Abstract
Allergic contact dermatitis (ACD) is driven by the activation and proliferation of allergen-specific memory T-lymphocytes and is currently diagnosed by patch testing with a selected panel of chemical allergens. The lymphocyte transformation test (LTT) can be used to monitor ex vivo T-lymphocyte responses to antigens, including contact allergens. The LTT is not viewed as being an alternative to patch testing, but it does seek to reflect experimentally skin sensitization to specific chemicals. The LTT is based on stimulation in vitro of antigen-driven T-lymphocyte proliferation. That is, exposure in culture of primed memory T-lymphocytes to the relevant antigen delivered in an appropriate configuration will provoke a secondary response that reflects the acquisition of skin sensitization. The technical aspects of this test and the utility of the approach for investigation of immune responses to contact allergens in humans are reviewed here, with particular emphasis on further development and refinement of the protocol. An important potential application is that it may provide a basis for characterizing those aspects of T-lymphocyte responses to contact allergens that have the greatest influence on skin sensitizing potency and this will be considered in some detail.
Introduction
Contact dermatitis is an inflammatory reaction induced in the skin following topical exposure to environmental or industrial low molecular weight (LMW) chemicals and can take two forms, i.e. irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD), which are clinically very similar and frequently indistinguishable. Irritation arises as a result of direct cell damage. In contrast, ACD develops in two phases. In the first phase, topical exposure of an inherently susceptible subject to a chemical allergen results in immunological priming and the acquisition of skin sensitization. If that sensitized individual is exposed subsequently, at the same or a different skin site, to the inducing chemical allergen, then an accelerated and more aggressive secondary immune response will be elicited. This in turn causes a local inflammatory reaction in the skin at the site of exposure that is characterized clinically as ACD (Cavani et al., Citation2007; Kimber et al., Citation2011; Nosbaum et al., Citation2009). Many hundreds of chemicals are known to act as contact allergens. However, they differ very considerably in their activity. In fact, contact allergens are believed to vary by up to five orders of magnitude with respect to their relative sensitizing potency and their ability to drive the acquisition of skin sensitization (Kimber et al., Citation2001; Lalko et al., Citation2011).
Diagnosis of ACD and identification of the causative chemical(s) relies on patch testing. Chemicals of interest are applied to the skin (usually on the subject’s back) at pre-determined concentrations. Some days later, following removal of the patch, any inflammatory reaction at the site of challenge is scored (Joy et al., Citation2013; Wahlberg & Lindberg, Citation2006). The cutaneous inflammatory reaction is driven initially by the local infiltration and activation of the previously expanded population of chemical allergen-specific memory T-lymphocytes. This in turn induces the recruitment of other immune cells and the release of cytokines and other inflammatory mediators that act in concert to cause the elicitation of a cutaneous inflammatory reaction (Martin, Citation2012). Based on clinical judgment, a positive patch test result can confirm that a subject is allergic to particular chemical(s) and to some extent provides a perspective on the extent of sensitization, the latter being based on the vigor of the induced skin reaction (Davis et al., Citation2013). Scoring of ACD reactions includes consideration of the size of erythematous reaction, the extent of edema and the presence of vesicles at the application site, with the strongest responses being associated with extreme blistering reactions. In addition, in an experimental context, the threshold dose of allergen required for elicitation of a discernible patch test reaction can also be a valuable metric in evaluation of the extent to which a subject is sensitized (Friedmann, Citation2007).
Patch testing is a practical and reliable technique for the diagnosis of ACD (Devos & van der Valk, Citation2002) where interpretation of the cutaneous response requires considerable experience and expert knowledge (Bruze et al., Citation1995). The patch test concentrations that are applied are considered carefully and represent a compromise between maintaining diagnostic sensitivity and ensuring that irritant reactions, that would confound the identification of an allergic reaction, are infrequent. Interpretation is not always straightforward and false positive and negative reactions can hinder accurate clinical interpretation (Sherertz et al., Citation2001). It is believed that patch tests can cause the acquisition of (iatrogenic) sensitization in a previously unresponsive subject; such cases are very rare (Jensen et al., Citation2006; Mowad, Citation2006). With the careful consideration of standard allergens and applied concentrations, the risk of inadvertent sensitization is extremely low, however active sensitization has been reported for some strong sensitizers such as p-phenylenediamine (PPD) (Devos & van der Valk, Citation2001).
The lymphocyte transformation test (LTT) is an experimental recall assay in which allergen-reactive memory T-lymphocytes drawn from peripheral blood of sensitized individuals are re-stimulated in vitro with relevant allergen to provoke a specific response (Nyfeler & Pichler, Citation1997). The LTT has the potential to characterize more fully the nature of immune responses to contact allergens in humans by providing additional information, such as, for example, identification of functional sub-populations responsive to allergen and cytokine secretion profiles. Since the concept of the LTT was first realized, there have been opportunities to modify/refine the method. The more important developments are considered here.
Immunobiology of allergic contact dermatitis
The central immunobiological event of ACD is the activation of allergen-specific T-lymphocytes. This requires initiation of a complex sequence of events, that is tightly regulated in time and space and that drives the acquisition and determines the potency of sensitization (Cavani et al., Citation2001; Kimber et al., Citation2012; Roberts & Aptula, Citation2008; Toncic et al., Citation2011). Initially, LMW chemicals must penetrate the skin gaining passage through the lipophilic strateum corneum and access to the viable epidermis and dermis (Berard et al., Citation2003). Free chemicals are of insufficient size to elicit an adaptive immune response; therefore, to acquire immunogenic potential, chemicals must form stable associations with proteins in the skin creating hapten–protein conjugates. For this reason chemical allergens are either naturally electrophilic or can be metabolized or otherwise converted into an electrophilic moiety. This phenomenon was recognized first by Landsteiner and Jacobs (Citation1935), who discovered that chemicals can act as sensitizers by binding to skin proteins.
Hapten–protein conjugates are recognized, internalized and processed by cutaneous dendritic cells (DC) [both epidermal Langerhans’ cells (LC) and dermal DC)]. Allergen bearing DC are stimulated to leave the skin and to migrate via afferent lymphatics to regional lymph nodes. Here allergen is presented to antigen-responsive T-lymphocytes that are induced to divide and differentiate. If clonal expansion of allergen-responsive T-lymphocytes is of sufficient magnitude then skin sensitization will be acquired. If the sensitized subject re-encounters the same chemical allergen, at the same or a different skin site, then a more vigorous and accelerated secondary immune response may be elicited, resulting in ACD (Becker & Knop, Citation1993). As indicated above, this cutaneous inflammatory reaction is characterized by an influx of lymphocytes and other leukocytes and the release of inflammatory mediators (Grabbe & Schwarz, Citation1998).
For allergen-specific T-lymphocyte activation and the elicitation of cellular cyto-toxicity that causes the clinical manifestations of ACD, the T-cell receptor (TCR) for antigen must recognize and interact with chemical haptens presented appropriately on peptides and in the context of major histocompatibility complex (MHC) gene products expressed on specialized antigen presenting cells (APC) (Martin, Citation2004; Martin et al., Citation2010; Thierse et al., Citation2005). Classical haptens can be presented by MHC Class I and/or Class II molecules as haptenated peptides, wherein epitopes recognized by TCR are generated via processing of chemically modified proteins by APC or, in some instances, by direct modification of peptides already associated with MHC determinants. In this way, protein-reactive chemicals can be recognized as covalently hapten-modified MHC-bound peptides by both CD8 and CD4 T-lymphocytes (Martin et al., Citation1992; Moulon et al., Citation1995; Ortmann et al., Citation1992; Vocanson et al., Citation2006). Non-classical haptens, including metal ions such as nickel, can interact directly in a non-covalent fashion with the MHC determinants or the TCR (Gamerdinger et al., Citation2003).
Dependent on the nature of the inducing contact allergen, skin exposure can result in the differentiation and expansion of allergen-specific effector CD8 T-lymphocytes (Type 1 cytotoxic T-cells; Tc1)—characterized by interferon (IFN)-γ release and of various functional sub-populations of CD4 T-lymphocytes, including T-helper (TH)-1, -2, and -17 cells and T-regulatory (Treg) cells—each with characteristic cytokine expression profiles (Kimber & Dearman, Citation2002; Saint-Mezard et al., Citation2004; Vocanson et al., Citation2005). Thus, expanded nickel-specific T-lymphocytes from allergic patients were found to comprise CD8 T-lymphocyte clones expressing IFNγ, whereas Treg cells expressing interleukin (IL)-10 performed a regulatory function in non-allergic patients (Cavani et al., Citation1998).
History of the lymphocyte transformation test in allergic contact dermatitis
Various manifestations of the LTT have been used for the characterization of (LMW) drug hypersensitivities (Pichler & Tilch, Citation2004) and in developing enhanced mechanistic understanding of skin sensitization and ACD, as considered here. Important advantages of this approach are that the cascade of events leading to T-lymphocyte priming has already occurred within the skin and lymph nodes, resulting in populations of memory T-lymphocytes in the peripheral blood that are easily accessible as they have already expanded in vivo. In addition, it is possible to adapt the method to explore aspects of naïve T-lymphocyte activation and priming in vitro, that may find utility in the development of methods for the prospective identification of potential skin sensitizers (Adler et al., Citation2011; Basketter et al., Citation2012; Gerberick & Robinson, Citation2000; Pichler, Citation2001).
One of the first attempts to study T-lymphocyte responses to chemical haptens using ex vivo human peripheral blood cells was published some 35+ years ago (Newman et al., Citation1977). That model was developed further to measure both primary and secondary proliferative responses of lymphocytes to trinitrophenol (TNP) by in vitro priming and subsequent re-stimulation with hapten-modified peripheral blood mononuclear cells (PBMC); studies that highlighted a requirement for MHC presentation as higher proliferative responses were recorded for autologous compared with allogeneic cultures (Seldin & Rich, Citation1978). The data suggested that, for an effective secondary proliferative response, T-lymphocytes required the TNP hapten to be presented by human leukocyte antigen (HLA) determinants and in particular by HLA-D.
Much of the pioneering work on development of an LTT focused on nickel. Nickel ACD has been studied extensively as a model of skin sensitization as nickel is one of the most prevalent contact allergens (Nguyen et al., Citation2008). Following restrictions on the use of nickel within consumer products by the European Union (EU) in 1994 the prevalence of nickel ACD has declined in EU countries (Jensen et al., Citation2002). However, it remains an important cause of skin sensitization and ACD. One interest was to determine whether activity in the LTT in nickel allergy could be of use in distinguishing between true clinical responses and false positive reactions to nickel in the patch test (Al-Tawil et al., Citation1981; Everness et al., Citation1990; Kimber et al., Citation1990; Silvennoinen-Kassinen, Citation1980; Svejgaard et al., Citation1978). The LTT correlated well with patch test results for nickel-positive individuals, but yielded a high percentage of positives in patch test-negative healthy controls (30%) (von Blomberg-van der Flier et al., Citation1987). One possible explanation might be that the LTT reveals sensitization to nickel below the threshold for a clinically discernible patch test reaction following challenge (that is, sub-clinical levels of sensitization). However, it would be very difficult in practice to distinguish a low level specific response in the LTT from a true-false positive response. Rasanen and Tuomi (Citation1992) also compared the use of patch testing with the nickel LTT in 21 nickel allergic patients and found that 95% and 86%, respectively, were identified as positive, indicating that in that series the LTT may have been somewhat less sensitive than patch testing. Nickel-specific CD4 T-lymphocytes have been isolated from peripheral blood and from the site of skin challenge of the same allergic patients (Kapsenberg et al., Citation1987). The TCR variable gene repertoire was shown to be the same in blood- and skin-derived T-lymphocyte clones specific for nickel (Werfel et al., Citation1997). Therefore, characterization of in vitro of nickel-reactive T-lymphocytes from peripheral blood reflects accurately the nature of the allergic response in the skin (Pacheco et al., Citation2013). This has important implications for analysis of T-lymphocytes from patients sensitive to other contact allergens where cells derived from blood samples, while biologically relevant to skin sensitization, are more conveniently obtained than from skin biopsies.
Following this early use in the study of nickel sensitized patients, the LTT was further developed to characterize allergen-specific responses to other contact allergens, including metal salts such as cobalt (Moed et al., Citation2005), chromium (Rasanen et al., Citation1991), MCI (methylchloroisothiazolinone) (Masjedi et al., Citation2003), DNCB (2,4-dinitrochlorobenzene) (Pickard et al., Citation2007), and PPD (Coulter et al., Citation2010; Kimber et al., Citation1990; Kneilling et al., Citation2010; McFadden et al., Citation2011). Although metals such as nickel can, for the purposes of the LTT, be readily dissolved in aqueous culture media in the form of metal salts, difficulties arise when using lipophilic chemicals (which applies to most contact allergens). For the latter, different strategies may be required.
Considerations, interpretations and limitations of lymphocyte transformation test
The clinical manifestations of ACD require the involvement of several cell types, including keratinocytes, LC, other skin-resident DC and T-lymphocytes (Novak et al., Citation1999; Sullivan et al., Citation1986). The most commonly used LTT protocol employs unfractionated PBMC populations that contain both T-lymphocytes and monocytes that can act together to mount a response without the need for addition of supplementary cell populations. The conventional LTT, as conducted using PBMC isolated from sensitized patients, is depicted in . The basic protocol for the LTT requires the enrichment of PBMC from whole blood using density gradient centrifugation (Pichler & Tilch, Citation2004). The PBMC fraction can then be characterized and cultured at a known cell density with a range of selected concentrations of chemical allergen delivered into culture (in a variety of formats) for between 5–7 days at 37 °C in an atmosphere of 5% CO2/95% air. The most widely applied method for measuring lymphocyte proliferation is [3H]-thymidine (TdR) incorporation that is usually added as a pulse in the final 16 h of culture (Cleaver, Citation1967). Cell proliferation is measured as a function of [3H]-TdR incorporation (β-scintillation counting) and stimulation indices (SI) can be calculated by reference to control populations cultured in the same way and for the same period in the absence of allergen. Within the compass of this broad design, modifications in protocol or technique can be introduced as depicted by dashed lines in ; such modifications can impact on the results obtained.
Figure 1. Schematic of conventional lymphocyte transformation test protocol (bold) for peripheral blood mononuclear cells (PBMC) in response to re-stimulation with chemical allergen with the addition of factors to consider (dashed lines) for improved assay design.
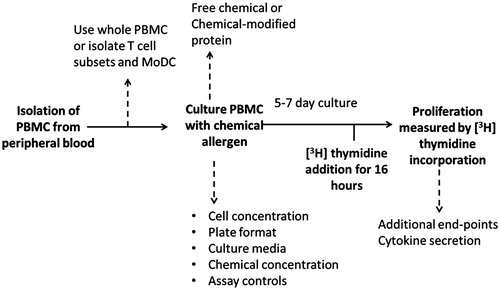
During the cell culture phase, important considerations include the use of autologous plasma versus human blood group AB-serum as a medium supplement, responder cell density, plate format, duration of assay (usually a 5–7 day stimulation window), concentration of chemical allergens (in whatever format), concentration and timing of [3H]-TdR addition and use of appropriate positive and negative controls (Mallone et al., Citation2011). Each of these variables has the potential to introduce subtle changes that can influence significantly the results obtained. These factors can, therefore, represent important causes of intra- and inter-laboratory variation.
Antigen-driven lymphocyte proliferation can be highly variable and requires an appropriate control to ensure that healthy cells have been isolated successfully. Tetanus toxoid (TT) provides one possible option, at least in those countries that have in place a tetanus vaccination program (Fryauff et al., Citation1999). Phytohemagglutinin (PHA) is a polyclonal T-lymphocyte mitogen, but a less exacting control than TT that induces antigen-specific recall responses (Sodomann et al., Citation1979).
Timing of lymphocyte transformation test relative to in vivo allergen-induced responses
Reproducibility and sensitivity are two important aspects of the LTT assay. High background proliferation may limit assay sensitivity and becomes an issue if PBMC populations contain a high frequency of activated proliferating cells that would, therefore, mask any additional effect of the allergen and result in low SI values. In particular, the timing of the drawing of blood relative to the time of sensitization or challenge could be an important factor. Little information about this is available, although research into drug hypersensitivity suggests that the timing of the LTT relative to clinical signs and patch testing is a key consideration (Kano et al., Citation2007). In that study patients were recruited that had previously shown adverse drug reactions, including: maculo-papular type of drug eruptions, Stevens–Johnson syndrome/toxic epidermal necrolysis and drug-induced hypersensitivity syndrome and/or drug rash and eosinophilia with systemic symptoms (DIHS/DRESS). False negatives were recorded in the LTT when the assay was conducted up to 3 weeks following onset of clinical signs for DIHS/DRESS patients. It was found that the optimal timing of the assay varied for different drugs and type of drug reaction. One explanation for this could be that the LTT is driven by memory T-lymphocyte responses and that the availability of such cells in blood would be significantly reduced if there was an on-going hypersensitivity reaction recruiting memory cells to the site of encounter with antigen. For drug hypersensitivities the Kano et al. (Citation2007) study, therefore, suggests that the LTT assay should be performed during remission and at least 4 weeks after a clinical reaction. The implication for the LTT as applied to skin sensitization is that the assay should ideally not be conducted during the 4 weeks following an allergic reaction or a positive patch test.
Approaches to optimization of the lymphocyte transformation test
The T-lymphocyte phenotype and activation state are important in the development of ACD and will impact significantly on proliferative responses recorded in LTT assays. As an alternative to unfractionated PBMC, purified CD8 and CD4 T-lymphocyte populations have been used in the LTT and cultured with autologous monocyte-derived DC to assess the cytokine secretion profiles of each subset in PPD allergic patients (Coulter et al., Citation2010). Although the use of purified T-lymphocyte subsets has the advantage of allowing assessment of the responsiveness of a particular cell subset, it is does not provide an holistic reflection of the immune response directed against a chemical allergen.
To overcome variability observed when freshly isolated peripheral blood cells are used and increase hapten-specific T-lymphocyte frequency, T-cell clones can be generated by limiting dilution and clonal expansion (Coulter et al., Citation2008). In such experiments, peripheral blood is taken from those individuals known to be allergic (patch test-positive) to PPD and proliferation measured following in vitro stimulation with the same allergen. In addition, in order to observe true antigen-specific responses and APC-T-lymphocyte interactions, a number of T-cell clones are generated from PPD-stimulated lymphocytes and cytokine secretion measured following further stimulation with autologous APC and PPD. However, the behavior of a clone in an LTT assay is not reflective of overall phenotype of anti-allergen immune responses in sensitized subjects as only one specific TCR is represented. In contrast, whole T-lymphocyte populations likely contain a pool of cells with varying characteristics potentially specific for several hapten–protein epitopes.
An alternative or supplementary strategy to improve sensitivity is the addition of certain cytokines to the culture media. The addition of cytokines such as IL-12/IL-7 and IL-4/IL-7 to the LTT culture media may improve sensitivity by increasing expression of co-stimulatory molecules on APC, thereby enhancing antigen-presentation (Rustemeyer et al., Citation2004) and increasing proliferative potential via IL-2 and cell survival (IL-7) (Kneilling et al., Citation2010). Again, use of such supplements may skew the overall response with respect to the overall quantity and quality of the induced T-lymphocytes by activating/suppressing specific T-lymphocyte subsets.
Chemical hapten presentation
Another very important consideration is the method selected to introduce the recall allergen; either by the addition of free chemical or as preformed chemical hapten–protein conjugates. Although the majority of skin sensitizing chemicals are inherently electrophilic, it is now appreciated that as many as one third of all contact allergens require either metabolic (pro-haptens) or abiotic activation such as auto-oxidation (pre-haptens) for the formation of hapten–protein conjugates (Chipinda et al., Citation2011). It is assumed that such conjugates form spontaneously in vitro in tissue culture with proteins such as human serum albumin (HSA) and that pre-haptens may undergo auto-oxidation in culture media. However, it is unlikely that culture conditions are necessarily optimal for conjugate formation and in addition for many chemical allergens the dynamic range of T-lymphocyte activation is relatively narrow with respect to free chemical, as such organic materials exhibit toxicity at relatively low concentrations. The creation in vitro of well-characterized chemical hapten–protein conjugates that can be added to cell cultures under defined conditions may circumvent some of these issues. Indeed, antibiotic-HSA conjugates have been used to investigate T-lymphocyte responses in drug-induced hypersensitivity reactions (Gaspard et al., Citation2000). In one report in which comparisons were made, higher concentrations of hapten conjugate than chemical allergen alone were required to stimulate T-lymphocyte proliferation, but this proliferative response was of a higher magnitude and without the associated cellular toxicity seen with direct addition of the free chemical (Jenkinson et al., Citation2010). However, at present there is little understanding of what properties an effective hapten–protein conjugate will possess.
Alternative methods to measure cellular proliferation
Bystander activation of non-specific T-lymphocytes during an antigen-specific T-lymphocyte response was first described during antiviral responses by CD8 T-lymphocytes (Tough et al., Citation1996), but has since been shown to also be exhibited by CD4 T-lymphocytes (Boyman, Citation2010). Bystander activation has been observed in patients with atopic dermatitis and it is likely that bystander cells are also activated in vivo in response to chemical contact allergens in ACD. During allergic skin responses in patients with atopic dermatitis the initial allergic response to aeroallergens is characterized by low numbers of antigen-specific TH2-type T-lymphocytes which subsequently switches to a TH0/TH1-type response following the activation of higher numbers of bystander T-lymphocytes in response to IL-12 produced by cells such as eosinophils and macrophages (Thepen et al., Citation1996). In the conventional LTT, PBMC are incubated with chemical contact allergen for 5–7 days and cell proliferation then measured by [3H]-TdR incorporation. One limitation of this method is that incorporation is measured in all proliferating cells and does not discriminate between allergen-specific and non-specific bystander cells. However, this may simply reflect the physiological response.
Alternative approaches to measure cellular proliferation in response to contact allergens have been developed that not only move away from using radiolabeled cells, but also aid in the identification and characterization of allergen-specific proliferating cells. 5-bromo-2deoxyuridine (BrdU), a non-radioactive thymidine analog detected by enzyme-linked immunosorbant assay (ELISA), can be used as an alternative read-out for the LTT. This endpoint has been used to detect drug-specific T-lymphocyte responses within peripheral blood from patients presenting with cutaneous adverse drug reactions to anticonvulsants (Hanafusa et al., Citation2012). Alternatively, cell cycle S-phase can be measured using flow cytometry with an anti-BrdU antibody (Gonchoroff et al., Citation1986). One advantage of using BrdU is that the phenotype of the proliferating population of cells can be identified, for example, as CD8 and CD4 T-lymphocytes, with cell surface markers alongside intracellular BrdU staining by flow cytometry.
CFSE (carboxyfluorescein diacetate succinimidyl ester) staining is another method that measures proliferation by flow cytometry with the added benefit that peaks in fluorescence intensity can determine how many cell divisions have occurred. CFSE passively diffuses into cells where acetate groups are cleaved by cellular esterases to yield highly fluorescent carboxy-fluorescein succinimidyl ester. This acquired cell fluorescent intensity is inherited by daughter cells and halved with each successive cell division with the advantage that it is not transferred to adjacent cells in a population (Fulcher & Wong, Citation1999; Quah et al., Citation2007). However, there is no information available regarding the use of this endpoint in the LTT for ACD, possibly as this technique in other scenarios displays considerably lower sensitivity than does [3H]-TdR incorporation, suggesting it may not be applicable to identification of a relatively small proportion of proliferating cells (Humphreys et al., Citation2003).
Another approach for measuring the proliferation of small cell numbers within in vitro assays is the marker Ki-67 (Soares et al., Citation2010). Ki-67 is a nuclear protein expressed during all active phases of cell division and has been shown to play a role in the regulation of cell division (Gerdes et al., Citation1984). When compared with BrdU and the CFSE-derivative Oregan Green (OG), the overall magnitude of Ki-67 expression in response to TT antigen correlated with BrdU and OG. The sensitivity of Ki-67 was found to be similar to OG; however, more sensitive than BrdU (Soares et al., Citation2010). Although Ki-67 expression is a commonly used measure of antigen-specific proliferation in other disciplines such as tumor immunology (Nakano et al., Citation2001), this marker has not been utilized fully as an endpoint in the LTT in ACD. In our experience, Ki-67 expression represents a useful tool when used together with standard [3H]-TdR incorporation as a means of identifying allergen-specific CD8 and CD4 T-lymphocyte subset proliferative responses in patients allergic to PPD and methylisothiazolinone (MI).
Allergen-driven activation and cytokine secretion
In addition to proliferation, activated T-lymphocytes also produce cytokines that can be measured by flow cytometry (intracellular production) or in cell supernatants via ELISA (secreted cytokine). The cytokine profile in response to contact allergens has been shown to vary for different allergens. Measurement of induced secretion of IFNγ and IL-12 provide markers of selective Type 1 (TH1/Tc1) responses, whereas IL-4, -5, -10 and -13 can be used as markers of preferential Type 2 (TH2/Tc2) responses. Subjects allergic to the contact allergen PPD displayed increased activity of Type 2 cytokines in both CD4 and CD8 T-lymphocytes in LTT assays (Coulter et al., Citation2010). In response to a mixture of MCI and MI, cellular proliferation has been characterized by secretion of both TH1 (IL-2 and IFNγ) and TH2 (IL-4, -5 and -13) cytokines (Masjedi et al., Citation2003).
The enzyme-linked immunospot assay (ELISpot) can be used as an alternative to ELISA and measures secreted cytokines on a per cell basis by immediate capture and immobilization of cytokine expressing cells. In this way the ELISpot can provide information on the numbers of antigen-specific cytokine secreting cells and the amount of secretion can be calculated by the area of the spots for each cell. ELISpot assays are a sensitive and specific in vitro assay for detecting cytokine profiles in response to contact allergens (Bordignon et al., Citation2008). In patients with ACD to nickel, the ELISpot assay was shown to improve detection of specific T-lymphocyte responses (Spiewak et al., Citation2007).
Secondary immune responses involve activation of allergen-specific memory T-lymphocytes and measurement of activation markers on T-lymphocytes provides an alternative non-radioactive endpoint for proliferation-related events. ACD is, at least in part, a cytotoxic T-cell-mediated immune response (Kehren et al., Citation1999; Kish et al., Citation2012). Granzyme B- and IFNγ production (measured by ELISA or ELISpot) have been used as endpoints for cytotoxicity drug-allergic responses (Porebski et al., Citation2013) and could also be used for ACD. Other phenotypic markers that have been associated with CD4 T-lymphocyte activation in PBMC derived from drug-allergic patients, such as the activation marker CD69, may also be applicable to T-lymphocyte responses in ACD (Beeler et al., Citation2008).
Immunomodulation
Altering the cellular composition of the PBMC culture is another way of potentially enhancing assay sensitivity by either blocking specific cellular molecules or by depleting certain cell populations. For example, blocking of CTLA-4 (cytotoxic T-lymphocyte antigen 4), the negative regulator of T-lymphocyte activation, was shown to improve the sensitivity of LTT assays where PBMC were derived from nickel allergic patients (Sugita et al., Citation2012). Depletion of CD25-expressing cells enhances the sensitivity of assays to detect immunogenic properties of weak antigens, potentially due to the removal of Treg (CD4+veCD25hiFoxP3+ve) cells that may suppress anti-hapten T-lymphocyte responses (Vocanson et al., Citation2008). A summary of the format and range of lymphocyte transformation type assays used to assess T-lymphocyte proliferation in response to chemical contact allergens is shown in .
Table 1. Summary of lymphocyte transformation tests used to assess T-cell responses to chemical contact allergens.
Future prospects
The LTT is more than an assay. If performed correctly, with appropriate controls, the LTT provides a potentially very useful tool for exploring the characteristics of human adaptive immune responses. Moreover, the LTT offers important opportunities for toxicologists to dissect out the cellular bases for the initiation and regulation of skin sensitization responses in humans. Of particular interest is use of the LTT for defining functional sub-populations of CD4 and CD8 T-lymphocytes responsive to chemical allergens and the contributions they make individually and in concert to the acquisition of skin sensitization.
One application of the LTT in which we are particularly interested is in identifying the important elements of the adaptive immune system that influence the extent to which skin sensitization is acquired. The expectation is that an appreciation of the relationship between the characteristics of adaptive immune responses to contact allergens and the extent of sensitization will allow extrapolation to defining the essential features of immune responses that govern the relative skin sensitizing potency of contact allergens. Identification of those key elements that drive relative potency may also facilitate the construction of mathematical paradigms for the characterization of skin sensitization responses.
We and others have speculated previously that the potency of skin sensitization might be a function of one or more of three aspects of the adaptive immune response. These are the: (a) vigor of T-lymphocyte proliferation and the extent of clonal expansion; (b) balance between various functional subsets of T-lymphocytes (including the relative number of antigen responsive CD4 and CD8 T-lymphocytes) and the relationship between Treg and effector cells; and (c) promiscuity of the immune response and number of clones responsive to inducing contact allergen (Kimber et al., Citation2012). The LTT gives us the opportunity, in humans, to attempt to define the critical immunological events that dictate the relative sensitizing potency of contact allergens.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Adler, S., Basketter, D., Creton, S., et al. 2011. Alternative (non-animal) methods for cosmetics testing: Current status and future prospects-2010. Arch. Toxicol. 85:367–485
- Al-Tawil, N. G., Marcusson, J. A., and Moller, E. 1981. Lymphocyte transformation test in patients with nickel sensitivity: An aid to diagnosis. Acta. Derm. Venereol. 61:511–515
- Basketter, D., Crozier, J., Hubesch, B., et al. 2012. Optimized testing strategies for skin sensitization - the LLNA and beyond. Regul. Toxicol. Pharmacol. 64:9–16
- Becker, D., and Knop, J. 1993. Mechanism in allergic contact dermatitis. Exp. Dermatol. 2:63–69
- Beeler, A., Zaccaria, L., Kawabata, T., et al. 2008. CD69 up-regulation on T–cells as an in vitro marker for delayed-type drug hypersensitivity. Allergy 63:181–188
- Berard, F., Marty, J. P., and Nicolas, J. F. 2003. Allergen penetration through the skin. Eur. J. Dermatol. 13:324–330
- Bordignon, V., Palamara, F., Cordiali-Fei, P., et al. 2008. Nickel-, palladium-, and rhodium- induced IFN-gamma and IL-10 production as assessed by in vitro ELISpot-analysis in contact dermatitis patients. BMC Immunol. 9:19
- Boyman, O. 2010. Bystander activation of CD4+ T-cells. Eur. J. Immunol. 40:936–939
- Bruze, M., Isaksson, M., Edman, B., et al. 1995. A study on expert reading of patch test reactions: Inter-individual accordance. Contact Dermatitis 32:331–337
- Cavani, A., Albanesi, C., Traidl, C., et al. 2001. Effector and regulatory T-cells in allergic contact dermatitis. Trends Immunol. 22:118–120
- Cavani, A., De Pita, O., and Girolomoni, G. 2007. New aspects of the molecular basis of contact allergy. Curr. Opin. Allergy Clin. Immunol. 7:404–408
- Cavani, A., Mei, D., Guerra, E., et al. 1998. Patients with allergic contact dermatitis to nickel and non-allergic individuals display different nickel-specific T cell responses. Evidence for the presence of effector CD8+ and regulatory CD4+ T-cells. J. Invest. Dermatol. 111:621–628
- Chipinda, I., Hettick, J. M., and Siegel, P. D. 2011. Haptenation: Chemical reactivity and protein binding. J. Allergy (Cairo) 2011:839682
- Cleaver, J. E. 1967. Thymidine metabolism and cell kinetics. Amsterdam: North-Holland Publishing Co
- Coulter, E. M., Jenkinson, C., Farrell, J., et al. 2010. Measurement of CD4+ and CD8+ T-lymphocyte cytokine secretion and gene expression changes in p-phenylenediamine-allergic patients and tolerant Individuals. J. Invest. Dermatol. 130:161–174
- Coulter, E. M., Jenkinson, C., Wu, Y., et al. 2008. Activation of T-cells from allergic patients and volunteers by p-phenylenediamine and Bandrowski's base. J. Invest. Dermatol. 128:897–905
- Davis, M. D., Hylwa, S. A., and Allen, E. M. 2013. Basics of patch testing for allergic contact dermatitis. Semin. Cutan. Med. Surg. 32:158–168
- Devos, S. A., and van der Valk, P. G. 2001. The risk of active sensitization to PPD. Contact Dermatitis 44:273–275
- Devos, S. A., and van der Valk, P. G. 2002. Epicutaneous patch testing. Eur. J. Dermatol. 12:506–513
- Everness, K. M., Gawkrodger, D. J., Botham, P. A., and Hunter, J. A. 1990. The discrimination between nickel-sensitive and non-nickel-sensitive subjects by an in vitro lymphocyte transformation test. Br. J. Dermatol. 122:293–298
- Friedmann, P. S. 2007. The relationships between exposure dose and response in induction and elicitation of contact hypersensitivity in humans. Br. J. Dermatol. 157: 1093–1102
- Fryauff, D. J., Mouzin, E., Church, L. W., et al. 1999. Lymphocyte response to tetanus toxin T-cell epitopes: Effects of tetanus vaccination and concurrent malaria prophylaxis. Vaccine 17:59–63
- Fulcher, D., and Wong, S. 1999. Carboxyfluorescein succinimidyl ester-based proliferative assays for assessment of T-cell function in the diagnostic laboratory. Immunol. Cell. Biol. 77:559–564
- Gamerdinger, K., Moulon, C., Karp, D. R., et al. 2003. A new type of metal recognition by human T cells: Contact residues for peptide-independent bridging of T-cell receptor and major histocompatibility complex by nickel. J. Exp. Med. 197:1345–1353
- Gaspard, I., Guinnepain, M. T., Laurent, J., et al. 2000. Il-4 and IFN-gamma mRNA induction in human peripheral lymphocytes specific for beta-lactam antibiotics in immediate or delayed hypersensitivity reactions. J. Clin. Immunol. 20:107–116
- Gerberick, G. F., and Robinson, M. K. 2000. A skin sensitization risk assessment approach for evaluation of new ingredients and products. Am. J. Contact Dermatitis 11:65–73
- Gerdes, J., Lemke, H., Baisch, H., et al. 1984. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 133:1710–1715
- Gonchoroff, N. J., Katzmann, J. A., Currie, R. M., et al. 1986. S-phase detection with an antibody to bromodeoxyuridine. Role of DNase pretreatment. J. Immunol. Meth. 93:97–101
- Grabbe, S., and Schwarz, T. 1998. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol. Today 19:37–44
- Hanafusa, T., Azukizawa, H., Matsumura, S., and Katayama, I. 2012. The predominant drug-specific T-cell population may switch from cytotoxic T-cells to regulatory T-cells during the course of anticonvulsant-induced hypersensitivity. J. Dermatol. Sci. 65:213–219
- Humphreys, N. E., Dearman, R. J., and Kimber, I. 2003. Assessment of cumulative allergen-activated lymph node cell proliferation using flow cytometry. Toxicol. Sci. 73:80–89
- Jenkinson, C., Jenkins, R. E., Aleksic, M., et al. 2010. Characterization of p-phenylenedi-amine-albumin binding sites and T-cell responses to hapten-modified protein. J. Invest. Dermatol. 130:732–742
- Jensen, C. D., Paulsen, E., and Andersen, K. E. 2006. Retrospective evaluation of the consequence of alleged patch test sensitization. Contact Dermatitis 55:30–35
- Jensen, C. S., Lisby, S., Baadsgaard, O., et al. 2002. Decrease in nickel sensitization in a Danish schoolgirl population with ears pierced after implementation of a nickel-exposure regulation. Br. J. Dermatol. 146:636–642
- Joy, N. M., Rice, K. R., and Atwater, A. R. 2013. Stability of patch test allergens. Dermatitis 24:227–236
- Kano, Y., Hirahara, K., Mitsuyama, Y., et al. 2007. Utility of the lymphocyte transformation test in the diagnosis of drug sensitivity: Dependence on its timing and the type of drug eruption. Allergy 62:1439–1444
- Kapsenberg, M. L., Res, P., Bos, J. D., et al. 1987. Nickel-specific lymphocyte-T clones derived from allergic nickel-contact dermatitis lesions in man: Heterogeneity based on requirement of dendritic antigen-presenting cell subsets. Eur. J. Immunol. 17:861–865
- Kapsenberg, M. L., Wierenga, E. A., Stiekema, F. E., et al. 1992. TH1 lymphokine production profiles of nickel-specific CD4+T-lymphocyte clones from nickel contact allergic and non-allergic individuals. J. Invest. Dermatol. 98:59–63
- Kehren, J., Desvignes, C., Krasteva, M., et al. 1999. Cytotoxicity is mandatory for CD8+ T-cell-mediated contact hypersensitivity. J. Exp. Med. 189:779–786
- Kimber, I., and Dearman, R. J. 2002. Allergic contact dermatitis: Cellular effectors. Contact Dermatitis 46:1–5
- Kimber, I., Basketter, D. A., Berthold, K., et al. 2001. Skin sensitization testing in potency and risk assessment. Toxicol. Sci. 59:198–208
- Kimber, I., Basketter, D. A., Gerberick, G. F., et al. 2011. Chemical allergy: Translating biology into hazard characterization. Toxicol. Sci. 120(Suppl 1):S238–268
- Kimber, I., Maxwell, G., Gilmour, N., et al. 2012. Allergic contact dermatitis: Commentary on the relationship between T-lymphocytes and skin sensitizing potency. Toxicology 291:18–24
- Kimber, I., Quirke, S., and Beck, M. H. 1990. Attempts to identify the causative allergen in cases of allergic contact dermatitis using an in vitro lymphocyte transformation test. Toxicol. In Vitro 4:302–306
- Kish, D. D., Gorbachev, A. V., Parameswaran, N., et al. 2012. Neutrophil expression of Fas ligand and perforin directs effector CD8 T-cell infiltration into antigen-challenged skin. J. Immunol. 189:2191–2202
- Kneilling, M., Caroli, U., Grimmel, C., et al. 2010. p-Phenylenediamine-specific lymphocyte activation test: A sensitive in vitro assay to detect p-phenylenediamine sensitization in patients with severe allergic reactions. Exp. Dermatol. 19:435–441
- Lalko, J. F., Kimber, I., Dearman, R. J., et al. 2011. Chemical reactivity measurements: Potential for characterization of respiratory chemical allergens. Toxicol. In Vitro 25:433–445
- Landsteiner, K., and Jacobs, J. 1935. Studies on the sensitization of animals with simple chemical compounds. J. Exp. Med. 61:643–656
- Mallone, R., Mannering, S. I., Brooks-Worrell, B. M., and on behalf of the Immunology of Diabetes Society, T. C. W. C. 2011. Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T-cell responses: Position statement of the T-Cell Workshop Committee of the Immunology of Diabetes Society. Clin. Exp. Immunol. 163:33–49
- Martin, S., Esser, P., Schmucker, S., et al. 2010. T-cell recognition of chemicals, protein allergens and drugs: Towards the development of in vitro assays. Cell. Mol. Life Sci. 67:4171–4184
- Martin, S., Ortmann, B., Pflugfelder, U., et al. 1992. Role of hapten-anchoring peptides in defining hapten-epitopes for MHC-restricted cytotoxic T cells. Cross-reactive TNP-determinants on different peptides. J. Immunol. 149:2569–2575
- Martin, S. F. 2004. T-lymphocyte-mediated immune responses to chemical haptens and metal ions: Implications for allergic and autoimmune disease. Int. Arch. Allergy Immunol. 134:186–198
- Martin, S. F. 2012. Allergic contact dermatitis: Xeno-inflammation of the skin. Curr. Opin. Immunol. 24:720–729
- Masjedi, K., Ahlborg, N., Gruvberger, B., et al. 2003. Methylisothiazo-linones elicit increased production of both T helper (TH)1- and TH2-like cytokines by peripheral blood mononuclear cells from contact allergic individuals. Br. J. Dermatol. 149:1172–1182
- McFadden, J. P., Yeo, L., and White, J. L. 2011. Clinical and experimental aspects of allergic contact dermatitis to p-phenylenediamine. Clinics Dermatol. 29:316–324
- Moed, H., Von Blomberg, M., Bruynzeel, D. P., et al. 2005. Improved detection of allergen-specific T-cell responses in allergic contact dermatitis through the addition of ‘cytokine cocktails'. Exp. Dermatol. 14:634–640
- Moulon, C., Vollmer, J., and Weltzien, H. U. 1995. Characterization of processing requirements and metal cross-reactivities in T-cell clones from patients with allergic contact dermatitis to nickel. Eur. J. Immunol. 25:3308–3315
- Mowad, C. M. 2006. Patch testing: Pitfalls and performance. Curr. Opin. Allergy Clin. Immunol. 6:340–344
- Nakano, O., Sato, M., Naito, Y., et al. 2001. Proliferative activity of intratumoral CD8+ T-lymphocytes as a prognostic factor in human renal cell carcinoma: Clinico-pathologic demonstration of antitumor immunity. Cancer. Res. 61:5132–5136
- Newman, W., Stoner, G. L., and Bloom, B. R. 1977. Primary in vitro sensitization of human T-cells. Nature 269:151–153
- Nguyen, S. H., Dang, T. P., Macpherson, C., et al. 2008. Prevalence of patch test results from 1970 to 2002 in a multi-centre population in North America (NACDG). Contact Dermatitis 58:101–106
- Nosbaum, A., Vocanson, M., Rozieres, A., et al. 2009. Allergic and irritant contact dermatitis. Eur. J. Dermatol. 19:325–332
- Novak, N., Haberstok, J., Geiger, E., and Bieber, T. 1999. Dendritic cells in allergy. Allergy 54:792–803
- Nyfeler, B., and Pichler, W. J. 1997. The lymphocyte transformation test for the diagnosis of drug allergy: Sensitivity and specificity. Clin. Exp. Allergy 27:175–181
- Ortmann, B., Martin, S., Von Bonin, A., et al. 1992. Synthetic peptides anchor T-cell-specific TNP epitopes to MHC antigens. J. Immunol. 148:1445–1450
- Pacheco, K., Barker, L., Maier, L., et al. 2013. Development of a validated blood test for nickel sensitization. J. Allergy Clin. Immunol. 132:767–769
- Pichler, W. J. 2001. Predictive drug allergy testing: An alternative viewpoint. Toxicology 158:31–41
- Pichler, W. J., and Tilch, J. 2004. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy 59:809–820
- Pickard, C., Smith, A. M., Cooper, H., et al. 2007. Investigation of mechanisms underlying the T-cell response to the hapten 2,4-dinitrochlorobenzene. J. Invest. Dermatol. 127:630–637
- Porebski, G., Pecaric-Petkovic, T., Groux-Keller, M., et al. 2013. In vitro drug causality assessment in Stevens-Johnson syndrome - alternatives for lymphocyte transformation test. Clin. Exp. Allergy 43:1027–1037
- Quah, B. J., Warren, H. S., and Parish, C. R. 2007. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat. Protoc. 2:2049–2056
- Rasanen, L., and Tuomi, M. L. 1992. Diagnostic value of the lymphocyte proliferation test in nickel contact allergy and provocation in occupational coin dermatitis. Contact Dermatitis 27:250–254
- Rasanen, L., Sainio, H., Lehto, M., and Reunala, T. 1991. Lymphocyte proliferation test as a diagnostic aid in chromium contact sensitivity. Contact Dermatitis 25:25–29
- Roberts, D. W., and Aptula, A. O. 2008. Determinants of skin sensitization potential. J. Appl. Toxicol. 28:377–387
- Rustemeyer, T., Von Blomberg, B. M., Van Hoogstraten, I. M., et al. 2004. Analysis of effector and regulatory immune reactivity to nickel. Clin. Exp. Allergy. 34:1458–1466
- Saint-Mezard, P., Berard, F., Dubois, B., et al. 2004. The role of CD4+ and CD8+ T-cells in contact hypersensitivity and allergic contact dermatitis. Eur. J. Dermatol. 14:131–138
- Seldin, M. F., and Rich, R. R. 1978. Human immune responses to hapten-conjugated cells. I. Primary and secondary proliferative responses in vitro. J. Exp. Med. 147:1671–1683
- Sherertz, E. F., Fransway, A. F., Belsito, D. V., et al. 2001. Patch testing discordance alert: False-negative findings with rubber additives and fragrances. J. Am. Acad. Dermatol. 45:313–314
- Silvennoinen-Kassinen, S. 1980. Lymphocyte transformation in nickel allergy: Amplification of T-lymphocyte responses to nickel sulphate by macrophages in vitro. Scand. J. Immunol. 12:61–65
- Soares, A., Govender, L., Hughes, J., et al. 2010. Novel application of Ki67 to quantify antigen-specific in vitro lymphoproliferation. J. Immunol. Meth. 362:43–50
- Sodomann, C. P., Rother, M., Havemann, K., and Martini, G. A. 1979. Lymphocyte proliferation to Phytohemagglutinin (PHA) in hepatitis B antigen-positive and -negative hepatitis. Res. Exp. Med. 175:95–107
- Spiewak, R., Moed, H., Von Blomberg, B. M., et al. 2007. Allergic contact dermatitis to nickel: Modified in vitro test protocols for better detection of allergen-specific response. Contact Dermatitis 56:63–69
- Sugita, K., Kabashima, K., Sawada, Y., et al. 2012. Blocking of CTLA–4 on lymphocytes improves the sensitivity of lymphocyte transformation tests in a patient with nickel allergy. Eur. J. Dermatol. 22:268–269
- Sullivan, S., Bergstresser, P. R., Tigelaar, R. E., and Streilein, J. W. 1986. Induction and regulation of contact hypersensitivity by resident, bone marrow-derived, dendritic epidermal cells: Langerhans cells and Thy-1+ epidermal cells. J. Immunol. 137:2460–2467
- Svejgaard, E., Morling, N., Svejgaard, A., and Veien, N. K. 1978. Lymphocyte transformation induced by nickel sulphate: An in vitro study of subjects with and without a positive nickel patch test. Acta. Derm. Venereol. 58:245–250
- Thepen, T., Langeveld-Wildschut, E. G., Bihari, I. C., et al. 1996. Biphasic response against aeroallergen in atopic dermatitis showing a switch from an initial TH2 response to a TH1 response in situ: An immunocytochemical study. J. Allergy Clin. Immunol. 97:828–837
- Thierse, H. J., Gamerdinger, K., Junkes, C., et al. 2005. T-cell receptor (TCR) interaction with haptens: Metal ions as non-classical haptens. Toxicology 209:101–107
- Toncic, R. J., Lipozencic, J., Martinac, I., and Greguric, S. 2011. Immunology of allergic contact dermatitis. Acta. Dermatovenerol. Croat. 19:51–68
- Tough, D. F., Borrow, P., and Sprent, J. 1996. Induction of bystander T-cell proliferation by viruses and Type I interferon in vivo. Science 272:1947–1950
- Vocanson, M., Cluzel-Tailhardat, M., Poyet, G., et al. 2008. Depletion of human peripheral blood lymphocytes in CD25+ cells allows for the sensitive in vitro screening of contact allergens. J. Invest. Dermatol. 128:2119–2122
- Vocanson, M., Hennino, A., Chavagnac, C., et al. 2005. Contribution of CD4+ and CD8+ T-cells in contact hypersensitivity and allergic contact dermatitis. Expert Rev. Clin. Immunol. 1:75–86
- Vocanson, M., Hennino, A., Cluzel-Tailhardat, M., et al. 2006. CD8+ T-cells are effector cells of contact dermatitis to common skin allergens in mice. J. Invest. Dermatol. 126:815–820
- von Blomberg-van der Flier, M., van der Burg, C. K., Pos, O., et al. 1987. In vitro studies in nickel allergy: Diagnostic value of a dual parameter analysis. J. Invest. Dermatol. 88:362–368
- Wahlberg, J. E., and Lindberg, M. 2006. Patch testing. Contact Dermatitis 4:366–390
- Werfel, T., Hentschel, M., Kapp, A., and Renz, H. 1997. Dichotomy of blood- and skin-derived IL-4-producing allergen-specific T-cells and restricted V® repertoire in nickel-mediated contact dermatitis. J. Immunol. 158:2500–2505
