Abstract
Helper T (TH) cells are an important part of the adaptive immune system. It is hypothesized that one type of helper T-cell, TH17 cells, play an important role in idiosyncratic drug-induced liver failure, and it was found that interleukin (IL)-17, the signature cytokine of TH17 cells, was elevated in most patients with idiosyncratic drug-induced liver failure. However, it was also found that IL-17 was elevated in some patients with acetaminophen (APAP)-induced liver failure. It is unlikely that APAP-induced liver failure is mediated by the adaptive immune system, but there are other cells such as macrophages and natural killer (NK) cells that also produce IL-17. Therefore, the phenotype of cells that produce IL-17 was studied in a mouse model of APAP-induced liver toxicity. To the authors’ surprise, it was found that most of the IL-17 producing cells in the liver were TH17 cells, and they were increased within hours of APAP treatment. This is too fast for a response of the adaptive immune system. These data suggest that TH17 cells can be part of the innate immune response; however, it is unclear what role they play in the pathogenesis of APAP-induced hepatotoxicity.
Introduction
Acetaminophen (paracetamol, APAP)-induced liver injury is not idiosyncratic, but the dose required to cause liver failure can vary significantly from one individual to another. For example, low-dose APAP treatment is believed to be safe and is usually not associated with liver damage; however, when used at the maximum recommended daily dose, it has been shown that APAP can result in the elevation of serum alanine aminotransferase (ALT) levels more than 3-times the upper limit of normal in over 40% of people (Watkins et al., Citation2006). APAP over-dose can lead to severe liver damage, and in the US it has been reported that more than 50% of cases of acute liver failure are due to APAP (Ostapowicz et al., Citation2002).
The mechanisms of APAP-induced hepatotoxicity have been extensively studied. APAP is mainly metabolized in the liver by Phase II pathways of sulfation and glucuronidation. The Phase I reaction that involves cytochromes P450 is only responsible for a very limited proportion of the metabolism of APAP; however, oxidation generates a highly reactive metabolite: N-acetyl-p-benzoquinone imine (NAPQI). NAPQI is detoxified by conjugation with glutathione. Although this conjugation prevents significant toxicity at low doses, with an overdose, the increased amount of NAPQI depletes glutathione (Nelson, Citation1990). Covalent binding of NAPQI to proteins leading to mitochondrial injury appears to be the ultimate mechanism leading to cell death (Kon et al., Citation2004; Reid et al., Citation2005).
Although it is generally accepted that APAP-induced hepatotoxicity is largely due to events occurring within the hepatocytes, some recent studies have focused on cells of the innate immune system and imply that these cells may also play an important role in the pathogenesis of this liver damage (Liu and Kaplowitz, Citation2006). However, a careful look at the current data suggests that these cells, i.e., Kupffer cells, neutrophils, and monocytes, likely play a protective role and are responsible for repairing tissue damage (Jaeschke et al., Citation2011). For example, it has been shown that complete depletion of Kupffer cells with the intravenous injection of liposome/clodronate caused a significant decrease in the hepatic expression of mRNA of modulatory cytokines and significantly increased susceptibility to APAP-induced liver injury (Ju et al., Citation2002), suggesting that Kupffer cells have protective function in the liver.
Our laboratory has postulated that most idiosyncratic drug-induced liver injury is mediated by the adaptive immune system. In order to test this hypothesis we studied the cytokine profile in patients with idiosyncratic drug-induced liver failure with an emphasis on cytokines related to TH17 cells. TH17 cells and their associated cytokines have been implicated in a number of human liver diseases, including autoimmune, viral, alcoholic, and ischemia/reperfusion injury (Hammerich et al., Citation2010). We found that most of the patients with idiosyncratic drug-induced liver failure had elevated levels of interleukin (IL)-17, the signature TH17 cytokine; however, many of the patients with APAP-induced liver failure also had increased levels of IL-17 (Li et al., Citation2010). Although several other cells such as macrophages and NK cells can produce IL-17, IL-21, another cytokine related to TH17 cells, was also elevated in patients with APAP-induced liver injury. In the present study, the involvement of IL-17/TH17 pathway in APAP-induced liver injury in a mouse model where the IL-17-producing cells in the liver could be phenotyped was investigated.
Materials and methods
Animals
Male Balb/c mice (15–20 g, 4–6-weeks-old) were purchased from Charles River (Montreal, Quebec, Canada) and kept 2/cage in a pathogen-free facility maintained at 22°C and with a 12:12 h light:dark cycle. The mice were given ad libitum access to standard mouse chow (Agribrands, Purina Canada, Strathroy, Ontario, Canada) and tap water for a week-long acclimatization period before starting an experiment. All animal procedures were performed according to the University of Toronto guidelines for the care and use of laboratory animals.
Chemicals, kits, and solutions
APAP, phorbol myristate acetate, ionomycin, Percoll solution, and RPMI 1640 medium were purchased from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum (FBS) and penicillin-streptomycin solution were purchased from Gibco, Invitrogen (Carlsbad, CA). All ELISA kits were purchased from R&D Systems (Minneapolis, MN). The ALT assay reagents were purchased from Thermo Fisher Scientific Inc. (Middletown, VA). Conjugated monoclonal antibodies against CD3 (phycoerythrin-Cy7 cyanine dye), CD4 (fluorescein isothiocyanate), and IL-17 (allophycocyanin) were purchased from eBioscience Inc. (San Diego, CA). CD1d tetramer (phycoerythrin) reagents were provided by the NIH tetramer Core Facility (Atlanta, GA).
APAP treatment and cell preparation
Animals were fasted for 15 h before experiments. The male mice were then given saline (vehicle, n = 4) or APAP dissolved in saline at a concentration of 300 mg/kg body weight (BW) (intraperitoneally [IP], n = 4). Two hours after the APAP (or vehicle) injection, all mice were euthanized by carbon dioxide asphyxiation. At necropsy, the liver was then perfused with phosphate-buffered saline (PBS, pH 7.4) and isolated, followed by immediate gall bladder removal. The liver was then carefully mashed on a BD Falcon 70 µm cell strainer using a syringe piston and centrifuged at 350 x g in 40–50 ml complete RPMI (RPMI containing 10% FBS and 1% penicillin/streptomycin). The cell pellets were re-suspended in 10 ml PBS and split into two 15 ml tubes. After centrifuging again, the samples were re-suspended in 9–10 ml of a 33% Percoll solution and then spun at 1150 x g for 20 min at room temperature. The cake on top of the Percoll was carefully removed, and the cell pellet at the bottom was re-suspended in 1 ml of complete RPMI. Following red blood cell lysis, lymphocytes were isolated, and the final cell concentration was adjusted to 2 × 107/ml.
Flow cytometric analysis of TH17 cells
IL-17-producing CD4+ lymphocytes were examined. Liver cells were suspended at a density of 1 × 106 cells/ml in complete culture medium. Cultures were stimulated for 5 h using 50 ng phorbol myristate acetate/ml and 750 ng ionomycin/ml in the presence of monensin, at 37°C and 5% CO2. Monensin is a standard reagent used for intracellular staining of cytokines; it inhibits the export of cytokines such as IL-17 so that they can be detected by flow cytometry. Without it, the levels of IL-17 remaining in the cells might be very low at the time of measurements.
All cells were then washed in PBS and pre-incubated with 2 µl of affinity-purified FcγR-bindinginhibitor/106 cells for 20 min on ice prior to staining. The recommended quantity of each primary antibody was combined in an appropriate volume of Flow Cytometry Staining Buffer (eBioscience Inc., San Diego, CA) and added to the cells. All samples were then incubated for at least 30 min in the dark on ice at 4°C and then washed with Flow Cytometry Staining Buffer (eBioscience). Following surface staining, cells were fixed and permeabilized using Permeabilization Reagent (eBioscience) and then stained with anti-IL-17 antibody. Stained cells were analyzed with a FACSCanto cytometer (BD Bioscience, San Jose, CA) and data were analyzed by FlowJo software (TreeStar, Ashland, OR). A minimum of 200,000 events per sample was routinely acquired.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA). The statistical test used for the ELISA results and flow cytometry analysis was a one-way analysis of variance (ANOVA). A two-tailed analysis was carried out with significance defined as a p-value < 0.05. All data were expressed as the mean ± SE.
Results and discussion
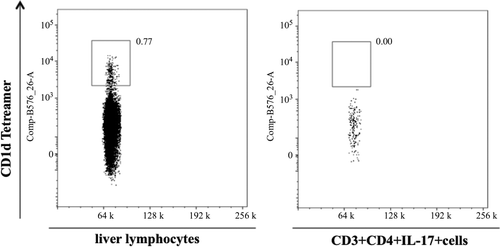
APAP-induced hepatotoxicity is generally not believed to be mediated by the adaptive immune system; however, an increase in pro-inflammatory TH17-related cytokines such as IL-17 and IL-21 was found in samples from patients with APAP-induced liver failure (Li et al., Citation2010). It was plausible that the source of this IL-17 was cells of the innate immune system such as NK cells (Lo et al., Citation2008). To test this hypothesis we treated mice with APAP and phenotyped the cells that produced IL-17. There was an increase in ALT () and this was associated with an increase in serum IL-17 levels as early as 2 h after APAP treatment (). The phenotype of IL-17-producing cells in APAP-induced acute liver injury was determined by flow cytometry. APAP-treated animals had significantly higher percentages of CD3+CD4+IL-17+ cells (0.64 ± 0.06%) as compared to the controls (0.15 ± 0.03%) (). In liver, it is known that a fraction of NKT cells also co-express CD3 and CD4 (Godfrey et al., Citation2000). However, in the current study, it was found that almost all of the CD3+CD4+IL-17+ cells did not bind to CD1d tetramer (), an NKT cell-specific marker. Such data indicate that these CD3+CD4+IL-17+ cells are TH17 cells rather than NKT cells.
Figure 1. Liver injury during APAP-induced liver toxicity. Balb/c mice were given saline or APAP (300 mg/kg) once by IP injection. At 0, 2, 6, and 24 h post-injection, mice were euthanized, blood was collected, and serum levels of ALT then measured. Results shown are the mean (± SE) of four mice.
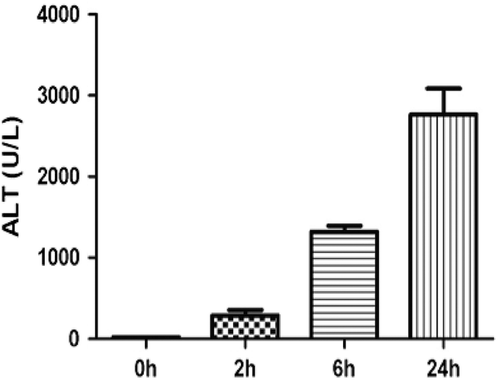
Figure 2. Serum IL-17 levels after APAP (300 mg/kg) treatment. Results shown are the mean (± SE) of four mice (** p < 0.01).
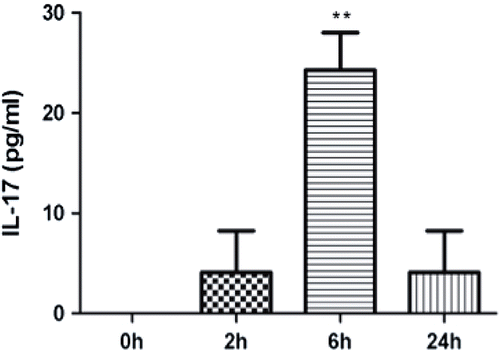
Figure 3. APAP treatment increased levels of IL-17-producing CD4+ T-cells in the liver after 2 h. (a) CD3 and CD4 double-positive cells were gated for further analysis. (b) Example of IL-17 expression compared between control and treatment animals; the x-axis is side scatter (SSC). (c) Percentage of CD4+IL-17+ T-cells in the liver 2 h after APAP treatment compared with controls. Results shown are the mean (± SE) from three mice. Similar results were obtained in three independent experiments (p < 0.03).
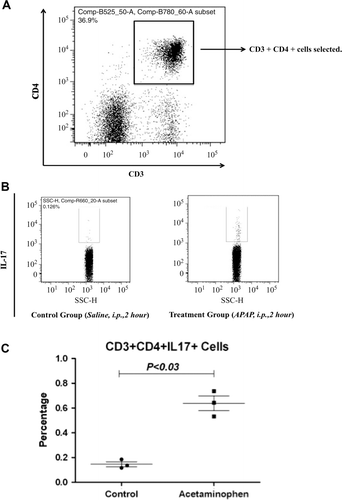
Figure 4. Comparison of staining for CD1d tetramer (NKT cell marker) in total hepatic lymphocytes (left panel) and hepatic lymphocytes that stain positive for CD3/CD4/IL-17 from (right panel). The x-axis is side scatter (SSC).
IL-17 and TH17 cells have been shown to play critical roles in various inflammatory and autoimmune diseases (Korn et al., Citation2009). Moreover, increasing evidence suggests that the TH17 pathway is also involved in different liver diseases, including acute liver failure, viral hepatitis, alcoholic liver disease, autoimmune hepatitis, and primary biliary cirrhosis (Hammerich et al., Citation2010). Recently, it has been reported that IL-17/IL-17R signaling is involved in the pathogenesis of Concanavalin A (ConA)-induced acute liver toxicity (Yan et al., Citation2012). The increased levels of IL-17 were found to parallel the severity of liver injury, and blockade of IL-17 or IL-17R significantly ameliorated ConA-induced acute liver injury.
Considering the fact that both IL-17 and IL-6 were increased at early time points, the adaptive immune system is unlikely to be the source of these cytokines. This suggested that the source of IL-17 is the innate immune system. However, the data in this study ( and ) strongly suggest that TH17 cells are the major source of IL-17 induced by the administration of APAP. It has been reported that IL-1ß signaling is activated by APAP (Williams et al., Citation2010), and it has been shown that IL-1ß can promote the expression of the chemokine CCL20 and recruit TH17 cells by interaction with its receptor, CCR6 (Hirata et al., Citation2010). Such a mechanism may explain the significant increase in TH17 cells observed in the current study. Very recently, TH17 cells have also been shown to be responsible for the innate IL-17-associated responses to bacteria (Geddes et al., Citation2011). Another recent paper suggests that most cells of the adaptive immune system have a counterpart in the innate immune system (Leslie, Citation2012); therefore, possibly, these cells should be referred to as TH17-like cells. However, it is not clear at this point whether the cells that we observed are the most common type of TH17 cells or uncommon. IL-6 is required for induction of such an early TH17 response. APAP-induced liver injury appears to be another example in which TH17 cells are part of the innate response, but it is not clear what role they play because the innate immune system appears to play a protective role in APAP-induced liver injury (Jaeschke et al., Citation2011).
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research. J.U. is the Canada Research Chair in Adverse Drug Reactions.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Geddes, K., Rubino, S. J., Magalhaes, J. G., Streutker, C., Le Bourhis, L., Cho, J. H., Robertson, S. J., Kim, C. J., Kaul, R., Philpott, D. J., Girardin, S. E. 2011. Identification of an innate T-helper type 17 response to intestinal bacterial pathogens. Nat. Med. 17:837–844.
- Godfrey, D. I., Hammond, K. J., Poulton, L. D., Smyth, M. J., Baxter, A. G. 2000. NKT cells: Facts, functions and fallacies. Immunol. Today 21:573–583.
- Hammerich, L., Heymann, F., Tacke, F. 2010. Role of IL-17 and TH17 cells in liver diseases. Clin. Dev. Immunol. 2011:345803.
- Hirata, T., Osuga, Y., Takamura, M., Kodama, A., Hirota, Y., Koga, K., Yoshino, O., Harada, M., Takemura, Y., Yano, T., Taketani, Y. 2010. Recruitment of CCR6-expressing TH17 cells by CCL 20 secreted from IL-1β-, TNFα-, and IL-17A-stimulated endometriotic stromal cells. Endocrinology 151:5468–5476.
- Jaeschke, H., Williams, C. D., Ramachandran, A., Bajt, M. L. 2011. Acetaminophen hepatotoxicity and repair: The role of sterile inflammation and innate immunity. Liver Int. 32:8–20.
- Ju, C., Reilly, T. P., Bourdi, M., Radonovich, M. F., Brady, J. N., George, J. W., Pohl, L. R. 2002. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem. Res. Toxicol. 15:1504–1513.
- Kon, K., Kim, J. S., Jaeschke, H., Lemasters, J. J. 2004. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 40:1170–1179.
- Korn, T., Bettelli, E., Oukka, M., Kuchroo, V. K. 2009. IL-17 and TH17 Cells. Annu. Rev. Immunol. 27:485–517.
- Leslie, M. 2012. Immunology. Crossover immune cells blur the boundaries. Science 336:1228–1229.
- Li, J., Zhu, X., Liu, F., Cai, P., Sanders, C., Lee, W. M., Uetrecht, J. 2010. Cytokine and autoantibody patterns in acute liver failure. J. Immunotoxicol. 7:157–164.
- Liu, Z. X., Kaplowitz, N. 2006. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin. Drug Metab. Toxicol. 2:493–503.
- Lo, C. K., Lam, Q. L., Sun, L., Wang, S., Ko, K. H., Xu, H., Wu, C. Y., Zheng, B. J., Lu, L. 2008. Natural killer cell degeneration exacerbates experimental arthritis in mice via enhanced IL-17 production. Arthritis Rheum. 58:2700–2711.
- Nelson, S. D. 1990. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 10:267–278.
- Ostapowicz, G., Fontana, R. J., Schiodt, F. V., Larson, A., Davern, T. J., Han, S. H., Mccashland, T. M., Shakil, A. O., Hay, J. E., Hynan, L., Crippin, J. S., Blei, A. T., Samuel, G., Reisch, J., Lee, W. M. 2002. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 137:947–954.
- Reid, A. B., Kurten, R. C., Mccullough, S. S., Brock, R. W., Hinson, J. A. 2005. Mechanisms of acetaminophen-induced hepatotoxicity: Role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J. Pharmacol. Exp. Ther. 312:509–516.
- Watkins, P. B., Kaplowitz, N., Slattery, J. T., Colonese, C. R., Colucci, S. V., Stewart, P. W., Harris, S. C. 2006. Aminotransferase elevations in healthy adults receiving 4 grams of aceta-minophen daily: A randomized controlled trial. JAMA 296:87–93.
- Williams, C. D., Farhood, A., Jaeschke, H. 2010. Role of caspase-1 and IL-1β in acetaminophen-induced hepatic inflammation and liver injury. Toxicol. Appl. Pharmacol. 247:169–178.
- Yan, S., Wang, L., Liu, N., Wang, Y., Chu, Y. 2012. Critical role of IL-17/IL-17 receptor axis in mediating ConA-induced hepatitis. Immunol. Cell. Biol. 90:421–428.