Abstract
Aspergillus fumigatus is a filamentous fungus that produces abundant pigmented conidia. Several fungal components have been identified as virulence factors, including melanin; however, the impact of these factors in a repeated exposure model resembling natural environmental exposures remains unknown. This study examined the role of fungal melanin in the stimulation of pulmonary immune responses using immunocompetent BALB/c mice in a multiple exposure model. It compared conidia from wild-type A. fumigatus to two melanin mutants of the same strain, Δarp2 (tan) or Δalb1 (white). Mass spectrometry-based analysis of conidial extracts demonstrated that there was little difference in the protein fingerprint profiles between the three strains. Field emission scanning electron microscopy demonstrated that the immunologically inert Rodlet A layer remained intact in melanin-deficient conidia. Thus, the primary difference between the strains was the extent of melanization. Histopathology indicated that each A. fumigatus strain induced lung inflammation, regardless of the extent of melanization. In mice exposed to Δalb1 conidia, an increase in airway eosinophils and a decrease in neutrophils and CD8+ IL-17+ (Tc17) cells were observed. Additionally, it was shown that melanin mutant conidia were more rapidly cleared from the lungs than wild-type conidia. These data suggest that the presence of fungal melanin may modulate the pulmonary immune response in a mouse model of repeated exposures to A. fumigatus conidia.
Introduction
Filamentous fungi are ubiquitous, saprophytic micro-organisms that acquire nutrients from decaying plant matter and water-damaged building materials. Conidia or spores formed by these fungi can be aerosolized following environmental disturbance. Certain conidia are sized within the respirable fraction and can be inhaled and deposited deep within the lungs (Eduard, Citation2009). In small numbers, conidia are rapidly phagocytosed and degraded by alveolar macrophages with little immunological consequence (Eduard, Citation2009; Latge, Citation1999). However, repeated exposures to high concentrations may lead to the persistence of conidia within the lung and induction of airway inflammation.
Among the filamentous fungi, the opportunistic pathogen, Aspergillus fumigatus, is an etiological agent of invasive aspergillosis, hypersensitivity pneumonitis, allergy, and asthma (Latge, Citation1999). A. fumigatus-associated diseases have been steadily on the rise due to more people living with HIV, greater numbers of organ transplants, and therapeutic interventions that result in increased numbers of immunosuppressed patients who are more susceptible to fungal infections (Denning, Citation1998). There has also been a steady increase in the incidence of allergy, including fungal allergies (Agarwal et al., Citation2009; Chaudhary & Marr Citation2011; Devereux, Citation2006; Simon-Nobbe et al., Citation2008). In order to improve diagnosis and treatment, it is necessary to determine both host- and fungal-specific factors that direct the development of protective and/or allergic immune responses to A. fumigatus-mediated diseases.
Previous reports identified numerous A. fumigatus-associated virulence factors including thermotolerance, production of secondary metabolites (gliotoxin) and proteases, as well as cell wall-associated molecules including α and β-glucans, galactomannans, and melanins (Inoue et al., Citation2009; Latge, Citation2001). Melanins are large, polymeric pigments associated with the cell wall that are highly resistant to acidic degradation, thereby contributing to the rigidity and integrity of the conidia (Pihet et al., Citation2009). They are responsible for the characteristic blue-green pigmentation in A. fumigatus wild-type (WT) conidia. Since fungi are primarily associated with external environments, melanin functions to protect the conidia from ultraviolet radiation and ensures the integrity of conidia under the stress of turgor pressure (Brakhage et al., Citation1999; Jacobson, Citation2000; Wheeler & Bell, Citation1988). Melanin has been proposed as a major virulence factor in A. fumigatus and other fungal species, including Cryptococcus neoformans (Dixon et al., Citation1987; Huffnagle et al., Citation1995; Jacobson, Citation2000; Jahn et al., Citation1997; Kwon-Chung et al., Citation1982; Tsai et al., Citation1998).
Using melanin knock-downs and albino mutants, melanins have been shown to enhance conidial survival by quenching reactive oxygen species (ROS), and preventing binding of complement protein C3 to the surface of the conidia (Jahn et al., Citation2000; Tsai et al., Citation1997). Melanin also protects conidia from the innate immune system by preventing phagolysosome acidification and inhibiting host-cell apoptosis (Jahn et al., Citation1997, Citation2000; Thywissen et al. Citation2011; Tsai et al., Citation1997, Citation1998; Volling et al. Citation2011). Further, conidia from melanin mutants exhibit decreased virulence in a mouse model of invasive aspergillosis (Langfelder et al., Citation1998; Tsai et al., Citation1998). The presence of melanin in A. fumigatus conidia has also been shown to attenuate the host pro-inflammatory cytokine response of human peripheral blood mononuclear cells, as albino mutant conidia induce higher levels of IL-6, TNFα, and IL-10 than WT conidia (Chai et al., Citation2010). Similar results have been shown with low melanin producing mutants of C. neoformans, as these conidia induce greater inflammatory responses, TNFα and CD4+ T-cell responses, and are cleared more rapidly (Huffnagle et al., Citation1995).
In this study, we examined murine pulmonary immune responses following multiple exposures to A. fumigatus conidia to determine the influence of melanin on the induction of allergy, asthma, and/or hypersensitivity pneumonitis. Multiple exposures to conidia were used in this study to resemble repeated natural environmental exposures. Two strains of A. fumigatus with melanin synthesis pathway mutations derived from a clinical isolate of A. fumigatus were used. The Δarp2 mutant has a single gene deletion for the tetrahydroxynapthalene reductase and exhibits tan pigmentation, while the Δalb1 mutant has a deletion of the gene coding for the polyketide synthase in the dihydroxynapthalene (DHN) melanin synthesis pathway and has an albino appearance (Tsai et al., Citation1999). These studies characterize the immune responses to the melanin-deficient conidia in an immunocompetent BALB/c murine model of repeated exposures. Our results show that lack of melanin in repeated conidial aspirations resulted in increased eosinophilia and decreased neutrophils and CD8+ IL-17 (Tc17) responses, as well as increased conidial clearance at early timepoints.
Materials and methods
Growth and handling of fungi
Fungal strains Aspergillus fumigatus B-5233 (wild-type [WT] parent strain), Δarp2, and Δalb1 were received as a gift from Dr June Kwon-Chung (NIAID, Bethesda, MD) (Tsai et al., Citation1999). Fungi were grown for 14 days on malt extract agar (MEA) plates at 25 °C. Fungal conidia were harvested from plates by applying 1 g of 0.5 mm glass beads (BioSpec Products Inc., Bartlesville, OK) and gently shaking. The bead/conidia mixture was collected in a tube and suspended in 1 ml sterile phosphate-buffered saline (PBS, pH 7.4). The beads were vortexed and the supernatant containing conidia collected and enumerated using a hemocytometer. To avoid the loss of fungal antigens, the conidia were subsequently diluted in sterile PBS, without washing to a final concentration of 4 × 107 conidia/ml (2 × 106 conidia/50 µl) for animal exposures, as previously reported (Templeton et al., Citation2011). Fresh conidial suspensions were prepared from 14-day-old cultures for each exposure.
MALDI-qTOF MS analysis of melanin mutant conidia
For positive ion matrix-assisted laser desorption/ionization quadrupole time-of-flight mass spectrometry (+MALDI qTOF MS) analysis, conidia were harvested as previously described (Hettick et al., Citation2008). Briefly, conidia (∼1 × 108) isolated from three plates of each A. fumigatus strain were mixed with 100 µl of 0.1 mm zirconium beads (BioSpec) and 1 ml of 50/50 acetonitrile/4% trifluoroacetic acid (TFA) in water. After three 1-min cycles of bead beating, the samples were centrifuged at 14,500 rpm for 10 min. The supernatant was mixed 1:1 with 10 mg/ml α-cyano-4-hydroxycinnamic acid (50/50 acetonitrile/0.1% TFA) and 1 µl spotted on the target plate and allowed to air dry. MALDI–qTOF mass spectra were acquired using a MALDI-SYNAPT MS (Waters Corporation, Milford, MA) qTOF mass spectrometer capable of mass resolution (m/Dm) of 14,000 and a mass accuracy of ±5 ppm. Spectra were acquired over the m/z range of 3000–14000 u. Composite mass spectra were the result of a 6.5--min acquisition with the frequency-tripled Nd:YAG laser (355 nm) operating at 200 Hz, with the laser pulse energy maintained just above the threshold for ion production. Mass spectra were acquired using a pre-determined ‘spiral’ pattern that was held constant for all sample deposits, ensuring that a reproducible surface area was irradiated for each sample.
Polyacrylamide gel electrophoresis
Conidial extracts were prepared by adding 2 ml PBS/0.1% Tween to each of four plates and the conidia were agitated from the surface using a sterile inoculating loop. The conidial suspension was centrifuged at 2000 rpm for 5 min. The supernatant was discarded and the pellet containing the conidia was resuspended in sodium bicarbonate buffer (pH 8.0) and rocked at 4 °C overnight. The sample was centrifuged and the pellet frozen at −80 °C overnight, then lyophilized. Following lyophilization, the sample was mixed with 0.1 mm glass beads and bead beat for three 1-min cycles using a mini bead-beater (BioSpec). Sodium bicarbonate buffer (2 ml) was added and the samples were again subjected to three 1-min bead-beating cycles, centrifuged, and the resulting supernatant was used for SDS-PAGE. Protein concentrations were determined using a BCA™ protein assay kit (Thermo Scientific, Waltham, MA). A 12% separating gel with a 4% stacking gel was used for SDS-PAGE analysis. Conidial extracts (30 µg) were mixed with Laemmli sample buffer and heated at 95 °C for 5 min. Samples were then separated by electrophoresis for 90 min at 100 V. The gel was then stained using Imperial Blue stain according to manufacturer instructions (Thermo Scientific).
Field emission scanning electron microscopy
A small sample of agar was isolated from 14-day-old culture plates of each A. fumigatus strain. The sample was air-dried for 3 days, attached to an aluminum mount with double-stick carbon tape, and sputter coated with gold/palladium. Images were collected on a Hitachi (Tokyo, Japan) S-4800 field emission scanning electron microscope.
Animals
Female BALB/c mice (5–7-weeks-old; The Jackson Laboratory, Bar Harbor, ME) were acclimated for ∼1 week before initial exposures. Mice were housed in groups of five in filtered, ventilated polycarbonate cages containing autoclaved hardwood chip bedding. The temperature in the animal facility was maintained at 68–72°F, the relative humidity at 36–57%, and a light/dark cycle of 12-h intervals. Mice were provided ad libitum access to NIH-31 modified 6% irradiated rodent diet (Harlan Teklad) and tap water. Sentinel mice were free of viral pathogens, parasites, mycoplasmas, Helicobacter, and cilia-associated respiratory (CAR) Bacillus. The National Institute for Occupational Safety and Health (NIOSH) animal facility is an environmentally controlled barrier facility fully accredited by the Association for the Assessment and Accreditations of Laboratory Animal Care International. All procedures were performed under a NIOSH Animal Care and Use Committee approved protocol (# 08-ST-M-015).
Animal exposures
Mice were exposed to fungal suspensions by involuntary aspiration as previously described (Rao et al., Citation2003). Briefly, mice were anesthetized using isoflurane (Webster Veterinary Supply Inc., Devens, MA) and then, while the mouse was suspended on a slant board, the tongue was held in full extension, and a 50 µl suspension of 2 × 106 conidia was placed at the base of the tongue. The tongue was restrained for two full breaths while the mice inhaled the conidial suspension, after which anesthetized mice were returned to the cage and allowed to recover.
To assess responses to repeated exposures used to resemble repeated natural environmental exposures, mice were aspirated 14-day-old conidia twice per week for 2 weeks, rested for 2 weeks, and then challenged a final time (). Three days post-challenge, the mice were sacrificed via an intraperitoneal (IP) injection of sodium pentobarbital (Sleepaway®, Fort Dodge Animal Health, Fort Dodge, IA).
Figure 1. Field emission scanning electron microscopy images. (A) WT, (B) Δarp2, and (C) Δalb1 conidia showing the presence of the RodA layer.

To examine the impact of innate immunity compared to adaptive immunity on the clearance of conidia, mice were either repeatedly exposed to conidia, as indicated in (adaptive immunity), or mock-exposed with sterile saline twice per week for 2 weeks, rested for 2 weeks, and then exposed a final time to the indicated conidia (innate immunity). The concentration of fungal conidia and the route of exposure for these experiments were conducted within the same parameters discussed earlier in this section. Mice were sacrificed at specific timepoints thereafter.
Histology
A group of five mice/fungal strain were repeatedly exposed, as indicated in . These mice were then sacrificed 72 h post-final exposure. The descending aorta and the inferior vena cava were severed, and then the lungs perfused by injecting 5 ml PBS followed by 5 ml 10% formalin buffered saline (FBS, Fisher Scientific, Fairlawn, NJ) into the right ventricle. The trachea was exposed, nicked, and a catheter was inserted into the trachea. With a syringe, 1 ml of 10% FBS was injected into the lungs, and the trachea was tied closed. The lungs were removed and fixed in 10% FBS for 3–5 days prior to histological processing. Tissue processing, embedding, hematoxylin and eosin (H&E), and Grocott’s Methenamine Silver (GMS) staining were done by the West Virginia University Tissue Bank (Morgantown, WV). To assess the frequency of germinated conidia in lung tissues of mice, 150 fields of view from GMS-stained mid-coronal sections of the lungs of each animal were examined for conidia, swollen conidia, and germ tube formation. Swollen conidia were defined by conidial swelling (2–3× normal size).
Flow cytometric analysis of bronchoalveolar lavage fluid (BALF)
Collection and preparation of BALF
To collect BALF, the lungs were first perfused with 10 ml PBS as previously described. The trachea was then exposed and a catheter was inserted and tied off with a suture. A syringe was attached to the catheter, and 1 ml PBS was injected into the lungs and subsequently removed. This process was repeated until 3 ml of BALF was collected. The pooled BALF was centrifuged for 5 min at 1500 rpm, the supernatant discarded, and the cell pellet resuspended and washed in 1 ml FACS buffer (PBS, 5% fetal bovine serum, and 0.05% sodium azide). The washed pellet was then re-suspended in FACS buffer and total cell numbers quantified via a hemocytometer.
Differential cell staining
All reagents were obtained from BD Biosciences (San Jose, CA) unless otherwise specified. One half of the cells (0.5 ml) were stained after first blocking using FACS buffer containing 10% rat serum and 2.5 µl of Fc-receptor blocking antibody (clone 2.4G2) to prevent non-specific binding of the subsequent fluorochrome-conjugated antibodies. After this step, the following antibodies were added at manufacturer recommended concentrations: rat anti-mouse Ly-6G [FITC], rat anti-mouse Siglec-F [PE], pan-leukocyte rat anti-mouse CD45 [PerCP], and rat anti-mouse CD11c [APC]. After staining for 30 min on ice in the dark, the cells were washed with FACS buffer and then fixed with BD Cytofix for 10 min. The cells were then re-washed and then re-suspended in FACS buffer for flow cytometry. Populations of cells were evaluated by flow cytometric analysis in a BD LSRII system (BD Biosciences). A minimum of 50,000 events per sample was acquired for analyses. Neutrophils were defined as CD45hiLy-6GhiCD11clow, eosinophils as Ly-6GlowSiglecFhiCD11clow, and alveolar macrophages as Ly-6GlowSiglecFhi-CD11chi, as previously reported (Stevens et al., Citation2007). Total numbers of each cell population were obtained by multiplying the frequency of a specific population by the total number of BALF cells recovered for each animal.
Intracellular cytokine staining
In a separate tube, BALF T-cells were quantified using rat anti-mouse CD8 [FITC] and CD4 [PerCP] antibodies. T-Cell cytokine production was determined by fluorescent intracellular cytokine staining (ICS), as previously described (Foster et al., Citation2007). Briefly, the BALF suspension was centrifuged for 5 min at 1500 rpm and washed in 1 ml RPMI 1640 complete medium (GIBCO, Grand Island, NY). The supernatant was discarded and a solution of Leukocyte Activation Cocktail with GolgiPlug in 0.2 ml complete medium was added to each sample for stimulation of cytokine production and simultaneous inhibition of cytokine secretion. Cells were incubated at 37 °C in 5% CO2 for 4 h. After incubation, the cells were washed in FACS buffer and stained for flow cytometry using rat anti-mouse CD4 [PerCP] and rat anti-mouse CD8 [FITC] on ice in the dark for 30 min. Cells were then washed in FACS buffer and centrifuged, and the cell pellets were re-suspended in BD Cytofix/Cytoperm for 10 min to allow for fixation and permeabilization required for subsequent ICS. Cells were washed with 1 ml of BD PermWash, and resuspended in PermWash. Each sample was divided equally into two tubes and stained with rat anti-mouse interferon (IFN)-γ [APC], rat anti-mouse tumor necrosis factor (TNF)-α [PE-Cy7], and rat anti-mouse interleukin (IL)-17 [PE], or with control isotype antibodies (eBioscience, San Diego, CA). Cell populations were analyzed on the BD LSRII system, with lymphocytes gated on the basis of low forward and side scatter, then subsequently gated on CD4+ or CD8+ populations to determine intracellular expression of cytokines. A minimum of 50,000 events per sample were acquired for analyses.
Data analysis methods
Analysis of flow cytometric samples was performed with FlowJo software (TreeStar, Ashland, OR). GraphPad Prism was used for generation of graphs and statistical analysis for clearance and conidial germination experiments (GraphPad Software, La Jolla, CA). The data were analyzed via two way analysis of variance (ANOVA) followed by Bonferroni post-test. Statistical analysis of intracellular cytokine producing cells was performed using SAS version 9.2 for Windows (SAS, Cary, NC). The data were log- transformed prior to analysis and ProcMix was used to run a one-way analysis of variance (ANOVA) with ‘experiment’ included as a random factor to measure statistical significance. Differences between experimental groups that resulted in a p value ≤0.05 were considered significant.
Results
MALDI mass spectra of melanin-deficient conidia are the same as wild-type A. fumigatus
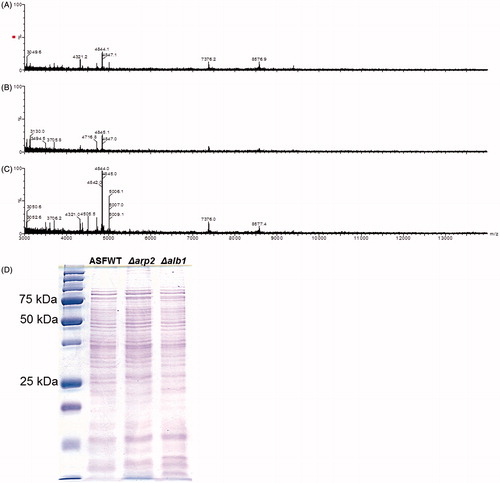
Although the genetic alterations in the melanin pathways of the strains used in this study have been previously characterized, it remains unknown if the gene deletions impacted any other proteins that could potentially alter the immune responses to these conidia. Therefore, the MALDI ‘fingerprint’ mass spectra of extracts from conidia of each strain were examined (Hettick et al., Citation2008). We previously demonstrated the utility of mass spectrometry to ‘fingerprint’ fungi for Aspergillus species and strain-specific discrimination. show the +MALDI qTOF MS fingerprint mass spectra for the different A. fumigatus strains. The mass spectra show the presence of multiple peptide/protein peaks, with prominent peaks at 4840, 7875, and 8575 u. These mass spectra are similar to the A. fumigatus spectral fingerprint previously reported (Hettick et al., Citation2008). The peak heights of protein/peptide signals in the Δalb1 mutant strain spectrum were greater than those of the WT and Δarp2 spectra (Supplemental Figures 1A–1C), an outcome consistent with previous observations that fungal-derived pigments suppress desorption/ionization processes during MALDI-TOF MS analyses (Buskirk et al., Citation2011).
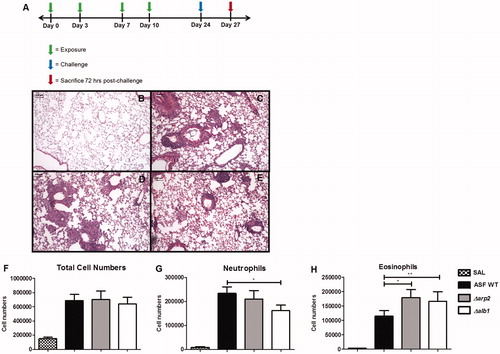
Figure 2. Exposure schedule and characterization of lung inflammation in repeatedly-exposed mice. (A) Exposure schedule, and representative H&E-stained lung sections from exposures to (B) saline only, (C) WT conidia, (D) Δarp2 conidia, or (E) Δalb1 conidia. Graphs indicate total polymorphonuclear cells in BALF from exposed mice. Total: (F) cell numbers; (G) neutrophils; and (H) eosinophils. Data are presented as mean (±SE) of four independent experiments. n = 20 mice/group. SAL, saline only exposures. Statistical differences indicated by asterisks (*p ≤ 0.02, **p ≤ 0.05), as determined by one-way ANOVA.
In addition to high resolution +MALDI qTOF MS analysis of the <15 kDa mass range, SDS PAGE was also performed to identify potential differences between high molecular weight proteins of the different strains. As illustrated in , the protein patterns of equally loaded melanin-deficient mutant conidial extracts were similar to that observed for WT A. fumigatus. However, the protein profiles for each of the strains contained observable differences, primarily in the density of some bands that were < 30 kDa. Despite these minor differences, the results suggest that global protein synthesis was not considerably altered by the gene deletions in the melanin-deficient conidia, and therefore should not significantly impact the immune responses following exposures.
Melanin-deficient conidia retain the Rodlet A layer
The interaction of fungi with immune cells occurs through the interaction of pattern recognition receptors (PRR) on host immune cells and the pathogen-associated molecular patterns (PAMP) on fungal conidia and hyphae. Non-germinated A. fumigatus conidia are known to contain a hydrophobic protein layer termed the Rodlet A (RodA) layer. This layer is immunologically inert and has been shown to protect the conidia from innate immune recognition by masking various PAMP and preventing recognition by the innate immune system (Aimanianda et al., Citation2009). Therefore, it was necessary to determine if the melanin-deficient conidia retained the RodA layer to ensure that any differences in the immune responses were not due to the differential accessibility of immunostimulatory cell wall components masked by the RodA layer. Field emission scanning electron microscopy () showed the surface of the albino (Δalb1) conidia appeared smoother than that of WT or Δarp2; however, this melanin mutant strain retained a RodA layer similar to that observed in WT conidia. Taken together with the mass spectrometry data and SDS-PAGE analysis, these data suggest that these strains differ primarily in melanin content.
Figure 3. +MALDI qTOF MS fingerprint mass spectra. (A) A. fumigatus WT. (B) Δarp2. (C) Δalb1. Spectra are representative of three independent fungal cultures. Spectra are presented on a fixed y-axis (% relative abundance) and optimized between the m/z range of 3000–14000 u. (D) SDS page banding pattern of conidial extracts.
Repeated exposures to A. fumigatus DHN-melanin-deficient conidia results in similar pathological inflammation compared to wild-type conidia
We then sought to determine if there was a difference in murine inflammatory response following repeated exposures to WT, Δarp2, or Δalb1 mutants in immunocompetent mice. Mice were repeatedly exposed to conidia via pharyngeal aspiration (). Moderate-to-severe inflammation and airway remodeling, resembling hypersensitivity pneumonitis, were evident in all mice regardless of the A. fumigatus strain (). The extensive granuloma formation, mucus production, bronchoalveolar lymphoid tissue (BALT) induction, and goblet cell hyperplasia were histologically similar between the three exposure groups.
Melanin-deficient conidial exposures result in different polymorphonuclear leukocyte responses
Airway cellularity after exposures was examined in BALF by flow cytometry. Total cell numbers were comparable between exposure groups; however, Δalb1 conidia exposures induced fewer neutrophils (). There was a concomitant increase in eosinophils in this group of animals (). Interestingly, eosinophils were also significantly increased in mice exposed to Δarp2 conidia when compared to WT-exposed mice ().
CD8+ IL-17+ T-cells are elevated in lungs of mice exposed to Δalb1 DHN-melanin-deficient conidia
To determine if there were differences in T-cell-mediated responses between melanin-mutant strains, BALF was analyzed for T-cells and intracellular cytokine staining using flow cytometry. A decreasing trend in CD8+ T-cell numbers that correlated with a decrease in melanin production was observed. However, there was no significant difference in CD4+ or CD8+ T-cell numbers (). Additionally, there was no significant difference in CD4+ T-cell cytokine staining of IFNγ and IL-17 (). An increase was observed in CD4+ TNFα staining, although this result was not statistically significant ().
Figure 4. T-cell cytokine production following multiple aspirations. CD4+ and CD8+ T-cell cytokine production in the BALF of mice exposed to WT, Δarp2, or Δalb1 conidia. CD4+ and CD8+ T-cells, CD4+ and CD8+ IFNγ+ cells. Data are presented as mean (±SE) of four independent experiments. n = 20. CD4+ and CD8+ IL-17+ and TNFα+, n = 10 mice/group. T-cells that were: (A) CD4+; (B) CD4+ IFNγ+; (C) CD4+ IL-17+; (D) CD4+ TNFα+; (E) CD8+; (F) CD8+ IFNγ+; (G) CD8+ IL-17+; and (H) CD8+ TNFα+. Statistical differences are indicated by asterisks (*p ≤ 0.01) as determined by one-way ANOVA.
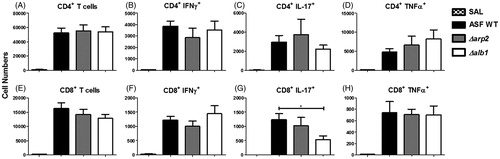
CD8+ T-cell IFNγ staining was slightly elevated, but not statistically significant, in mice exposed to Δalb1 conidia (). Interestingly, there was a significant decrease in the Δalb1 induced CD8+IL17+ (Tc17) cell population when compared to both WT and Δarp2 exposure groups (). CD8+ TNFα staining was consistent between exposure groups ().
Melanin-deficient conidia are cleared more rapidly from the lungs of both sensitized and non-sensitized mice
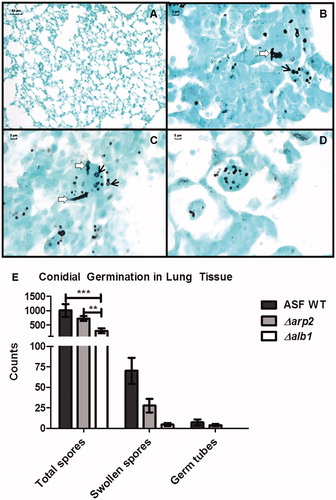
Histological examination of samples from repeatedly aspirated mice demonstrated that a larger number of WT conidia remained intracellular in the lungs of mice at the time of sacrifice than in mice exposed to the melanin mutant conidia ( and Supplementary Table 1). Additionally, there were greater numbers of swollen conidia in the WT (7% swollen conidia) and Δarp2-exposed mice (3.8%) than in Δalb1-exposed mice (1.5%). A higher frequency of germ tube formation was also observed in mice exposed to WT and Δarp2 conidia. No germ tubes were identified in any lung sections of the mice exposed to Δalb1 conidia (). These results were not due to differential viability, as each fungal strain exhibited similar viability prior to aspiration (data not shown).
Figure 5. Conidial germination in the lung tissue of mice repeatedly exposed as indicated in . GMS stained lung section following exposures to (A) saline only, (B) WT conidia, (C) Δarp2 conidia, and (D) Δalb1 conidia. Black arrows indicate swollen conidia (2–3× the size of resting conidia). White arrows indicate the formation of germ tubes. (E) Quantification of total conidia, swollen conidia, or germ tube formation in WT-, Δarp2-, and Δalb1-exposed mice. n = 4 mice for WT and Δalb1. n = 5 mice for Δarp2. Statistical significance is indicated by asterisks (***p < 0.0001, **p < 0.01), as determined by two-way ANOVA followed by a Bonferroni post-test.
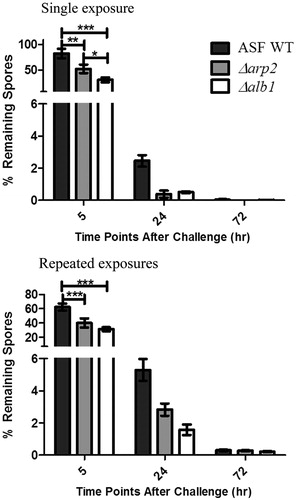
To determine the lung clearance kinetics of each strain, we compared mice that were aspirated a single time (innate immunity—four exposures to saline only and challenged with conidia) to mice repeatedly aspirated (adaptive immunity—exposed as indicated in ). The number of WT conidia that remained in the lung was significantly greater than Δarp2 and Δalb1 conidia at 5 h post-final exposure in both single and repeated exposure mice (). By 24 h post-final exposure, >94% of conidia were cleared in both single and multiple exposure mice despite the presence or absence of melanin (). By 72 h, >99% of the fungal conidia were removed from all mice, irrespective of melanin content. Interestingly, there were greater numbers of conidia remaining in the lungs of mice that repeatedly aspirated conidia at the 24 and 72 h timepoints compared to single exposure mice. This result was not dependent on the presence of melanin in the fungal conidia.
Figure 6. Rate of conidial clearance in exposed mice. Mice were exposed to WT, Δarp2, or Δalb1 conidia. Left panel shows clearance following a single conidial exposure in mice mock-exposed to saline 4-times and then challenged with the indicated fungal strain. Right panel shows adaptive clearance in mice repeatedly exposed to conidia, as indicated in . Data are presented as the average ±standard error of measure of two independent experiments. n = 10 mice/group. Data from each strain were normalized to the total amount of conidia obtained from mice sacrificed immediately after exposure. n = 5 mice/group. Statistical significance is indicated by asterisks (***p < 0.0001, **p < 0.001, and *p < 0.05), as determined by two-way ANOVA followed by a Bonferroni post-test.
Discussion
Aspergillus fumigatus is responsible for a wide spectrum of human illnesses ranging from allergic rhinitis to invasive aspergillosis. The fungal-specific factors that contribute to airway immune responses, evasion of immune recognition, and induction of allergic responses have not yet been completely elucidated. While it is known that fungal proteases are common allergens, additional components that may lead to fungal allergic sensitization are less understood (Lamhamedi-Cherradi et al., Citation2008; Latge, Citation1999; Robinson et al., Citation1990). Fungal melanin has been previously shown to be an important virulence factor in invasive aspergillosis mouse models, yet there is limited information on the impact of melanin in an immunocompetent animal model (Langfelder et al., Citation1998; Tsai et al., Citation1998). Tsai et al. used an immunocompromised mouse model to show that a single exposure to albino mutant conidia exhibited decreased virulence, with only 0–10% mortality by Day 21 post-infection compared to 70–80% mortality by Day 7 post-infection with wild-type (WT) conidia. By genetically reconstituting the albino mutant, toxicity could be restored to the WT mortality of 80–100% by Day 7. The present study aimed to determine the impact of A. fumigatus pigmentation on the pulmonary immune response in immunocompetent mice following repeated pharyngeal aspiration exposures. Multiple exposures were used to mimic repeated human fungal exposures that may lead to allergy, asthma, and/or hypersensitivity pneumonitis induction (Eduard, Citation2009).
Two A. fumigatus mutant strains with alterations in their melanin synthetic pathways were used in addition to the WT strain to examine the impact of fungal pigmentation on the pulmonary immune response. While the WT conidia are melanized, the Δarp2 mutant exhibits light brown coloration, and the Δalb1 conidia lack pigmentation and appear white. Previous studies have shown that the melanin mutant conidia differ from WT conidia primarily in melanin content and the smoothness of the outer cell wall surface (Jahn et al., Citation1997; Tsai et al., Citation1998). Our FESEM results confirmed that Δalb1 conidia have smooth outer cell wall morphology. While there were observable differences in the density of some SDS-page protein bands less than 30 kDa, there did not appear to be significant differences in the protein profile of melanin mutant conidia compared to WT. Using a previously reported mass spectrometry method to ‘fingerprint’ fungi (Hettick et al., Citation2008), we were able to demonstrate similar proteomic signatures of these three strains. Based on these observations, the single gene deletions in the mutant conidia do not appear to significantly alter the protein profile of the mutant strains.
Structurally, A. fumigatus conidial walls are covered with a hydrophobic rodlet protein layer composed of RodA and RodB proteins with melanin polymers intercalated throughout the underlying spore wall (Paris et al., Citation2003). Together, these layers provide the conidia its structural rigidity. Importantly, the rodlet layer is thought to protect the conidia from innate immune recognition by pattern recognition receptors (PRR). Previous reports have shown that in vitro exposure of primary dendritic cells and macrophages to the RodA protein did not induce cellular maturation/activation. RodA did not stimulate production of inflammatory cytokines, antibody, or protect from infection in an invasive aspergillosis model. However, ΔRodA mutant conidia or swollen WT conidia were more inflammatory and capable of activating innate immune cells (Aimanianda et al., Citation2009).
Previous studies have reported contradicting results concerning the presence of RodA layer in melanin-deficient A. fumigatus conidia (Jahn et al., Citation1997; Pihet et al., Citation2009; Thywissen et al., Citation2011). Pihet et al. used atomic force microscopy and reported the absence of a rodlet layer on naturally isolated A. fumigatus melanin mutant conidia. Others have examined the surface of laboratory-derived albino conidia with scanning electron microscopy and reported the RodA layer remains intact in albino conidia (Jahn et al., Citation1997; Thywissen et al., Citation2011). Similarly, we observed that the rodlet layer was intact in the melanin mutant strains and appeared in highly organized tight bundles.
With repeated exposure to fungal conidia, we found similar levels of inflammation, granuloma formation, BALT induction, goblet cell hyperplasia, and airway remodeling in each exposure group. However, the melanin mutant conidia stimulated greater numbers of eosinophils in the airways that suggested a shift in the type of immune response. Previously, eotaxin-2 and IL-5 were demonstrated to cooperatively regulate airway eosinophilia in the lungs (Ochkur et al., Citation2007; Yang et al., Citation2003). Although these factors were not measured in the current study, their role cannot be ruled out. Interestingly, we could only detect a weak A. fumigatus specific serum anti-body response (data not shown). Additional studies are needed to determine the fungal-specific factors, which may impact allergic sensitization, or the mechanisms of pulmonary tolerance in response to A. fumigatus. However, it is apparent that the presence of melanin does not significantly impact pathological inflammation in the lungs.
It has been previously reported that a T-helper (TH)-1 response, consisting of CD4+ T-cells and IFNγ, is necessary for the efficient clearance of fungal conidia (Latge, Citation1999; Rivera et al., Citation2006). Therefore, the present study evaluated the T-cells to determine the type of response occurs following repeated A. fumigatus exposures. While TNFα and IFNγ have been extensively characterized and known to be required for protective immunity against fungi, IL-17 has recently been recognized as important for fungal immunity (Wuthrich et al., Citation2012). IL-17 is associated with chronic inflammation, autoimmune disorders, and allergy, and is known to aid in recruitment and subsequent activation of neutrophils and macrophages to the site of inflammation (Korn et al., Citation2009; Souwer et al., Citation2010). In the present study, the levels of CD4+ IFNγ+, TNFα+, or IL-17+ (TH17) cells were all increased, yet comparable between the WT-, Δarp2-, and Δalb1-exposed mice. These data further demonstrate that the extent of melanization does not appear to affect these cell populations.
Less is known about the roles of CD8+ T-cells in immune responses to fungi. Previously, CD8+ T-cell responses were shown to be partly dependent on conidial germination (Carvalho et al., Citation2012; Templeton et al., Citation2011). While we did not observe changes in CD8+IFNγ+ cell recruitment, CD8+IL17+ (Tc17) cells were significantly reduced in mice exposed to Δalb1 conidia compared to WT. These results have not been previously shown in models of A. fumigatus fungal exposures and may indicate a novel function for Tc17 cells in the immune responses to filamentous fungi; however, further experiments are required to confirm the role of these cells. Tc17 cells are a unique sub-set of CD8+ T-cells associated with anti-viral immunity (viral clearance), pulmonary inflammatory responses, systemic lupus erythematosus, control of tumor growth, and contact dermatitis (Garcia-Hernandez Mde et al., Citation2010; Hamada et al., Citation2009; Henriques et al., Citation2010; Yeh et al., Citation2010; Zhao et al., Citation2009). Tc17 cells also demonstrate functional plasticity, and are reported to produce pro-inflammatory cytokines and chemokines responsible for the enhanced early recruitment of macrophages, natural killer cells, and neutrophils (Garcia-Hernandez Mde et al., Citation2010; Hamada et al., Citation2009; Yen et al., Citation2009). The decrease in Tc17 cells in the Δalb1 conidia exposed mice correlated with the significant decrease in neutrophils. It is possible that the decrease in this cytokine in Δalb1-exposed mice is related to the later timepoint (72 h post-challenge) examined in these studies. Further experiments to examine the kinetics of IL-17 induction will be critical in determining its role in A. fumigatus-mediated immune responses and the mechanisms associated with its regulation.
Although the results reported in the current study have been observed in previous murine studies of A. fumigatus exposure using C57BL/6J mice (Murdock et al., Citation2012), correlations between eosinophil recruitment to the lungs and IL-17 production were not identified in the present study. These differences may be due to genetic variations between C57BL/6J and BALB/c mice as previous BALB/c murine models have shown that IL-17 depletion resulted in the increase of pulmonary eosinophilia (Hellings et al., Citation2003). Other variables including the method of conidial delivery and exposure interval may also play a role in these reported differences. Future experiments will aim to fully characterize the influence of IL-17 expressing cell types on inflammatory cell recruitment in response to A. fumigatus exposures.
In immunocompromised models, A. fumigatus albino mutants are more rapidly phagocytosed and degraded than WT spores (Jahn et al., Citation1997; Langfelder et al., Citation1998; Tsai et al., Citation1998). We confirmed that, after a single exposure, the clearance of melanin mutant conidia is also more rapid in immunocompetent mice. The ability to efficiently clear conidia appeared to be hindered in mice after repeated aspiration, irrespective of melanin content. In a study by Murdock et al. (Citation2012), it was reported that repeated exposures did not enhance conidial clearance. It may be possible that repeated exposures results in the induction of a tolerance response to lessen the extent of inflammation, and subsequent tissue injury over time. The presence of IL-17 has also been shown to inhibit A. fumigatus clearance (Nembrini et al., Citation2009; Werner et al., Citation2009; Zelante et al., Citation2009). In accordance with these studies, our decreased clearance results may correlate with the decreased Tc17 and airway neutrophil recruitment in response to Δalb1 conidia. Future experiments to examine IL-10 and IL-17 secretion following repeated exposures to melanin-deficient conidia would aid in determining the mechanisms affected by the presence or absence of melanin.
The retention of WT conidia observed in our experimental model further validates previous studies that have shown WT A. fumigatus conidia to contain factors that inhibit phagocytosis. Melanin can quench ROS produced by phagocytic cells as well as inhibit complement protein C3 binding to the surface of the conidia (Jahn et al., Citation1997, Citation2000; Tsai et al., Citation1997). The presence of melanin in A. fumigatus also prevents host cell apoptosis and phagolysosome acidification, thereby protecting the conidia from release into the extracellular environment for subsequent phagocytosis and acidic degradation within the phagocyte (Thywissen et al., Citation2011).
In summary, the current studies showed that melanin in A. fumigatus conidia protect the conidia from rapid clearance, modifies airway immune responses, and yet does not appear to have a noticeable effect on inflammation. Although melanin has been shown to be an important virulence factor in invasive disease models, it appears to be less significant in an immunocompetent model. This is likely due to the efficiency of the innate immune system to clear fungal conidia within 72 h, regardless of melanin content. However, this is the first report to illustrate the presence Tc17 cells within the lungs in response to A. fumigatus exposures, thereby suggesting a potential role for these cells in the immune response to filamentous fungi.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported in part by an interagency agreement with the National Institute of Environmental Health Sciences (CDC IAA#12-NS12-01).
Supplementary Material
Download PDF (115 KB)Acknowledgements
The authors wish to thank Dr June Kwon-Chung (NIAID, Bethesda, MD) for providing the Aspergillus fumigatus fungal strains used in this study. The authors also wish to thank Diane Schwegler-Berry for help with the preparation and analysis of FESEM samples, Michael Kashon for statistical advice, and Angela Rae Lemons for reviewing the content of this manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Agarwal, R. A., Aggarwal, A. N., Gupta, D., and Jindal, S. K. (2009). Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with bronchial asthma: Systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 13:936–944
- Aimanianda, V., Bayry, J., Bozza, S., et al. (2009). Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460:1117–1121
- Brakhage, A. A., Langfelder, K., Wanner, G., et al. (1999). Pigment biosynthesis and virulence. Contrib. Microbiol. 2:205–215
- Buskirk, A. D., Hettick, J. M., Chipinda, I., et al. (2011). Fungal pigments inhibit the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis of darkly pigmented fungi. Anal. Biochem. 411:122–128
- Carvalho, A., De Luca, A., Bozza, S., et al. (2012). TLR3 essentially promotes protective class I-restricted memory CD8+ T-cell responses to Aspergillus fumigatus in hematopoietic transplanted patients. Blood 119:967–977
- Chai, L. Y., Netea, M. G., Sugui., J., et al. (2010). Aspergillus fumigatus conidial melanin modulates host cytokine response. Immunobiology 215:915–920
- Chaudhary, N., and Marr, K. A. (2011). Impact of Aspergillus fumigatus in allergic airway diseases. Clin. Transl. Allergy 1:4
- Denning, D. W. (1998). Invasive aspergillosis. Clin. Infect. Dis. 26:781–803
- Devereux, G. (2006). The increase in the prevalence of asthma and allergy: Food for thought. Nat. Rev. Immunol. 6:869–874
- Dixon, D. M., Polak, A., and Szaniszlo, P. J. (1987). Pathogenicity and virulence of wild-type and melanin-deficient Wangiella dermatitidis. J. Med. Vet. Mycol. 25:97–106
- Eduard, W. (2009). Fungal spores: A critical review of the toxicological and epidemiological evidence as a basis for occupational exposure limit setting. Crit. Rev. Toxicol. 39:799–864
- Foster, B., Prussin, C., Liu, F., et al. (2007). Detection of intracellular cytokines by flow cytometry. Curr. Protoc. Immunol. 78:6.24.1–6.24.21
- Garcia-Hernandez Mde, L., Hamada, H., Reome, J. B., et al. (2010). Adoptive transfer of tumor-specific Tc17 effector T-cells controls the growth of B16 melanoma in mice. J. Immunol. 184:4215–4227
- Hamada, H., Garcia-Hernandez Mde, L., Reome, J. B., et al. (2009). Tc17, a unique subset of CD8 T-cells that can protect against lethal influenza challenge. J. Immunol. 182:3469–3481
- Hellings, P. W., Kasran, A., Liu, Z., et al. (2003). IL-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am. J. Respir. Cell Mol. Biol. 28:42–50
- Henriques, A., Ines, L., Couto, M., et al. (2010). Frequency and functional activity of TH17, Tc17 and other T-cell subsets in Systemic Lupus Erythematosus. Cell. Immunol. 264:97–103
- Hettick, J. M., Green, B. J., Buskirk, A. D., et al. (2008). Discrimination of Aspergillus isolates at the species and strain level by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry fingerprinting. Anal. Biochem. 380:276–281
- Huffnagle, G. B., Chen, G. H., Curtis, J. L., et al. (1995). Down-regulation of the afferent phase of T-cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J. Immunol. 155:3507–3516
- Inoue, K., Koike, E., Yanagisawa, R., et al. (2009). Pulmonary exposure to soluble cell wall β-(1,3)-glucan of Aspergillus induces pro-inflamatory response in mice. Int. J. Immunopathol. Pharmacol. 22:287–297
- Jacobson, E. S. (2000). Pathogenic roles for fungal melanins. Clin. Microbiol. Rev. 13:708–717
- Jahn, B., Boukhallouk, F., Lotz, J., et al. (2000). Interaction of human phagocytes with pigmentless Aspergillus conidia. Infect. Immun. 68:3736–3739
- Jahn, B., Koch, A., Schmidt, A., et al. (1997). Isolation and characterization of a pigmentless-conidium mutant of Aspergillus fumigatus with altered conidial surface and reduced virulence. Infect. Immun. 65:5110–5117
- Korn, T., Bettelli, E., Oukka, M., and Kuchroo, V. K. (2009). IL-17 and TH17 cells. Annu. Rev. Immunol. 27:485–517
- Kwon-Chung, K. J., Polacheck, I., and Popkin, T. J. (1982). Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J. Bacteriol. 150:1414–1421
- Lamhamedi-Cherradi, S. E., Martin, R. E., Ito, T., et al. (2008). Fungal proteases induce TH2 polarization through limited dendritic cell maturation and reduced production of IL-12. J. Immunol. 180:6000–6009
- Langfelder, K., Jahn, B., Gehringer, H., et al. (1998). Identification of a polyketide synthase gene (pksP) of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence. Med. Microbiol. Immunol. 187:79–89
- Latge, J. P. (1999). Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310–350
- Latge, J. P. (2001). The pathobiology of Aspergillus fumigatus. Trends Microbiol. 9:382–389
- Murdock, B. J., Falkowski, N. R., Shreiner, A. B., et al. (2012). IL-17 drives pulmonary eosinophilia following repeated exposure to Aspergillus fumigatus conidia. Infect. Immun. 80:1424–1436
- Nembrini, C., Marsland, B. J., and Kopf, M. (2009). IL-17-producing T-cells in lung immunity and inflammation. J. Allergy Clin. Immunol. 123:986–994
- Ochkur, S. I., Jacobsen, E. A., Protheroe, C. A., et al. (2007). Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflamation with characteristics of severe asthma. J. Immunol. 178:7879–7889
- Paris, S., Debeaupuis, J. P., Crameri, R., et al. (2003). Conidial hydrophobins of Aspergillus fumigatus. Appl. Environ. Microbiol. 69:1581–1588
- Pihet, M., Vandeputte, P., Tronchin, G., et al. (2009). Melanin is an essential component for the integrity of the cell wall of Aspergillus fumigatus conidia. BMC Microbiol. 9:177
- Rao, G. V., Tinkle, S., Weissman, D. N., et al. (2003). Efficacy of a technique for exposing the mouse lung to particles aspirated from the pharynx. J. Toxicol. Environ. Health 66:1441–1452
- Rivera, A., Ro, G., van Epps, H. L., et al. (2006). Innate immune activation and CD4+ T-cell priming during respiratory fungal infection. Immunity 25:665–675
- Robinson, B. W., Venaille, T. J., Mendis, A. H., and McAleer, R. (1990). Allergens as proteases: An Aspergillus fumigatus proteinase directly induces human epithelial cell detachment. J. Allergy Clin. Immunol. 86:726–731
- Simon-Nobbe, B., Denk, U., Poll, V., et al. (2008). The spectrum of fungal allergy. Int. Arch. Allergy Immunol. 145:58–86
- Souwer, Y., Szegedi, K., Kapsenberg, M. L., and de Jong, E. C. (2010). IL-17 and IL-22 in atopic allergic disease. Curr. Opin. Immunol. 22:821–826
- Stevens, W. W., Kim, T. S., Pujanauski, L. M., Hao, X., and Braciale, T. J. (2007). Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J. Immunol. Meth. 327:63–74
- Templeton, S. P., Buskirk, A. D., Law, B., et al. (2011). Role of germination in murine airway CD8+ T-cell responses to Aspergillus conidia. PLoS One 6:e18777
- Thywissen, A., Heinekamp, T., Dahse, H. M., et al. (2011). Conidial dihydroxynaphthalene melanin of the human pathogenic fungus Aspergillus fumigatus interferes with the host endocytosis pathway. Front. Microbiol. 2:96
- Tsai, H. F., Chang, Y. C., Washburn, R. G., et al. (1998). The developmentally regulated alb1 gene of Aspergillus fumigatus: Its role in modulation of conidial morphology and virulence. J. Bacteriol. 180:3031–3038
- Tsai, H. F., Washburn, R. G., Chang, Y. C., and Kwon-Chung, K. J. (1997). Aspergillus fumigatus arp1 modulates conidial pigmentation and complement deposition. Mol. Microbiol. 26:175–183
- Tsai, H. F., Wheeler, M. H., Chang, Y. C., and Kwon-Chung, K. J. (1999). A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 181:6469–6477
- Volling, K., Thywissen, A., Brakhage, A. A., and Saluz, H. P. (2011). Phagocytosis of melanized Aspergillus conidia by macrophages exerts cytoprotective effects by sustained PI3K/Akt signalling. Cell. Microbiol. 13:1130–1148
- Werner, J. L., Metz, A. E., Horn, D., et al. (2009). Requisite role for the dectin-1 β-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 182:4938–4946
- Wheeler, M. H., and Bell, A. A. (1988). Melanins and their importance in pathogenic fungi. Curr. Top. Med. Mycol. 2:338–287
- Wuthrich, M., Deepe, G. S. Jr., and Klein, B. (2012). Adaptive immunity to fungi. Annu. Rev. Immunol. 30:115–148
- Yang, M., Hogan, S. P., Mahalingam, S., et al. (2003). Eotaxin-2 and IL-5 cooperate in the lung to regulate IL-13 production and airway eosinophilia and hyper-reactivity. J. Allergy. Clin. Immunol. 112:935–943
- Yeh, N., Glosson, N. L., Wang, N., et al. (2010). Tc17 cells are capable of mediating immunity to vaccinia virus by acquisition of a cytotoxic phenotype. J. Immunol. 185:2089–2098
- Yen, H. R., Harris, T. J., Wada, S., et al. (2009). Tc17 CD8 T-cells: Functional plasticity and subset diversity. J. Immunol. 183:7161–7168
- Zelante, T., De Luca, A., D'Angelo, C., et al. (2009). IL-17/TH17 in anti-fungal immunity: What's new? Eur. J. Immunol. 39:645–648
- Zhao, Y., Balato, A., Fishelevich, R., et al. (2009). TH17/Tc17 infiltration and associated cytokine gene expression in elicitation phase of allergic contact dermatitis. Br. J. Dermatol. 161:1301–1306
