Abstract
Endosulfan, a chlorinated hydrocarbon insecticide/acaricide, is a member of a cyclodiene sub-group of poisons to a wide variety of insects and mites. It is also toxic to humans and animals, but there is limited knowledge about endosulfan-related splenic and overall immunotoxicity. The aim of this study was to review pathological findings of endosulfan toxicity in the spleen and to examine potential protective effects of the anti-oxidant Vitamin C (Vit C). Here, after 6-week exposures, the spleens of New Zealand White rabbits were examined grossly and histopathologically and tissue caspase-3 activity was assessed immunohistochemically. Rabbits in four groups were used: Group END were given by oral gavage a sub-lethal dose of endosulfan (1 mg/kg) in corn oil daily for 6 weeks; Group END + C received the same dose of endosulfan daily and Vit C (20 mg/kg) every other day by gavage during this period; Group Vit C received oral corn oil daily and 20 mg/kg Vit C every other day; and Group OIL received corn oil daily for 6 weeks. Analyses of the tissues collected 1 week after the final dosing revealed lymphocyte depletion and necrosis in spleens of the hosts that received the pesticide (END only and END + C); hemorrhage and slight neutrophilic infiltration was also noted. Caspase-3 immunoreactivity was marked in lymphocytes in all spleens of rabbits in both END groups. Overall, these toxicities were mitigated by Vit C co-treatment; in END + C hosts, markedly decreased depletion of lymphocytes, inflammation and caspase-3 immunoreactivity were observed. However, even with mitigation, the level of toxicity present was still greater than any seen in the spleens of hosts that received OIL or Vit C alone. These results revealed endosulfan could cause toxicity in the rabbit spleen, characterized by depletion of lymphocytes, inflammation, necrosis and hemorrhage, and that this toxicity could begin to be mitigated by Vit C co-treatment.
Keywords:
Introduction
Endosulfan is a member of the cyclodiene organocholorine pesticides used worldwide in agriculture due to its low volatility, chemical stability, lipid solubility and slow rate of biotransformation and degradation (Rose et al. Citation1999). Although endosulfan is banned in some countries, it is still commonly used thoroughout the world. Numerous studies have reported that endosulfan has been detected in different foods (Strandberg and Hites Citation2001). Further evidence of human endosulfan exposure in regulated countries was confirmed by its presence in the blood or human milk (Arrebola et al. Citation2001; Harris et al. Citation2001).
Mechanisms of cell death fall into two major types; necrosis and apoptosis. The latter is the counterpart and counterbalance to mitosis in cell population determination. Apoptosis plays a key role in the control of cellular populations in the immune system (Cohen et al. Citation1995). Apoptosis can be triggered by a variety of stimuli, including cytokines, hormones, viruses and toxins. In this process, proteases play a critical role, with cysteine-dependent aspartate-directed proteases (caspases) being the most recognized of the proteases (Earnshaw et al. Citation1999). Caspase-3 has been implicated as a key mediator of apoptosis (Kuida et al. Citation1996).
Immunity is necessary for a healthy life and requires a series of delicately balanced, complex multicellular physiological mechanisms that allow an individual to distinguish foreign material from the self and either neutralize or eliminate it (Burns et al. Citation1996). The immune system consists of two distinct but inter-related entities, humoral and cell-mediated immunity (Elgert Citation1996). Organs of the immune system are divided into primary and secondary organs. The spleen is a secondary organ and it has been further sub-classified as either a storage or defense spleen. Although the spleen may serve both functions, in many species one function often predominates. The spleen of the rabbit and humans is an example of a defense spleen (Saercy Citation2001).
Vitamin C (ascorbic acid) is essential for both cellular growth and differentiation; it is also important for optimal functioning of the immune system (Ai et al. Citation2006; Wintergerst et al. Citation2006; Maggini et al. Citation2010). Infections and delayed wound healing are common findings of clinical Vitamin C deficiency (Carr and Frei Citation1999; Bruno et al. Citation2006; Wintergerst et al. Citation2006). It is also known that the anti-oxidant effects from this molecule contributes to protection of immune (and most other types of cells) from exogenous or endogenous reactive oxygen species that can form during toxicant exposure and/or during ongoing inflammatory responses in a host (Wintergerst et al. Citation2006).
Among its numerous toxicities, endosulfan is immunotoxic and inhibits the metabolic activity of peripheral blood phagocytes in various animal models (Pistl et al. Citation2001; Bharath et al. Citation2011; Singh et al. Citation2011). Gross paleness and atrophy in the spleen were reported after dermal administration of endosulfan to rabbits (Bakili Citation1997). Decreased spleen/body weight ratio after sub-chronic (22 week) oral treatment with endosulfan and other immunosuppressive effects such as paleness/atrophy of the spleen, marked suppression of humoral and cell-mediated immune responses, significant decrease of lymphocyte activation, changes in delayed-hypersensitivity responses and suppression of TH1 or TH2 cytokines levels have also been reported (Pistl et al. Citation2001; Bakili Citation1997; Banerjee and Hussain Citation1986; Pal et al. Citation2009). Nevertheless, knowledge about specific toxicity of endosulfan into the spleen at the histopathologic and immunohistochemical levels remains unknown.
Accordingly, the aim of the study presented here was to investigate whether sub-acute dosing with endosulfan-induced pathological changes and apoptosis in the spleen. This study, using a rabbit model, also investigated whether concurrent administration of Vit C could modulate any effects induced by this toxicant.
Materials and methods
Animals
The experiment was approved by the Institutional Animal Use and Care Committee of the Akdeniz University and performed in accordance with the National Institutes of Health Guidelines for the Care and Handling of Animals. New Zealand white rabbits (male, 6–8 months old, 2.8–3.7 kg) were obtained from Akdeniz University (Antalya, Turkey) for use in the study. All rabbits were housed individually in plastic cages in a pathogen-free facility maintained at 22°C with a 60% relative humidity and a 12-h light:dark cycle.
Rabbits were provided ad libitum access to a diet of standard rabbit chow (88% dry matter, 9% ash, 16% crude protein, 15% crude fiber; 2600 kcal/kg of metabolizable energy) and filtered tap water. Body weights were recorded weekly throughout the experimental period and doses of endosulfan and Vit C were adjusted accordingly. The physical condition of each rabbit was assessed daily for any obvious signs of toxicities/morbidity.
Chemicals
Commercial endosulfan (94.1% purity, 33% β and 67% α isomers, respectively) was used in this study (Fertil Kimya, Konya, Turkey). A stock solution containing 3 mg endosulfan was dissolved in 1 ml corn oil. Vitamin C (Bayer, Istanbul, Turkey) was dissolved in tap water at a level of 20 mg/ml. Doses of endosulfan and Vitamin C were adjusted according to changes in animal weight; because the stock solutions could be adjusted with vehicle to accommodate the changing weights of the hosts, in no case did the volume of either agent delivered during each gavage ever exceed 6 ml/animal. Each stock was prepared daily and used fresh.
Experimental design
Twenty-four rabbits were randomly allocated into four groups (n = 6/group). Rabbits in the END group were to be given a sub-lethal concentration (1 mg/kg BW/day) of endosulfan in corn oil. Those in the END + C group were to receive endosulfan (1 mg/kg) daily and also Vitamin C (20 mg/kg) on alternate days. Rabbits in the Vit C group were to receive corn oil daily and Vitamin C on alternate days. Rabbits in the OIL control group were to receive only the corn oil daily. All dosings were by oral gavage; dosing lasted a total of 6 weeks. The dose of ascorbic acid was equivalent to double the human therapeutic dose (Khan and Sinha Citation1994); the dose of endosulfan was selected based on sub-lethal toxication, as determined in earlier studies from our faculty (Ozdem et al. Citation2011).
Histopathological evaluation
One week after the final treatment, all rabbits were euthanized by intravenous injection of an overdose of pentobarbital (I.E. Ulugay, Istanbul) and then subjected to necropsy. Tissue samples of the spleen were fixed in 10% formalin, embedded in paraffin and sections cut (to 5 μm). Several of the slides were then stained with hematoxylin and eosin (H&E). Slides from each animal were then examined in a blinded manner under light microscopy to assess any overt toxicities in the organ.
The histopathology findings were scored for severity of lesions in all animals in each group using a scale ranging between 0–3, wherein 0 = no lesion, 1 = mild (slight hyperemia and a slight number of cells that exhibited necrotic changes (such as karyopyknosis and karyorexis;), 2 = moderate (small group of necrotic cells) and 3 = severe (large excessive necrotic areas).
Immunohistochemical evaluation
For evaluation of apoptosis, several slides from each rabbit were stained for the presence of caspase-3 by immunohistochemistry; specifically, a Caspase-3 (CPP32) Ab-4 kit was used (Thermo Scientific, Boston, MA) according to manufacturer instructions. To evaluate the percentage of immuno-positive cells, 100 cells in 10 different high-powered fields on each slide were examined (under 40 × objective) using a Nikon E600 trinocular microscope (Nikon, Tokyo, Japan); photomicrographs were taken using an in-line Nikon Coolpix P7100 camera. From the number of caspase+ cells/100 cells analyzed, average expression levels were calculated for rabbits in each treatment group. A minimum of three slides/rabbit was evaluated in all groups.
Statistical analysis
Data are presented in terms of means ± SD. A one-way analysis of variance (ANOVA) test was used to detect differences between groups in the analyses of the caspase+ cell counts. For within-group determination of differences, a non-parametric Duncan multiple comparison method was used. All analyses were done using SPSS 13.0 (SPSS, Chicago, IL). A p value < 0.05 was accepted as statistically significant.
Results
After 6 weeks of treatment, no mortality or clinical symptoms were observed in any group during the experiment. At necropsy, no overt pathological findings were noticed in any of the spleens. No splenic lesions were seen, except in the rabbits in the END groups. In the organ of these rabbits, there were degenerative and necrotic changes; histopathology scores are provided in . These spleens also appeared atrophic and characterized by an effacement of germinal centers, diminution of the white pulp and congestion in the red pulp. Microscopically, lymphocyte depletion and necrotic areas were seen. Karyopyknosis and karyorexis were commonly seen within the splenic lymphocytes that were still present. In addition, hemorrhage and a slight degree of neutrophil infiltration was also noticed in some of the spleens in this group (). Decreased cellularity in the periarteriolar lymphoid sheath (PALS) region was a common finding in the END group. In two rabbits, slight increases in the presence of plasma cells, macrophages and pigmented macrophages, in addition to neutrophil infiltration, were observed. There was no evidence of fibrosis in any groups’ spleens. Furthermore, rabbits in the OIL and Vit C groups consistently had normal tissue architecture in their spleens.
Figure 1. Representative spleen histopathology from rabbits in different groups. (A) A spleen with normal histology in Vit C group, H&E stain; 40 × magnification. Note: Rabbits in the OIL and Vit C groups had similar histologies. (B) A spleen with marked necrosis in END group (arrow heads), H&E stain; 10 × magnification. (C) A spleen with slight lymphocyte depletion in END + C group, H&E stain; 20 × magnification. Representative spleen immunohistochemistry from rabbits in different groups. (D) A spleen with scattered caspase-3+ cells in Vit C group, Streptavidin-biotin peroxidase method; Harris hematoxylin counterstain. 40 × magnification. (E) A spleen with numerous caspase-3 immunoreactions (indicating significant apoptosis) among cells in rabbits in the END groups. Streptavidin-biotin peroxidase method; Harris hematoxylin counter stain. 40 × magnification. (F) A spleen with a slight degree of caspase-3 immunoreactions indicating a decrease in apoptosis among cells present in the END + C group, Streptavidin-biotin peroxidase method; Harris hematoxylin counterstain. 40 × magnification.
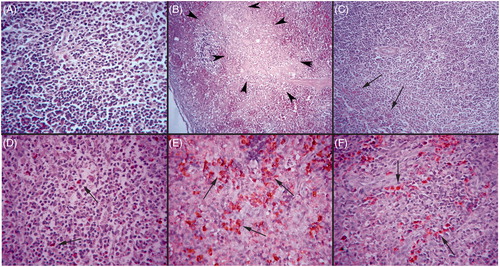
Table 1. Percentages of caspase-3+ cells and histopathology scores in the spleen.
A presence of caspase-3+ cells was marked and severe in the spleens of all rabbits in both groups that received END for the 6-week period. When evaluated, the spleens of rabbits that received END alone had 39.71 ± 5.37% caspase-3+ cells, while those that received ENDO in conjunction with Vit C had 13.33 ± 4.17% caspase-3+ cells (). Slight apoptotic activity was also seen in some cells in spleens of the OIL control (7.66 ± 2.16%) and Vit C only (5.83 ± 1.47%) rabbits. This data showed there was a significant ameliorating effect of Vit C on this particular finding; however, the protection was not complete as the percentages of caspase-3+ cells was still significantly greater than that in spleens of the other two ‘control’ populations of rabbits ().
These results illustrated that endosulfan could potentially cause immune suppression in the rabbit by inducing lymphocyte depletion, inflammatory reactions, necrosis and hemorrhage in their spleen. Furthermore, this study showed that such toxicity was decreased by concurrent Vit C treatment in that the Vit C reduced histopathologic and immunohistochemical lesions in the spleens of the rabbits in the END + C group.
Discussion
Endosulfan can cause toxic effects in different organs (Mor and Ozmen Citation2003, Citation2010a,Citationb; Ozmen et al. Citation2010; Ozmen Citation2011; Ozmen and Mor Citation2012, Citation2015). Some authors reported an immunosuppressive effect of endosulfan also (Bakili, Citation1997; Pistl et al., Citation2001; Bharath et al. Citation2011; Singh et al. Citation2011), but there is a little information about spleen lesions in this toxication. Data reported here demonstrate that exposure to endosulfan results in pathological changes in spleen in rabbits.
The function of the spleen is dependent on the systemic circulation; as such, it lacks afferent lymphatic vessels. When endosulfan is present in excess amounts, it will automatically be circulated and affect all the body’s organs, including lymphoid organs. An examination of histopathological features here revealed some morphological changes in the spleen of the endosulfan exposed rabbits. Changes in the spleen as an immune system organ were examined in this study and possible immunotoxic effects of this chemical were reported.
The immune system is a complex network of interacting regulatory genes, hormones and cells that has evolved in multicellular organisms for the purpose of maintaining a homeostasis against a dynamic battery of foreign environmental agents and pathogens. Environmental pollutants can interfere with the normal function of the immune system, leading to a broad range of disorders. Widespread use of the endosulfan increases the chance that more individuals will be exposed to it, potentially at toxic levels. Immunotoxicity of endosulfan manifests as a decrease in immune response capacity, including the suppression of immune cell function and atrophy of immune organs (Bakili Citation1997; Pistl et al. Citation2001; Pal et al. Citation2009; Singh et al. Citation2011). This study adds to a growing list of publications reporting on the adverse physiological effects of endosulfan. Our study showed that endosulfan caused necrotic changes and increased apoptotic activity in the spleen.
The role of toxin-induced apoptosis has been a major theme in toxicological evaluation, although the relative contribution of apoptosis caused by toxins remains unclear. Our previous studies showed that cytotoxic effects of endosulfan in different organs of rabbits are mediated by inducing apoptosis (Mor and Ozmen Citation2003, Citation2010a,Citationb; Ozmen et al. Citation2010; Ozmen Citation2011; Ozmen and Mor Citation2012, Citation2015). This study supported that endosulfan – apart from already being shown by others to be neurotoxic, hepatotoxic, cardiotoxic and nephrotoxic – is also immunotoxic. The immuno-histochemical study outcomes here showed there was a significantly higher expression of active caspase-3 in the spleens of rabbits after endosulfan exposures. Consequently, those lymphocytes would (and did) exhibit morphological characteristics of cells undergoing apoptosis, such as karyopyknosis, apoptotic bodies, etc. Hence, the function of these spleens was likely impaired by the death of their constituent lymphocytes.
There are reports in the literature about immunotoxic effects of endosulfan (Bakili Citation1997; Kurkure et al. Citation1993); however, specific effects in/on the spleen remain relatively unknown. This study reported here provided the first evidence of endosulfan inducing apoptosis in lymphocytes in the spleen and suggested to us the potential existence of a novel cytotoxic mechanism of action for endosulfan.
Different nutrients, especially anti-oxidants, have been demonstrated as being required for adequate immune responses in animal and human studies (Bruno et al. Citation2006; Wintergerst et al. Citation2006; Pal et al. Citation2009). Nutrient deficiency affects innate, adaptive and cellular immune responses and suppresses immune function, with the resulting dysregulation of the co-ordinated host response to something like an infection being impaired and, accordingly, leading to an increase in the virulence of the challenging pathogen(s). Vitamin C is a major component of the body anti-oxidant system and is known to provide protection from the toxic effects of many exogenous/endogenous toxicants, including reactive oxygen species (ROS). While ROS are generated as by-products of normal aerobic respiration, they are also readily formed during inflammation and during host cell responses to exposure to environmental toxicants (McGregor and Biesalski Citation2006; Pavlovic and Sarac Citation2010). Because the data here showed that Vit C co-treatment appeared to block/mitigate toxicities in rabbits, we surmise that the splenotoxic effects of endosulfan may be mediated in great part by the formation of ROS and/or other agents that are subject to chemical reduction in situ.
With regard to the actual splenotoxicity noted in the END-treated rabbits, caspase-positive lymphocytes/cells are normally found scattered throughout the parenchyma, even in a normal rabbit spleen. The findings here in the control rabbit spleens were in agreement with those of a previous study (Roitt Citation1994). In the control spleen, a slight presence of caspase-3+ cells was present in the white pulp. It is known that T- and B-cells in the spleen are largely separated into different anatomical compartments: B-cells in the follicle, germinal center and marginal zone and T-cells in the peri-arteriolar lymphocyte sheath and marginal zones (Roitt Citation1994). Because of the marginal zone localization of caspase-3+ cells in the END-treated host, it is surmised that B-cells were the primary cell type being affected by endosulfan. Clearly, future studies of B-cell numbers/functionality will need to be performed to verify this is the case in the rabbit and other animal hosts to validate the novel mechanism of toxic action for this agent.
Acknowledgements
The author would like to acknowledge Dr Firdevs Mor for kindly providing support during the study.
Disclosure statement
The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Ai Q, Mai K, Tan B, Xu W, Zhang W, Ma M, Liufu Z. 2006. Effects of dietary vitamin C on survival, growth, and immunity of large yellow croaker Pseudosciaena Crocea. Aquaculture 261:327–336.
- Arrebola FJ, Martinez Vidal JL, Fernandez-Gutierrez A. 2001. Analysis of endosulfan and its metabolites in human serum using gas chromatography-tandem mass spectrometry. J Chromatog Sci. 39:177–182.
- Bakili RA. 1997. Acute Dermal Toxicity Study to Rabbit. Gujarat, India: Department of Toxicology, Jai Research Foundation, Project ID #1026/JRF/97; 6/14/97.
- Banerjee BD, Hussain QZ. 1986. Effect of sub-chronic endosulfan exposure on humoral and cell-mediated immune responses in albino rats. Arch Toxicol. 59:279–284.
- Bharath BK, Anjaneyulu Y, Srilatha CH. 2011. Imuunomodulatory effect of Ocimum sanctum against endosulfan-induced immunotoxicity. Vet World 4:25–27.
- Bruno EJ Jr, Ziegenfuss, TN, Landis J. 2006. Vitamin C: Research update. Curr Sports Med Rep. 5:177–181.
- Burns LA, Meade BJ, Munson AE. 1996. Toxic responses of the immune system. In: Klaassen CD, Ed. Casarett and Doul’s toxicology: the basic science of poisons. 5th ed. New York: McGraw Hill. p. 355–402.
- Carr AC, Frei B. 1999. Toward a new recommended dietary allowance for Vitamin C-based on anti-oxidant and health effects in humans. Am J Clin Nutr. 69:1086–1107.
- Cohen MG, Macfarlane M, Fearnhead HO, Sun X, Dinsdale D. 1995. Mechanisms of cell death, with particular reference to apoptosis. In: Matteis FD, Smith LL, Eds. Molecular and cellular mechanisms of toxicity. Boca Raton (FL): CRC Press. p. 185–205.
- Earnshaw WC, Martins LM, Kaufmann SH. 1999. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Ann Rev Biochem. 68:383–424.
- Elgert KD. 1996. Immunology Understanding the Immune System. Bethesda (MD): NIH Publication No. 07–5423, pp. 22–464.
- Harris CA, Woolridge MW, Hay AW. 2001. Factors affecting the transfer of organochlorine pesticide residues to breast milk. Chemosphere 43:243–256.
- Khan PK, Sinha SP. 1994. Impact of higher doses of Vitamin C in modulating pesticide genotoxicity. Teratol Carcinogen Mutagen. 14:175–181.
- Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA. 1996. Decreased apoptosis in the brain and premature lethality in CP1-deficient mice. Nature 384:368–372.
- Kurkure NV, Bhandarkar AG, Joshi MV, Sadekar RD, Bhagwat SS. 1993. Immuno-suppressive and histotoxic effects of endosulfan in chicks. Indian J Anim Sci. 63:1258–1260.
- Maggini S, Wenzlaff S, Hornig D. 2010. Essential role of Vitamin C and zinc in child immunity and health. J Internal Med Res. 38:386–414.
- McGregor GP, Biesalski HK. 2006. Rationale and impact of Vitamin C in clinical nutrition. Curr Opin Clin Nutr Metab Care 9:697–703.
- Mor F, Ozmen O. 2003. Acute endosulfan poisoning in cattle. Vet Human Toxicol. 45:323–324.
- Mor F, Ozmen O. 2010a. Effect of Vitamin C in reducing the toxicity of endosulfan in liver in rabbits. Exp Toxicol Pathol. 62:75–80.
- Mor F, Ozmen O. 2010b. Endosulfan-induced neurotoxicity and serum acetylcholinesterase inhibition in rabbits: Protective effect of Vitamin C. Pest Biochem Physiol. 96:108–112.
- Ozdem S, Nacitarhan C, Gulay MS, Hatipoglu FS, Ozdem SS. 2011. The effect of ascorbic acid supplementation on endosulfan toxicity in rabbits. Toxicol Ind Health 27:437–446.
- Ozmen O. 2011. Pathology of endosulfan. In: Stoytcheva M, Ed. Pesticides in the modern world – effects of pesticides exposure. Rijeka, Croatia: Intech Publication. p. 289–307.
- Ozmen O, Mor F. 2012. Apoptosis in adult rabbit testes during subacute endosulfan toxicity. Pest Biochem Physiol. 102:129–133.
- Ozmen O, Mor F. 2015. Effects of Vitamin C on pathology and caspase-3 activity of kidneys with subacute endosulfan toxicity. Biotech Histochem. 90:25–30.
- Ozmen O, Sahinduran S, Mor F. 2010. Pathological and immunohistochemical examination of the pancreas in subacute endosulfan toxicity in rabbits. Pancreas 39:367–370.
- Pal R, Ahmed T, Kumar V, Suke SG, Ray A, Banerjee BD. 2009. Protective effects of different antioxidants against endosulfan-induced oxidative stress and immunity in albino rats. Indian J Exp Biol. 47:723–729.
- Pavlovic V, Sarac M. 2010. A short overview of Vitamin C and selected cells of the immune system. Cent Eur J Med. 6:1–10.
- Pistl J, Kovalkovicova N, Kacmar P, Kovalkovičová N, Kačmár P, Kusová I, Mikula I, Šutiaková I. 2001. Effect of endosulfan on peripheral sheep leukocytes in vitro. Vet Human Toxicol. 43:78–82.
- Roitt IM, editor. 1994. The acquired immune response. In: Essential immunology. Boston (MA): Blackwell Scientific. p. 145–240.
- Rose RL, Hodgson E, Roe RM. 1999. Pesticides. In: Marquart H, Schafer S, McClellan R, Welsch F, Eds. Toxicology. Baltimore (MD): Academic Press. p. 165–191.
- Saercy GP. 2001. Hemopoietic system. In: Carlton WW, McGavin MD, Eds. Thomson’s special veterinary pathology. 3rd ed.. St. Louis (MO): Mosby. p. 325–381.
- Singh ND, Sharma AK, Dwivedi P, Kumar M, Patil RD 2011. Immunosuppressive effect of combined citrinin and endosulfan toxicity in pregnant Wistar rats. Vet Arch. 81:751–763.
- Strandberg B, Hites RA. 2001. Concentration of organochlorine pesticides in wine corks. Chemosphere 44:729–735.
- Wintergerst ES, Maggini S, Hornig DH. 2006. Immune-enhancing role of Vitamin C and zinc and effect on clinical conditions. Nutr Metab. 50:85–94.
