Abstract
The toxic effects of highly carcinogenic mycotoxins, especially aflatoxins (AF), on key antigen-presenting cells, such as dendritic cells (DC), are largely unknown. To elucidate the effect of AF on DC function, porcine monocyte-derived DC (MoDC) were treated with a mixture of several AF (i.e., AFB1, AFB2, AFG1, and AFG2) and the phagocytic capacity, the membrane expression level of several DC activation markers, the T-cell proliferation-inducing capacity, and the cytokine secretion pattern were assessed. As compared to untreated MoDC, AF significantly up-regulated the expression of the co-stimulatory molecules CD25 and CD80/86. However, the phagocytic activity of MoDC was not affected by AF treatment. While the cytokine secretion pattern of AF-treated MoDC was similar to control MoDC, the T-cell proliferation-inducing capacity of MoDC was increased upon aflatoxin treatment. The results indicate that a mixture of naturally occurring AF enhances the antigen-presenting capacity of DC, which could explain the observed immunotoxicity of AF by breaking down tolerance and further emphasizes the need to reduce the admissible level of AF in agricultural commodities.
Introduction
Even in our super-sanitized world, many potential carcinogenic mycotoxins are present, in particular aflatoxins (AF) (Reddy et al., Citation2009; Wild & Turner, Citation2002). These potent carcinogens are mainly produced by Aspergillus sp., and frequently contaminate many agricultural products, such as corn and cereals. The consumption of AF-contaminated food/feed affects animal and public health. AF are classified into four types based on structure: AFB1, AFB2, AFG1, and AFG2 (), all of which are concomitantly present on Aspergillus-infested crops. Although the exact proportion of AFB1, -B2, -G1, and -G2 in Aspergillus-contaminated feed and foods is unclear, these molds predominantly produce AFB1 and to a lesser extent the other three AF in feed (Cervino et al., Citation2008; Joubrane et al., Citation2011; Li et al., Citation2009). AFB1 is the most immune-disruptive aflatoxin, as a lower bioavailability and less toxic nature for AFB2, -G1, and -G2 have been reported (Cusumano et al., Citation1990; Hoogenboom et al., Citation2001; Mehrzad et al., Citation2011). Although the occurrence of AF-contaminated food/feed has decreased substantially in more affluent, but not in developing, countries due to the presence of extensive monitoring programs, the contamination of agricultural commodities by AF-producing fungi nevertheless remains a serious threat for animal and public health (Martins et al., Citation2007; Hernández Hierro et al., Citation2008; Pukkala et al., Citation2009).
Figure 1. Chemical structures of the four tested aflatoxins (AF). Note that AFB2 and AFG2 are derivatives of AFB1 and AFG1, respectively. OMe = methoxy.
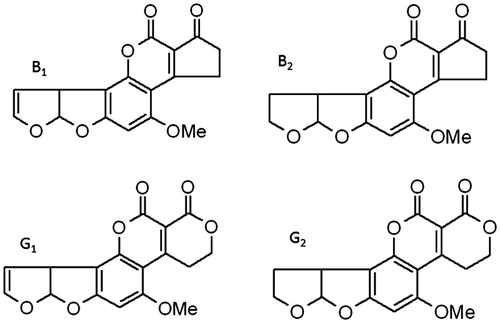
Besides their known carcinogenicity, AF cause immunosuppression in domestic animals and humans by impairing the innate and cell-mediated immunity (Chaytor et al., Citation2011; Gong et al., Citation2004; Luongo et al., Citation2013). Both cytolysis by natural killer (NK) cells and several macrophage functions, including phagocytosis, production of reactive oxygen species (ROS), and intracellular killing, are impaired by AF (Qureshi et al., Citation1998; Liu et al., Citation2002). In addition, AF dysregulate neutrophil functions in a bovine model, resulting in a decreased phagocytosis and intracellular ROS production (Mehrzad et al., Citation2011). Further, pathogen recognition is impaired in human leukocytes by AF treatment (Malvandi et al., Citation2013). Exposure of pigs to AF-contaminated feed increases their susceptibility to infection and reduces vaccine-induced protection, mainly through the dysregulation of T-cell polarization (Meissonnier et al., Citation2008; Venturini et al., Citation1996). This led to the assumption that AF interfere with the function of antigen-presenting cells, such as dendritic cells (DC), as these cells drive the polarization of naïve T-cells (Meissonnier et al., Citation2008). The knowledge concerning the impact of these AF on DC-T-cell co-operation and, thus, immune regulation is very limited.
DC link innate and acquired immunity and as such are pivotal in the induction of immune responses to control and eliminate pathogens (Joffre et al., Citation2009). These professional antigen-presenting cells (APC) are localized at peripheral tissues where they act as immune sentinels continuously patrolling and sampling environmental antigens (Huang et al., Citation2001; Joffre et al., Citation2009). Upon antigen encounter and processing, DC mature up-regulating MHC-II and co-stimulatory molecules to efficiently present antigen to naïve T-cells. Moreover, their cytokine secretion pattern dictates the polarization of naïve CD4+ T-cells into T-helper (TH)- 1, TH2, or TH17 effector cells or regulatory cells, which in turn drives the ensuing immune response against invading pathogens (Huang et al., Citation2001; Joffre et al., Citation2009). Little is known about the effect of mixed AF on DC function. To mimic the natural exposure of the immune system to AF, we prepared a mixture of naturally-occurring levels of AFB1, AFB2, AFG1, and AFG2 (mAF) and examined their impact on function of swine monocyte-derived DC (MoDC). Swine were used here, as pigs represent an important economically relevant animal model, whose immune system closely resembles that of humans (Dawson et al., Citation2013; Fairbairn et al., Citation2011; Meurens et al., Citation2012).
Materials and methods
Aflatoxins
AFB1, AFB2, AFG1, and AFG2 (mAF) were separately purchased from Sigma-Aldrich Chemie (Deisenhofen, Germany); all were Aspergillus-derived, >98% pure, and free of lipopolysaccharide and contaminant chemicals (according to supplier). Each was first separately dissolved in 96% ethanol (at 0.1 mg/ml) according to Mehrzad et al. (Citation2011). Further dilutions were then made with sterile, endotoxin-free Dulbecco’s phosphate-buffered saline (DPBS, pH 7.3; Gibco, Merelbeke, Belgium). Aliquots of the AFB1, AFB2, AFG1, and AFG2 solutions were then combined and 10 μl of this AF mixture added to the cell cultures to obtain final overall concentrations of each agent in the well of, respectively, 2.0, 1.0, 0.5 and 0.5 ng/ml. The mixture of these environmentally relevant levels of AF was chosen to mimic as closely as possible in vivo situations (see Discussion).
Generation of porcine monocyte-derived dendritic cells (MoDC)
Heparinized blood samples were obtained from the external jugular vein of three separate Belgian Landrace piglets (female, 8–20-weeks-old) kept as blood donors under standard conditions at the Faculty of Veterinary Medicine (Merelbeke, Belgium). Blood sampling was performed from each of the three piglets over the span of 1-month (i.e., 1 week between each host). All animal experiments were in accordance with the local animal welfare regulations and were approved by the Ethical Committee of the Faculty of Veterinary Medicine.
Peripheral blood mononuclear cells (PBMC) were isolated from each whole blood sample by lymphoprep density gradient centrifugation (Axis-Shield, Oslo, Norway). Monocytes were further enriched to a purity of >95% by immuno-magnetic bead selection (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) using anti-CD172a monoclonal antibody (mAb; clone 74-12-15a; Pescovitz et al., Citation1984) and goat anti-mouse microbeads together with LS separation columns (Miltenyi Biotec). The CD172a+ monocytes were subsequently cultured in 24-well plates (Nunc, Thermo Fisher Scientific, Langenselbold, Germany) at a density of 5.0 × 105 cells/ml in phenol-red free Dulbecco’s modified Eagle’s Medium (DMEM; Gibco), supplemented with 10% (v/v) fetal calf serum (FCS; Greiner, Wemmel, Belgium), 100 U/penicillin/ml (Gibco), 100 μg streptomycin/ml (Gibco), recombinant porcine (rp) GM-CSF (17.5 ng/ml; Inumaru et al., Citation1998) and rpIL-4 (5 ng/ml; R&D Systems, Minneapolis, MN), and then incubated at 37 °C in a humidified atmosphere at 5% CO2 to generate MoDC as previously described (Devriendt et al., Citation2010). On Day 3 of the culture period, MoDC were fed by addition of fresh medium supplemented with rpGM-CSF and rpIL-4 at the same concentrations. On Day 4 or 5 of the culture period, cells with long membrane protrusions, a typical feature of immature DC, dominated the cell culture.
Phagocytosis assay
Samples of immature MoDC isolated from each of the piglets were incubated with mAF for 1, 2, 12, and 24 h at 37 °C in the humidified 5% CO2 atmosphere in six separate wells; after the treatment period these wells were then pooled to collect the MoDC for phagocytosis assays. At the end of each respective period, 5.0 × 106 fluorescein isothiocyanate (FITC)-loaded polystyrene micro-particles (1.0 μm diameter; Sigma) were added to the stimulated MoDC (10 microparticles/DC) and the cells incubated for 3 h at 37 °C under 5% CO2. Subsequently, the cells were harvested on ice by flushing, washed with ice-cold PBS, and microparticle internalization was assessed by flow cytometry with a FACSCanto flow cytometer (BD Biosciences, Erembodegem, Belgium) with a minimum event count of 20 000/sample. For each sample, flow cytometric analyses of phagocytosis were performed by assessing the fluorescence intensity of 20 000 cells within the live, singlet cell gate. The mean fluorescent intensity (MFI) was calculated using FACSDiva 6.1.3 software (BD Biosciences). The results were presented as the percentage of phagocytosed microparticles relative to that in mock-stimulated MoDC (control MoDC). Relative phagocytic activity was calculated as 100 × (phagocytosis by AF-treated MoDC/phagocytosis by control [0 ng AF/ml] MoDC).
Cell surface expression of co-stimulatory molecules and activation markers
Cell surface expression of co-stimulatory molecules and activation markers upon stimulation were assessed in samples of each piglet’s MoDC with medium in six separate wells and the above-mentioned levels of mAF for 12 and 24 h using flow cytometry (i.e. after the treatment period these wells were then pooled to collect the MoDC for staining). These assays were performed using mAb against MHC-II (MSA3, IgG2a; Lunney et al., Citation1994), CD40 (G28-5, IgG1, anti-human; Bimczok et al., Citation2007), CD25 (K231.3B2, IgG1; Bailey et al., Citation1992), and a human CTLA4-muIgG2a fusion protein (all from Ancell, Bayport, MN), respectively, followed by R-phycoerythrin- and AlexaFluor-647-conjugated isotype-specific anti-mouse secondary antibodies (Life Technologies, Merelbeke, Belgium).
In brief, the MoDC were harvested after the culturing with the AF and washed in staining medium (DMEM + 1% FCS) and then incubated with pre-titrated saturating concentrations of each primary Ab for 20 min at 4 °C. Cells stained with isotype-matched irrelevant mAb (Life Technologies) were used to assess non-specific binding. After washing, the cells were stained for 20 min at 4 °C with the secondary Ab in staining medium. The cells were then washed and propidium iodide (5 μg/ml) was added to help in exclusion of dead cells during analyses. Flow cytometry data were then acquired and analyzed as above. Expression of each marker was defined by quantifying the fluorescence intensity of 20 000 cells within the live, singlet cell gate. Relative marker expression (%) was calculated as 100 × [(MFItreatment − MFIcontrol)/MFIcontrol].
T-cell proliferation assay
The T-cell stimulatory capacity of MoDC was analyzed in an allogenic T-cell proliferation assay. T-cells were isolated from each piglet’s PBMC fraction by enriching CD6+ cells to a purity of >95% by positive immunomagnetic selection with anti-CD6 mAb (IgG1, clone a38b2; Saalmüller et al., Citation1994) and goat anti-mouse microbeads, together with LS columns (Miltenyi Biotech). The MoDC were then treated with the above-mentioned levels of mAF (or left untreated) for 12 and 24 h. The MoDC were then harvested by flushing, washed, counted with a counting chamber (Neubauer) in nigrosin solution, and co-cultured at titrated numbers with 2.0 × 105 allogenic CD6+ T-cells in round-bottom 96-well microtiter plates (Nunc). In all cases, a 1:30 (DC:T) ratio was used for the proliferation assays; each DC:T sample was analyzed in triplicate. CD6+ T-cells stimulated with 5 μg/ml ConA (Sigma) only were used as a positive control. All cultures were performed in proliferation medium (DMEM supplemented with 10% FCS, penicillin/streptomycin, and 50 μM 2-mercaptoethanol) at 37 °C in a 5% CO2 humidified atmosphere. After 5 days of culture, the cells were pulse-labeled with 1 μCi/well [3H]-thymidine (Amersham ICN, Bucks, UK) for an additional 18 h. Cells were then harvested onto glass fiber filters (Perkin-Elmer Life Sciences, Brussels, Belgium) and [3H]-thymidine incorporation measured using a β-scintillation counter (Perkin-Elmer). Results were calculated in terms of a stimulation index (SI) wherein SI = mean cpm of co-cultures DCtreatment/mean cpm of co-cultures DCcontrol.
Analysis of the cytokine secretion profile by ELISA
MoDC from each piglet were generated and stimulated with mAF as above. After 12 and 24 h, cell-free culture supernatants were collected and levels of porcine tumor necrosis factor (TNF)-α, interleukin (IL)-1β, -6, -8, and -10 cytokines then determined with commercially available ELISA kits (TNFα, IL-1β, IL-6, and IL-8: R&D Systems; IL-10: Life Technologies, Merelbeke, Belgium) according to manufacturer instructions. All samples were diluted by ½ (TNFα, IL-1β, IL-6, IL-10) or ¼ (IL-8) in reagent diluent prior to use in the assays. Optical densities were then measured in an ELISA plate reader (MTX Lab systems, Vienna, VA) at 450 nm. Cytokine concentrations in the medium were calculated using DeltaSOFT JV 2.1.2 software (BioMetallics, Princeton, NJ) with a 4-parameter curve-fitting algorithm applied for standard curve calculations. The limits of detection of the TNFα, IL-1β, IL-6, IL-8, and IL-10 kits were, respectively, 125, 62.5, 4.3, 125, and 3.0 pg/ml.
Statistical analyses
Statistical analyses were performed with SPSS 20 (IBM, Armonk, NY). Effects of mAF on phagocytosis of microparticles, DC marker expression, T-cell proliferation, and cytokine secretion were assessed with a non-parametric Mann-Whitney test. Significance was accepted at p < 0.05.
Results
mAF do not impair the phagocytic capacity of MoDC
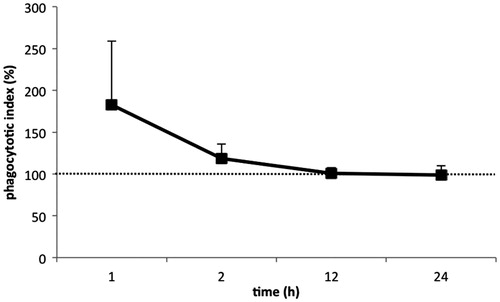
As AF disrupts phagocytosis of particles by innate immune cells, this study sought to assess the effect of AF on DC-mediated phagocytosis of microparticles. Before the addition of polystyrene microparticles, MoDC from each piglet were treated for different lengths of time with a mixture of several AF. At the low concentrations used here, the mAF did not affect cell viability (data not shown). After 1 h of treatment, the mAF caused the cells to display a slight but not significant increase in internalization of the microparticles (). However, as the treatment length increased (i.e. 2, 12, or 24 h), the mean phagocytic activity of the cells from the three piglets returned to a level unchanged as compared to that in mock-treated MoDC. Note: For the benefit of the Readers, all data from each piglet is also provided in Supplemental Table S1.
Figure 2. Effect of naturally occurring levels of AF on phagocytic activity of porcine monocyte-derived dendritic cells (MoDC). MoDC isolated from each of three piglets were separately treated for different periods of time with a mixture of several aflatoxins (mAF) containing AFB1 (2.0 ng/ml), AFB2 (1.0 ng/ml), AFG1 (0.5 ng/ml), and AFG2 (0.5 ng/ml). At the end of each period, 5.0 × 106 fluorescent 1.0-μm microparticles were added to each set of MoDC to assess if there were effects on phagocytic activity induced by the treatments. Data are presented as mean (±SEM, n = 3) percent (%) change in phagocytic activity relative to values for each piglet’s corresponding untreated MoDC. Dashed line = phagocytic activity of control non-AF-treated MoDC.
mAF enhance the expression of activation markers by DC
DC need to up-regulate the cell surface expression of peptide-MHC complexes and co-stimulatory molecules to efficiently present processed antigens to naïve T-cells. Thus, we wanted to elucidate the effect of mAF on the activation of porcine MoDC by assessing the expression of cell surface markers MHC-II, CD40, CD80/86, and CD25. The latter is considered an important marker for porcine DC activation (Devriendt et al., Citation2013). As indicated in , mAF treatment had a clear time-dependent effect on surface expression of these markers on the cells isolated from the three piglets. After 12 h of mAF treatment, there was an increase in the mean expression of CD80/86 and CD25 as compared to that by control (i.e. 0 ng AF/ml) DC, although this was only significant for CD25 (p = 0.037) (). Prolonged exposure (24 h) of the cells to the toxin mixture further increased the mean CD80/86 expression (p = 0.019), whereas the effect on CD25 expression disappeared (). Note: For the benefit of the readers, all data from each piglet is also provided in Supplemental Table S1.
Figure 3. Aflatoxins affect the cell surface expression of DC activation markers. MoDC isolated from each of three piglets were separately treated with a mixture of several aflatoxins (mAF containing AFB1 [2.0 ng/ml], AFB2 [1.0 ng/ml], AFG1 [0.5 ng/ml], and AFG2 [0.5 ng/ml]) for 12 and 24 h and then analyzed for expression of CD40, CD80/86, CD25, and MHC-II by flow cytometry. Data are presented as mean (±SEM, n = 3) percent (%) change in expression relative to values for each piglet’s corresponding untreated MoDC. *p < 0.05 to untreated cells. imm = immature (untreated or control) MoDC.
![Figure 3. Aflatoxins affect the cell surface expression of DC activation markers. MoDC isolated from each of three piglets were separately treated with a mixture of several aflatoxins (mAF containing AFB1 [2.0 ng/ml], AFB2 [1.0 ng/ml], AFG1 [0.5 ng/ml], and AFG2 [0.5 ng/ml]) for 12 and 24 h and then analyzed for expression of CD40, CD80/86, CD25, and MHC-II by flow cytometry. Data are presented as mean (±SEM, n = 3) percent (%) change in expression relative to values for each piglet’s corresponding untreated MoDC. *p < 0.05 to untreated cells. imm = immature (untreated or control) MoDC.](/cms/asset/bb2849d2-897b-47ad-8523-f8752cde8b39/iimt_a_916366_f0003_c.jpg)
mAF enhance the T-cell stimulatory activity of DC
To further assess if mAF-mediated phenotypic DC activation was linked to a functional activation, the effect of mAF on the ability of DC to induce T-cell proliferation was examined. Up-regulation of CD80/CD86 by mAF was accompanied by an enhanced T-cell stimulatory capacity of the DC (). After 12 h of MoDC exposure to the mAF, the resultant mean increase in T-cell stimulatory ability still showed a large variation; after a 24 h incubation, this increase was more consistent, resulting in significantly enhanced T-cell stimulatory capacity as compared to that seen with non-AF-treated DC (p = 0.002). Note: For the benefit of the readers, all data from each piglet is also provided in Supplemental Table S1.
Figure 4. Aflatoxins affect the T-cell stimulatory capacity of porcine MoDC. MoDC isolated from each of three piglets were separately treated with a mixture of several aflatoxins (see legend) for 12 and 24 h. The mAF-treated MoDC were subsequently co-cultured with 2.0 × 105 CD6+ T-cells at a 1:30 (DC:T) ratio. T-cell proliferation was measured via [3H]-methyl thymidine incorporation. Data are presented as mean (±SEM, n = 3) stimulation index of the three individual sets of piglet cells analyzed. *p < 0.05 vs non-AF-treated MoDC.
![Figure 4. Aflatoxins affect the T-cell stimulatory capacity of porcine MoDC. MoDC isolated from each of three piglets were separately treated with a mixture of several aflatoxins (see Figure 3 legend) for 12 and 24 h. The mAF-treated MoDC were subsequently co-cultured with 2.0 × 105 CD6+ T-cells at a 1:30 (DC:T) ratio. T-cell proliferation was measured via [3H]-methyl thymidine incorporation. Data are presented as mean (±SEM, n = 3) stimulation index of the three individual sets of piglet cells analyzed. *p < 0.05 vs non-AF-treated MoDC.](/cms/asset/96c54172-1cdf-40e4-9610-3a38d00a5996/iimt_a_916366_f0004_c.jpg)
mAF do not affect cytokine secretion by MoDC
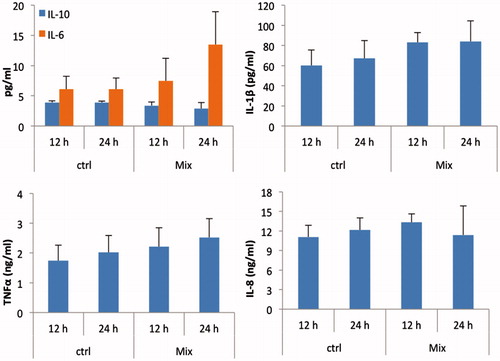
As the mAF increased the ability of DC to activate T-cells, and as cytokines play a crucial role in activation and polarization of T-cells, we next determined the effect of the mAF on DC cytokine secretion pattern. As shown in , among the cells isolated from the three piglets, there was a small increase in secretion (i.e. mean levels in culture medium) of inflammatory IL-1β, IL-6, and TNFα, and a slight decrease in that of anti-inflammatory IL-10 (). However, these concentrations were not significantly different from those in the culture medium of the mock-treated MoDC. Note: For the benefit of the readers, all data from each piglet is also provided in Supplemental Table S1.
Figure 5. Cytokine expression pattern of AF-exposed MoDC. MoDC isolated from each of three piglets were separately treated with either 0 ng/ml (ctrl) or a mixture of aflatoxins (see legend) for 12 and 24 h. Cell culture supernatants harvested at the end of each incubation period were analyzed for the presence of IL-1β, IL-6, IL-8, IL-10, and TNFα by ELISA. Data are presented as mean (±SEM, n = 3) levels of each cytokine in the medium (at each timepoint) from the three individual sets of piglet cells analyzed. Ctrl = non-AF-treated MoDC.
Discussion
The study here examined the effects of a mixture of different aflatoxins (AF) at levels that may be obtained by consumption of environmentally-contaminated feed and foods. The AF and their toxic effects on key immune cells is a threat to animal and human health worldwide (CAST, Citation1989; Luongo et al., Citation2013; Reddy et al., Citation2009; Viegas et al., Citation2013). Recent data indicate that the chronic exposure of piglets to low mycotoxin levels still could affect the intestinal immune system and prolong infection with intestinal pathogens (Devriendt et al., Citation2009; Oswald et al., Citation2003). Based on the chemical structure and properties of Group B and G AF, they can easily pass through the plasma membrane of (for example) intestinal epithelial cells and rapidly appear in the blood circulation (Battacone et al., Citation2003; Gallo et al., Citation2008; Martins et al., Citation2007). Although the expression of major AF-metabolizing enzymes in intestinal epithelial cells is yet to be explored, detoxification of AF by these cells is minimal, and these highly carcinogenic AF can easily reach the blood stream. Thereafter, the AF are converted to highly oxidative epoxides that bind to proteins, DNA, and/or RNA, causing oxidative stress and disturbing normal cellular processes (Bernabucci et al., Citation2011; Mehrzad et al., Citation2011). Indeed, the toxicity of AF not only depends on their intact form but also on their biotransformation to reactive hydroxylated derivatives (Ayed-Boussema et al., Citation2012; Tulayakul et al., Citation2007). In human immune cells, cytochrome P450 (CYP) enzymes are involved in metabolism of AF and this CYP-dependent biotransformation of AF might be required for their effect on porcine DC (Bahari et al., Citation2014). DC are the most potent APC with the unique ability to activate naïve T-cells and elucidating the impact of mixed AF on DC functions key to dictating the nature of the immune response could help to understand the immunotoxicity caused by low doses of mycotoxins.
Indeed, ingestion of AF-contaminated feed by piglets resulted in an altered T-cell proliferation, presumably due to a direct effect of AF on DC (Meissonnier et al., Citation2008). Basically, T-cell activation by DC requires three inter-related signals: (1) the interaction of the T-cell receptor with peptide-MHC complexes on the DC surface; (2) DC have to up-regulate the expression of co-stimulatory molecules, such as CD40 and CD80/86, to fully activate naïve T-cells; and (3) secretion of cytokines which will influence the polarization of the activated T-cells. If one of those signals is absent, T-cell activation and consequently T-cell proliferation will be impaired (Huang et al., Citation2001). The observed broad effects of mAF on DC clearly suggested that AF did provide some signals in DC. To the best of our knowledge, this is the first study elucidating the effects of mAF on the function of porcine DC.
We challenged DC with a dose of mAF corresponding to approximately what can be found in mold-contaminated feed and, hence, could reflect naturally occurring levels in young piglets. Indeed, when animals are fed feedstuffs contaminated at the upper limit of 10 μg/kg feed on a dry matter basis which is tolerated by the EU Feed legislation (2002), and considering the daily feed intake of piglets, total AF levels in the blood stream could reach ∼10 ng/ml. Although there are differing proportions of AFB1, AFB2, AFG1, and AFG2 in Aspergillus-contaminated feed, aflatoxinogenic molds predominantly produce AFB1 and to a lesser extent the other three AF in feed (Cervino et al., Citation2008; Joubrane et al., Citation2011; Li et al., Citation2009). Worldwide, occupational exposure to AF is alarming; a level of even ∼8 ng AFB1/ml in blood of swine ‘workers’ whose exposure was by the inhalation, oral, and/or dermal routes, has been reported (Viegas et al., Citation2013). Further, in countries where mycotoxin-monitoring programs are absent, livestock may encounter far higher levels of the AF than the doses used here. Moreover, as the four AF types (in varying proportions) are present in Aspergillus-infested feed/food, and a mixture of environmentally relevant levels of these AF were used here to investigate effects on DC function, the approach in this study closely mimicked in vivo conditions that reflect levels often encountered by piglets. Taken together, these factors make the dose rationale used here relevant.
The ability of DC to take up antigens is central to their function. Using flow cytometric assays we assessed the phagocytosis capacity of AF-exposed DC. Previous studies indicated that AF could decrease the phagocytotic activity of neutrophils and macrophages (Mehrzad et al., Citation2011; Moon et al., Citation1999). In contrast, the phagocytotic capacity of DC was modestly increased at short lengths of exposure tested here (i.e. 1 h), possibly reflecting a response of the DC to mycotoxin sensing. Intriguingly, a 12 or 24 h treatment period did not influence microparticle uptake by the MoDC; this implied little interference by the AF with membrane and actin cytoskeleton dynamics, two well-known key mechanisms necessary for efficient phagocytosis (Goodridge et al., Citation2012).
To justify the broad effects of AF on DC, we demonstrated there was a relationship between the mAF and cell-surface marker expression in porcine DC and confirmed that the mAF modified DC phenotype. Indeed, expression of CD25 and co-stimulatory CD80/86 were each increased, indicating a phenotypical activation of the mAF-treated DC. Remarkably, the mAF enhanced the T-cell proliferation-inducing capacity of the MoDC. This is in contrast with a diminished cell-mediated immunity in AFB1-fed piglets observed by Meissonnier et al. (Citation2008). This increased T-cell proliferation-inducing activity might be due to the effect of mAF on the secretion of cytokines.
However, the DC cytokine secretion profile did not show a decreased IL-10 secretion. The increased T-cell stimulatory capacity of mAF-treated MoDC also did not seem to be a result of increased levels of pro-inflammatory cytokines, as mAF treatment only slightly increased the secretion of these cytokines by the porcine DC. This is in line with previous data wherein the mAF did not modulate IL-1β and TNFα expression in swine alveolar macrophages (Liu et al., Citation2002). However, as we assayed only a limited amount of cytokines, a potential effect of the mAF on other cyto-/chemokines cannot be precluded. Because the naturally-occurring level of mAF did not affect the monitored pro- and anti-inflammatory cytokine secretion pattern of the DC, one critical unanswered question is how mAF-treated MoDC mediated an increase in T-cell proliferation. Our results sufficiently support the point that the observed increased T-cell proliferation induced by mAF-treated DC could mainly result from the increased expression of the DC activation markers CD25 and CD80/86. The enhanced antigen-presenting capacity of steady-state DC induced by naturally-occurring levels of AF could result in a breakdown of immunological tolerance and provide a mechanism for the observed immunotoxicity of AF in mammals. Further functional assays are in progress to elucidate molecular mechanisms behind this phenomenon.
In conclusion, the results here indicate that a mixture of naturally-occurring AF dysregulates the antigen-presenting capacity of porcine DC. This immunotoxic effect at low levels could impact animal and public health and further emphasizes the need to reduce the admissible level of AF in agricultural products.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Supplementary material available online
Supplementary Table S1
Final_S1__porcine_dc_paper_.doc
Download MS Word (73.5 KB)Acknowledgements
The authors wish to acknowledge Dr. S. Inumaru (Institute of Animal Health, Ibaraki, Japan) for kindly providing the rpGM-CSF, Dr. H. J. Rothkötter (Institute of Anatomy, Magdeburg, Germany) for the anti-CD40 hybridoma SN, and Dr. A. Saalmüller (University of Veterinary Medicine, Vienna, Austria) for the 74-12-15a, MSA3, and a38b2 mAb. Ghent University, Ferdowsi University of Mashhad, the FWO-Vlaanderen and the IWT-Vlaanderen are all acknowledged for their financial support.
References
- Ayed-Boussema, I., Pascussi, J. M., Maurel, P., et al. 2012. Effect of aflatoxin B1 on nuclear receptors, PXR, CAR, and AhR and their target cytochromes P450 mRNA expression in primary cultures of human hepatocytes. Int. J. Toxicol. 31:86–93
- Bahari, A., Mehrzad, J., Mahmoudi, M., et al. 2014. Effect of aflatoxin B1 on cytochrome P450 isoforms in human lymphocytes and monocytes. Immunopharmacol. Immunotoxicol. 36:1–10
- Bailey, M., Stevens, K., Bland, P. W., and Stokes, C. R. 1992. A monoclonal antibody recognising an epitope associated with pig IL-2 receptors. J. Immunol. Meth. 153:85–91
- Battacone, G., Nudda, A., Cannas, A., et al. 2003. Excretion of aflatoxin M1 in milk of dairy ewes treated with different doses of aflatoxin B1. J. Dairy Sci. 86:2667–2675
- Bernabucci, U., Colavecchia, L., Danieli, P. P., et al. 2011. Aflatoxin B1 and fumonisin B1 affect the oxidative status of bovine peripheral blood mononuclear cells. Toxicol. In Vitro 25:684–891
- Bimczok, D., Rau, H., Wundrack, N., et al. 2007. Cholera toxin promotes the generation of semi-mature porcine monocyte-derived dendritic cells that are unable to stimulate T-cells. Vet. Res. 38:597–612
- Cervino, C., Asam, S., Knopp, D., et al. 2008. Use of isotope-labeled aflatoxins for LC-MS/MS stable isotope dilution analysis of foods. J. Agric. Food Chem. 56:1873–1879
- Chaytor, A. C., See, M. T., Hansen, J. A., et al. 2011. Effects of chronic exposure of diets with reduced concentrations of aflatoxin and deoxynivalenol on growth and immune status of pigs. J. Anim. Sci. 89:124–135
- Council for Agricultural Science and Technology (CAST). 1989. Mycotoxins: Economic and Health Risks. Taskforce Report # 116. Ames, IA: Council for Agricultural Science and Technology
- Cusumano, V., Costa, G. B., and Seminara, S. 1990. Effects of aflatoxins on rat peritoneal macrophages. Appl. Eviron. Microbiol. 56:3482–3484
- Dawson, H. D., Loveland, J. E., Pascal, G., et al. 2013. Structural and functional annotation of the porcine immunome. BMC Genomics 14:332–347
- Devriendt, B., Gallois, M., Verdonck, F., et al. 2009. The food-contaminant fumonisin B1 reduces the maturation of porcine CD11R1+ antigen-presenting cells and antigen-specific immune responses, leading to a prolonged intestinal ETEC infection. Vet. Res. 40:40–54
- Devriendt, B., Goddeeris, B. M., and Cox, E. 2013. FcγR expression profile on porcine dendritic cells depends on the nature of the stimulus. Vet. Immunol. Immunopathol. 152:43–49
- Devriendt, B., Verdonck, F., Summerfield, A., et al. 2010. Targeting of Escherichia coli F4 fimbriae to Fcγ receptors enhances the maturation of porcine dendritic cells. Vet. Immunol. Immunopathol. 135:188–198
- Fairbairn, L., Kapetanovic, R., Sester, D. P., and Hume, D. A. 2011. The mononuclear phagocyte system of the pig as a model for understanding human innate immunity and disease. J. Leukocyte Biol. 89:855–871
- Gallo, A., Moschini, M., and Masoero, F. 2008. Aflatoxins absorption in the gastrointestinal tract and in the vaginal mucosa in lactating dairy cows. Ital. J. Anim. Sci. 7:53–63
- Gong, Y., Hounsa, A., Egal, S., et al. 2004. Post-weaning exposure to aflatoxin results in impaired child growth: A longitudinal study in Benin, West Africa. Environ. Health. Perspect. 112:1334–1338
- Goodridge, H. S., Underhill, D. M., and Touret, N. 2012. Mechanisms of Fc receptor and dectin-1 activation for phagocytosis. Traffic 13:1062–1071
- Hernández Hierro, J. M., Garcia-Villanova, R. J., Rodríguez Torrero, P., and Toruno Fonseca, I. M. 2008. Aflatoxins and Ochratoxin A in red paprika for retail sale in Spain: Occurrence and evaluation of a simultaneous analytical method. J. Agric. Food Chem. 56:751–756
- Hoogenboom, L. A., Tulliez, J., Gautier, J. P., et al. 2001. Absorption, distribution, and excretion of aflatoxin-derived ammoniation products in lactating cows. Food Addit. Contam. 18:47–58
- Huang, Q., Liu, D., Majewski, P., et al 2001. The plasticity of dendritic cell responses to pathogens and their components. Science 294:870–875
- Inumaru, S., Kokuho, T., Denham, S., et al. 1998. Expression of biologically active recombinant porcine GM-CSF by baculovirus gene expression system. Immunol. Cell Biol. 76:195–201
- Joffre, O., Nolte, M. A., Sporri, R., and Reis e Sousa, C. 2009. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 227:234–247
- Joubrane, K., El Khoury, A., Lteif, R., et al. 2011. Occurrence of aflatoxin B1 and Ochratoxin A in Lebanese cultivated wheat. Mycotoxin Res. 27:249–2457
- Li, F. Q., Li, Y. W., Wang, Y. R., and Luo, X. Y. 2009. Natural occurrence of aflatoxins in Chinese peanut butter and sesame paste. J. Agric. Food Chem. 57:3519–3524
- Liu, B. H., Yu, F. Y., Chan, M. H., and Yang, Y. L. 2002. The effects of mycotoxins, fumonisin B1 and aflatoxin B1, on primary swine alveolar macrophages. Toxicol. Appl. Pharmacol. 180:197–204
- Lunney, J. K., Walker, K., Goldman, T., et al. 1994. Overview of the first international workshop to define swine leukocyte cluster of differentiation (CD) antigens. Vet. Immunol. Immunopathol. 43:193–206
- Luongo, D., Russo, R., Balestrieri, A., et al. 2013. In vitro study of AFB1 and AFM1 effects on human lymphoblastoid Jurkat T-cell model. J Immunotoxicol. (Epub ahead of print)
- Malvandi, A. M., Mehrzad, J., and Saleh-moghaddam, M. 2013. Biologically relevant doses of mixed aflatoxins B and G up-regulate MyD88, TLR2, TLR4 and CD14 transcripts in human PBMC. Immunopharmacol. Immunotoxicol. 35:528–532
- Martins, H. M., Mendes Guerra, M. M., and d’Almeida Bernardo, F. M. 2007. Occurrence of aflatoxin B1 in dairy cow feed over 10 years in Portugal (1995–2004). Rev. Iberoam. Micol. 24:69–71
- Mehrzad, J., Klein, G., Kamphues, J., et al. 2011. In vitro effects of very low levels of aflatoxin B1 on free radicals production and bactericidal activity of bovine blood neutrophils. Vet. Immunol. Immunopathol. 141:16–25
- Meissonnier, G. M., Pinton, P., Laffitte, J., et al. 2008. Immunotoxicity of aflatoxin B1: Impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol. Appl. Pharmacol. 231:142–149
- Meurens, F., Summerfield, A., Nauwynck, H., et al. 2012. The pig: A model for human infectious diseases. Trends Microbiol. 20:50–57
- Moon, E. Y., Rhee, D. K., and Pyo, S. 1999. Inhibition of various functions in murine peritoneal macrophages by aflatoxin B1 exposure in vivo. Int. J. Immunopharmacol. 21:47–58
- Oswald, I. P., Desautels, C., Laffitte, J., et al. 2003. Mycotoxin fumonisin B1 increases intestinal colonization by pathogenic Escherichia coli in pigs. Appl. Environ. Microbiol. 69:5870–5874
- Pescovitz, M. D., Lunney, J. K., and Sachs, D. H. 1984. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J. Immunol. 133:368–375
- Pukkala, E., Martinsen, J. I., Lynge, E., et al. 2009. Occupation and cancer-follow-up of 15 million people in five Nordic countries. Acta Oncol. 48:646–790
- Qureshi, M. A., Brake, J., Hamilton, P. B., et al. 1998. Dietary exposure of broiler breeders to aflatoxin results in immune dysfunction in progeny chicks. Poult. Sci. 77:812–819
- Reddy, K. R., Abbas, H. K., Abel, C. A., et al. 2009. Mycotoxin contamination of commercially important agricultural commodities. Toxins Rev. 28:154–168
- Saalmüller, A., Aasted, B., Canals, A., et al. 1994. Analyses of monoclonal antibodies reactive with porcine CD6. Vet. Immunol. Immunopathol. 43:243–247
- Tulayakul, P., Dong, K. S., Li, J. Y., et al. 2007. The effect of feeding piglets with the diet containing green tea extracts or coumarin on in vitro metabolism of aflatoxin B1 by their tissues. Toxicon 50:339–348
- Venturini, M. C., Quiroga, M. A., Risso, M. A., et al. 1996. Mycotoxin T-2 and aflatoxin B1 as immunosuppressors in mice chronically infected with Toxoplasma gondii. J. Comp. Pathol. 115:229–237
- Viegas, S., Veiga, L., Figueredo, P., et al. 2013. Occupational exposure to aflatoxin B1 in swine production and possible contamination sources. J. Toxicol. Environ. Health 76:944–951
- Wild, C. P., and Turner, P. C. 2002. The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis 17:471–481
