shingu K. 2011b. Intravenous anesthetic propofol suppresses prostaglandin E2 production in murine dendritic cells. J. Immunotoxicol. 8:359–366.
- Itagaki, K., Barton, B. E., Murphy, T. F., Taheri, S., Shu, P., Huang, H., Jordan, M. L. 2010. Eicosanoid-induced store-operated calcium entry in dendritic cells. J. Surg. Res. 169:301–310.
- Jiang, Q., Yin, X., Lill, M. A., Danielson, M. L., Freiser, H, Huang, J. 2008. Long-chain carboxychromanols, metabolites of Vitamin E, are potent inhibitors of cyclooxygenases. Proc. Natl. Acad. Sci. USA 105:20464–20469.
- Jiang, Y. J., Lu, B., Choy, P. C., Hatch, G. M. 2003. Regulation of cytosolic phospholipase A2, cyclooxygenase-1 and -2 expression by PMA, TNFα, LPS and M-CSF in human monocytes and macrophages. Mol. Cell Biochem. 246:31–38.
- Jiang, Z., Yin, X., Jiang, Q. 2011. Natural forms of Vitamin E and 13′-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively. J. Immunol. 186:1173–1179.
- Jozefowski, S., Biedron, R., Bobek, M., Marcinkiewicz, J. 2005. Leukotrienes modulate cytokine release from dendritic cells. Immunology 116:418–428.
- Lewis, R. A., Austen, K. F., Soberman, R. J. 1990. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. New Engl. J. Med. 323:645–655.
- Lutz, M. B., Kukutsch, N., Ogilvie, A.L., Rossner, S., Koch, F., Romani, N., Schuler, G. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77–92.
- Machida, I., Matsuse, H., Kondo, Y., Kawano, T., Saeki, S., Tomari, S., Obase, Y., Fukushima, C., Kohno, S., 2004. Cysteinyl leukotrienes regulate dendritic cell functions in a murine model of asthma. J. Immunol. 172:1833–1838.
- Mak, T., Saunders, M. E., (Eds.). 2006. The Immune Response: Basic and Clinical Principles. Burlington, VT: Elsevier Academic Press.
- Miyahara, N., Ohnishi, H., Matsuda, H., Miyahara, S., Takeda, K., Koya, T., Matsubara, S., Okamoto, M., Dakhama, A., Haribabu, B., Gelfand, E. W. 2008. Leukotriene B4 receptor 1 expression on dendritic cells is required for the development of TH2 responses and allergen-induced airway hyper-responsiveness. J. Immunol. 181:1170–1178.
- Needleman, P., Turk, J., Jakschik, B. A., Morrison, A. R., Lefkowith, J. B. 1986. Arachidonic acid metabolism. Annu. Rev. Biochem. 55:69–102.
- Ogawa, K., Hirai, M., Katsube, T., Murayama, M., Hamaguchi, K., Shimakawa, T., Naritake, Y., Hosokawa, T., Kajiwara, T. 2000. Suppression of cellular immunity by surgical stress. Surgery 127:329–336.
- Okunishi, K., Dohi, M., Nakagome, K., Tanaka, R., Yamamoto, K. 2004. A novel role of cysteinyl leukotrienes to promote dendritic cell activation in the antigen-induced immune responses in the lung. J. Immunol. 173:6393–6402.
- Radmark, O., Shimizu, T., Jornvall, H., Samuelsson, B. 1984. Leukotriene A4 hydrolase in human leukocytes. Purification and properties. J. Biol. Chem. 259:12339–12345.
- Radmark, O., Werz, O., Steinhilber, D., Samuelsson, B. 2007. 5-Lipoxygenase: Regulation of expression and enzyme activity. Trends Biochem. Sci. 32:332–341.
- Rehfeldt, W., Resch, K., Goppelt-Struebe, M. 1993. Cytosolic phospholipase A2 from human monocytic cells: Characterization of substrate specificity and Ca2+-dependent membrane association. Biochem. J. 293:255–261.
- Rigouzzo, A., Girault, L., Louvet, N., Servin, F., De-Smet, T., Piat, V., Seeman, R., Murat, I., Constant, I. 2008. The relationship between bi-spectral index and propofol during target-controlled infusion anesthesia: A comparative study between children and young adults. Anesth. Analg. 106:1109–1116.
- Robbiani, D. F., Finch, R. A., Jager, D., Muller, W. A., Sartorelli, A. C., Randolph, G. J. 2000. The leukotriene C4 transporter MRP1 regulates CCL19 (MIP-3β, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell 103:757–768.
- Rola-Pleszczynski, M., Stankova, J. 1992. Leukotriene B4 enhances IL-6 production and IL-6 messenger RNA accumulation in human monocytes in vitro: Transcriptional and post-transcriptional mechanisms. Blood 80:1004–1011.
- Rossi, A., Cuzzocrea, S., Sautebin, L. 2009. Involvement of leukotriene pathway in the pathogenesis of ischemia-reperfusion injury and septic and non-septic shock. Curr. Vasc. Pharmacol. 7:185–197.
- Sethi, G., Shanmugam, M. K., Ramachandran, L., Kumar, A. P., Tergaonkar, V. 2012. Multifaceted link between cancer and inflammation. Biosci. Rep. 32:1–15.
- Shin, E. H., Lee, H. Y., Bae, Y. S. 2006. Leukotriene B4 stimulates human monocyte-derived dendritic cell chemotaxis. Biochem. Biophys. Res. Commun. 348:606–611.
- Slade, M. S., Simmons, R. L., Yunis, E., Greenberg, L. J. 1975. Immunosuppression after major surgery in normal patients. Surgery 78:263–372.
- Sompayrac, L., (Ed.). 2003. How immune system works? Malden, MA: Blackwell Publishing.
- Spanbroek, R., Hildner, M., Steinhilber, D., Fusenig, N., Yoneda, K., Radmark, O., Samuelsson, B., Habenicht, A. J. 2000. 5-lipoxygenase expression in dendritic cells generated from CD34+ hematopoietic progenitors and in lymphoid organs. Blood 96:3857–3865.
- Thivierge, M., Stankova, J., Rola-Pleszczynski, M. 2009. Cysteinyl-leukotriene receptor type 1 expression and function is down-regulated during monocyte-derived dendritic cell maturation with zymosan: Involvement of IL-10 and prostaglandins. J. Immunol. 183:6778–6787.
- Vanlersberghe, C., Camu, F. 2008. Propofol. Handbook Exp. Pharmacol. 187:227–252.
- Vasileiou, I., Xanthos, T., Koudouna, E., Perrea, D., Klonaris, C., Katsargyris, A., Papadimitriou, L. 2009. Propofol: A review of its non-anesthetic effects. Eur. J. Pharmacol. 605:1–8.
- Werz, O., Burkert, E., Fischer, L., Szellas, D., Dishart, D., Samuelsson, B., Radmark, O., Steinhilber, D. 2002. Extracellular signal-regulated kinases phosphorylate 5-lipoxygenase and stimulate 5-lipoxygenase product formation in leukocytes. FASEB J. 16:1441–1443.
- Yoshimoto, T., Soberman, R. J., Spur, B., Austen, K. F. 1988. Properties of highly purified leukotriene 3β synthase of guinea pig lung. J. Clin. Invest. 81:866–871.