Abstract
The influence of size of nanoparticles (NP), especially in regard to pulmonary toxicity, has been widely investigated. In general, NP with smaller diameters are more pro-inflammatory in vivo, at least in terms of neutrophil influx. Nevertheless, the influence of size of NP on polymorphonuclear neutrophil (PMN) cell biology is poorly documented. In the study here, it was decided to determine if AgNP with a diameter of 70 nm (AgNP70) will alter the biology of human PMN similarly to AgNP20 previously reported to induce apoptosis and inhibit de novo protein synthesis. The results here indicated that, in contrast to AgNP20, AgNP70 delayed PMN apoptosis. However, both AgNP20 and AgNP70 inhibited de novo protein synthesis. Both forms of AgNP did not significantly increase reactive oxygen species (ROS) production, but AgNP20 significantly increased the cell production of the CXCL8 chemokine (IL-8). In addition, AgNP20, but not AgNP70, induced the release of albumin and matrix metalloproteinase-9 (MMP-9/gelatinase B) into culture supernatants. Consistent with this latter observation, gelatinase activity was increased by AgNP20, as assessed by zymography. From these outcomes, it is concluded that two NP with different initial diameters can possess similar – as well as distinct – biological properties in modulating human PMN functions. These outcomes are testimony to the complexity of the modes of action of NP at the cellular level.
Introduction
There is increasing evidence that nanoparticles (NP) can possess different biological activities according to their biophysical and chemical characteristics, including composition, surface area and diameters (Anderson et al. Citation2015; Mohamud et al. Citation2014; Seiffert et al. Citation2015). Since one of the major sources of human exposure to NP is via inhalation, several studies have investigated the potential pulmonary toxicity of NP in a variety of in vivo airway models of inflammation. Two major observations can be made from such studies with NP: (i) NP with smaller diameters trend to reach the smaller airways vs NP with greater diameters and (ii) increased granulocyte counts, especially neutrophils (PMN) in bronchoalveolar lavage (BAL) fluid, are associated with NP-induced airway inflammation (Carvalho et al. Citation2011; Seiffert et al. Citation2015).
Despite this, the literature dealing with the direct effects of NP on PMN is not well documented, even if our group (Babin et al. Citation2013; Goncalves and Girard Citation2014; Goncalves et al. Citation2010; Poirier et al. Citation2014) and others (Abrikossova et al. Citation2012; Bartneck et al. Citation2010; Chekanov et al. Citation2013; Couto et al. Citation2014; Moeller et al. Citation2012; Papatheofanis and Barmada Citation1991) reported that different kinds of NP can activate human PMN, leading to distinct modulatory activities on functional responses including reactive oxygen species (ROS) production, cytokine formation, apoptosis and degranulation. Among these studies, it was previously reported that silver NP (AgNP) with a diameter of 20 nm (AgNP20) induced human neutrophil apoptosis and inhibited de novo protein synthesis (Poirier et al. Citation2014). However, despite a long involvement in this area of research, how a given NP with a different diameter will alter the biology of PMN is a neglected area of research and, to the best of our knowledge, has never been investigated. Because of this latter study dealing with AgNP20, and since AgNP are largely used in various industrial sectors, including textile and hygiene products (Arora et al. Citation2012; Pratsinis et al. Citation2013; Sotiriou and Pratsinis Citation2010), as well as in medicine (Chaloupka et al. Citation2010; Franci et al. Citation2015), in addition to the recent proposition that more attention should be paid to potential toxicity of AgNP (Wang et al. Citation2015), it was decided here to determine how AgNP with a diameter of 20 nm and of 70 nm (AgNP70) would alter the biology of human PMN.
Materials and methods
Chemicals
The cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-8 were purchased from PeproTech Inc. (Rocky Hill, NJ). The plant lectin Viscum album agglutinin-I (VAA-I), ultrapure lipopolysaccharides (LPS) from Escherichia coli, trypan blue, arsenic trioxide, formyl-methionyl-leucyl phenylalanine (fMLF), cycloheximide (CHX), dextran and CM-H2DCFDA were bought from Sigma (St-Louis, MO). Silver nanoparticles (Pelco® citrate Biopure™ silver) with a diameter of 70 nm (AgNP70) or of 20 nm (AgNP20) were purchased from Ted Pella (Redding, CA). Ficoll-Hypaque was purchased from GE Healthcare (Uppsala, Sweden). RPMI 1640, HEPES, Hank’s balanced salt solution (HBSS), penicillin and streptomycin were all bought from Life Technologies (Grand Island, NY). The lactase dehydrogenase (LDH)-Cytotoxicity Assay Kit II was purchased from Abcam® (Toronto, Ontario, Canada).
Size distribution and zeta potential measurements
The size distribution and surface charge (zeta potential) of the AgNP were determined by dynamic light scattering using a Malvern Zetasizer Nano-ZS (Model ZEN3600, Malvern Instruments Inc., Westborough, MA). Measurements were performed using 10 and 100 μg/ml AgNP in pure water.
Transmission electron microscopy (TEM)
The AgNP70 suspension obtained from the manufacturer was examined using a Hitachi H-7100 transmission electron microscope as previously described (Poirier et al. Citation2014).
Neutrophil isolation
Neutrophils (PMN) were isolated from venous blood of healthy volunteers by dextran sedimentation followed by centrifugation over Ficoll-Hypaque as previously described (Babin et al. Citation2013; Poirier et al. Citation2014; Simard et al. Citation2011). Blood donations were obtained from informed and consenting individuals according to institutionally approved procedures. Cell viability was monitored by trypan blue exclusion and was always > 98%. Purity was confirmed by cytology from cytocentrifuged preparations colored by the Hema 3 stain set (Fisher Scientific, Pittsburgh, PA).
Cell viability and cell shape changes
Freshly isolated human PMN (10 × 106 PMN/ml in RPMI 1640 containing 25 mM HEPES and 1% penicillin-streptomycin solution, supplemented with 10% heat-inactivated autologous serum) were treated for 0–24 h with or without increasing concentrations of AgNP70 (0–100 μg/ml), as previously reported with other NP (Arora et al. Citation2009; Babin et al. Citation2013; Poirier et al. Citation2014; Rosas-Hernandez et al. Citation2009). Cell viability and membrane integrity were monitored by trypan blue exclusion and LDH assays, respectively. The morphological cell shape changes were observed under light microscopy (400×) and photomicrographs were taken using an Eclipse TS100 camera (Nikon, Tokyo, Japan).
Determination of apoptosis
The basal or spontaneous apoptotic rate was evaluated by cytology as previously published (Girard et al. Citation1997; Goncalves et al. Citation2010). Neutrophils (10 × 106 PMN/ml) were incubated at 37 °C in 5% CO2 in 96-well plates for 24 h in the presence of the above buffer (control), AgNP70 or the pro-apoptotic plant lectin VAA-I (1 μg/ml) (Savoie et al. Citation2000) or AgNP20 also known to induce spontaneous apoptosis in human PMN apoptosis (Poirier et al. Citation2014). Cells were then cytocentrifuged onto microscope slides and colored with Hema-3 stain. A total of ≈300 cells/slide were evaluated in a blinded manner. Apoptosis evaluation was based on nucleus morphology (at 400 × magnification). Results were expressed as the percentage of PMN undergoing apoptosis.
Detection of intracellular ROS
Cells (10 × 106 PMN/ml) were suspended in HBSS containing 10 μM CM-H2DCFDA (a general oxidative stress indicator) for 15 min at 37 °C as previously published (Goncalves and Girard Citation2014; Simard et al. Citation2011). Cells were then washed twice with HBSS before being incubated in the presence of buffer or AgNP for 15, 30 or 60 min. Phorbol 12-myristate 13-acetate (PMA; 10−7 M) was used as a positive control (Karlsson et al. Citation2000). After each incubation, the cells were washed three times with HBSS and then re-suspended in phosphate-buffered saline (PBS, pH 7.4) for flow cytometric analysis using a FACScan system (BD Biosciences, San Jose, CA). A total of 10 000 events/sample was acquired. Total ROS production by the cells was expressed as mean fluorescence intensity (MFI).
Metabolic labeling and de novo protein synthesis assay
Cells (10 × 106 PMN/ml in RPMI 1640 medium supplemented with 10% autologous serum) were metabolically labeled with 4.625 MBq of Redivue Pro-Mix L-[35S] in vitro cell labeling mix (GE Healthcare, Baie d’Urfé, QC, Canada) in the presence or absence of 10 or 100 μg/ml AgNP70 or AgNP20, 5 μM of the positive control arsenic trioxide (ATO) or 10 μg/ml cycloheximide (CHX, a potent protein synthesis inhibitor) for 24 h. Neo-synthesis of protein was monitored by 1D-SDS-PAGE as previously described (Binet et al. Citation2006; Poirier et al. Citation2014).
Detection of albumin and MMP-9 by Western blotting
Neutrophils (10 × 106 PMN/ml in RPMI-1640) were incubated with buffer, fMLF (10−8M) or 100 μg/ml AgNP20, 100 μg/ml AgNP70 for 30 or 60 min at 37 °C. Cells were then centrifuged at 13 000 rpm for 10 min at 4 °C. Pellets were discarded and supernatants (equivalent to 1 × 106 cells for detection of albumin and MMP-9 protein released from granules) were separated over a 7.5% SDS-PAGE gel before being electrotransferred to nitrocellulose membranes that, in turn, were blocked for 1 h at room temperature with TBS-Tween containing 5% non-fat dry milk (Carnation, Don Mills, ONT, Canada). After washing the membranes with TBS-Tween (0.15%), a fixed dilution (1:1000 in TBS-Tween [0.15%]) of anti-MMP-9/gelatinase B and anti-albumin antibodies was applied to the membranes. After incubation overnight at 4 °C, the membranes were washed with TBS-Tween and then incubated for 1 h at room temperature with a goat anti-rabbit IgG HRP secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA; diluted 1:20 000 in TBS-Tween + 5% non-fat dry milk) or a goat anti-mouse IgG HRP secondary antibody (Jackson ImmunoResearch; diluted 1:20 000 in TBS-Tween + 5% non-fat dry milk), followed by several washes. Protein expression was ultimately revealed using Luminata Forte Western HRP Substrate (Millipore (Canada) Ltd, Etobicoke, ONT, Canada). Chemiluminescence was revealed using a chemiDocTM MP Imaging system (Imagelab 5.2.1) from Bio-Rad Laboratories (Canada) Ltd. (Mississauga, ONT, Canada).
Zymography assay
Neutrophils were treated with buffer (control), AgNP or fMLF (positive control) for 30 min and the samples were then centrifuged at 2000 rpm for 10 min at 4 °C. The pellets were discarded and the supernatants (30 μl, corresponding to ≈50 000 cells) were mixed with 10 μl of a non-reducing buffer (40% glycerol, SDS 8%, 1 M Tris-HCl [pH 6.8]) and then separated over 10% acrylamide gels containing 0.2% gelatin. The gels were then washed twice for 30 min with 2.5% Triton X-100 and incubated overnight in digestion buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM CaCl2). Thereafter, the gels were stained with 0.1% Coomassie blue solution and de-stained to determine the gelatinase activity, i.e. visualized by apparition of white zones in blue-stained gel as previously documented (Babin et al. Citation2013).
IL-8 production
The concentration of IL-8 in culture supernatants was determined using a commercial ELISA kit (Life Technologies, Carlsbad, CA). Freshly-isolated human PMN in RPMI 1640 supplemented with 5% heat-inactivated fetal calf serum were incubated in 24-well plates in the presence or absence of the indicated agonists at 37 °C in 5% CO2 for 20 h. Supernatants were harvested after centrifugation and stored at −70 °C before performing the ELISA. The level of detection for the kit was ≤ 5 pg IL-8/ml.
Statistical analysis
All data were expressed as mean ± SEM. A one-way analysis of variance (ANOVA; Dunnett multiple-comparison test) was performed using Prism software (v 5.01; GraphPad, San Diego, CA). Differences were considered statistically significant at: *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.005 vs control.
Results
Characterization of AgNP70 and lack of necrotic activity in human neutrophils
According to the manufacturer data sheet, the starting diameter of the AgNP70 was 68.5 [± 4.2] nm; this was confirmed by TEM (). However, some aggregates of ∼400 nm could also be observed (arrowhead). Next, the AgNP70 was characterized by DLS suspended in pure water and compared with the AgNP20 (both at 10 and 100 μg/ml). As illustrated in , AgNP20 possessed a size of 32.1 [± 0.3] nm (100%) or 34.9 [± 1.5] nm (∼99%) at 10 or 100 μg/ml, respectively. In the same order, the size of AgNP70 was 84.5 [± 0.1] nm (100%) and 89.7 [± 1.5] nm (100%). The values of the zeta potentials were −49.3 [± 0.5], −41.3 [± 1.7], −46.4 [± 0.4] and −50.0 [± 0.6] mV for AgNP20 at 10 and 100 μg/ml and for AgNP70 at 10 or 100 μg/ml. In the same order, the PDI were 0.110 [± 0.003], 0.170 [± 0.006], 0.140 [± 0.006] and 0.180 [± 0.010], confirming the observed stability of the suspensions.
Figure 1. Transmission electronic microscopy (TEM) image of primary AgNP70 stock solution and evaluation of cell viability/morphology in AgNP70-induced human PMN. (A) Samples of the manufacturer stock solution was used for characterization by TEM to confirm the primary diameter of the NP was ∼70 nm. Freshly isolated human PMN were incubated for 24 h in the presence of buffer (controls), 10 or 100 μg/ml AgNP70, 65 ng/ml GM-CSF (GM) or 100 μg/ml AgNP20 and then (B) cell necrosis was evaluated by measuring levels of LDH released into the culture supernatants, (C) number of cells remaining in plates were determined and (D) cell morphology was evaluated using optical microscopy (400×). Results shown are means ± SEM (n = 3 [B]; n = 5 [C]). Both low and high controls were used according to manufacturer recommendations. AgNP20 = silver nanoparticles of 20 nm; arrowhead = aggregates; arrows = increased cell size in AgNP20-induced PMN. Pictures in (D) are from one representative experiment out of five done with different blood donors.
![Figure 1. Transmission electronic microscopy (TEM) image of primary AgNP70 stock solution and evaluation of cell viability/morphology in AgNP70-induced human PMN. (A) Samples of the manufacturer stock solution was used for characterization by TEM to confirm the primary diameter of the NP was ∼70 nm. Freshly isolated human PMN were incubated for 24 h in the presence of buffer (controls), 10 or 100 μg/ml AgNP70, 65 ng/ml GM-CSF (GM) or 100 μg/ml AgNP20 and then (B) cell necrosis was evaluated by measuring levels of LDH released into the culture supernatants, (C) number of cells remaining in plates were determined and (D) cell morphology was evaluated using optical microscopy (400×). Results shown are means ± SEM (n = 3 [B]; n = 5 [C]). Both low and high controls were used according to manufacturer recommendations. AgNP20 = silver nanoparticles of 20 nm; arrowhead = aggregates; arrows = increased cell size in AgNP20-induced PMN. Pictures in (D) are from one representative experiment out of five done with different blood donors.](/cms/asset/61c2a9ac-bbbc-4d83-8990-79dc3730704b/iimt_a_1106622_f0001_c.jpg)
Table 1. Size and Zeta potential of AgNP20 and AgNP70.
Cell viability was next determined by measuring LDH release from the AgNP70-treated PMN. As illustrated in , after 24 h, AgNP70 did not cause a significant increase in LDH release from PMN treated with 10 or 100 μg/ml vs untreated cells, exactly as previously demonstrated for AgNP20 (Poirier et al. Citation2014). As expected, the positive technical control for the assay led to a marked release of LDH. To support this, the number of cells remaining in the plates after the 24 h incubation (period of time at which apoptosis is normally determined in human PMN) was evaluated – as was their viability (by trypan blue exclusion). Cell viability was always > 96% (data not shown); as shown in , AgNP70 did not significantly alter the number of cells remaining in the plates after 24 h incubation with 10 or 100 μg/ml AgNP70. In parallel, whether or not AgNP70 could alter the cell size of the PMN, as previously observed with AgNP20 (Poirier et al. Citation2014) was determined. Unlike AgNP20, AgNP70 did not increase cell size (), suggesting human PMN responded differently to the two test diameters of the same AgNP.
AgNP70 inhibit human PMN spontaneous apoptosis by a de novo protein synthesis – and ROS production-independent mechanisms
Because it was previously demonstrated that AgNP20 are pro-apoptotic in human PMN, the role of AgNP70 in this biological process was next investigated. As illustrated in , AgNP70 were found to reduce levels of apoptotic PMN at a concentration of 100 μg/ml (19.3 [± 4.8]% of apoptotic cells vs 45.8 [± 2.9]% observed, as expected, in control cells or spontaneous apoptosis) compared to AgNP20 that induce apoptosis (83.8 [± 2.3]%). However, as with AgNP20, AgNP70 did not significantly alter basal levels of spontaneous apoptosis when PMN were treated at a dose of 10 μg/ml.
Figure 2. AgNP70 and AgNP20 have opposing effect on human neutrophil apoptosis, but both act as potent inhibitors of de novo protein synthesis without inducing ROS production. Freshly isolated human PMN were incubated with the indicated agonist for the specified periods of time and apoptosis (A) and then de novo protein synthesis (B) and ROS production (C) were determined (see Materials and methods). Results shown are means ± SEM (n = 4); (C) Results are from one representative experiment out of four performed with different blood donors. VAA, Viscum album agglutinin-I (1 μg/ml); PMA, phorbol-12-myristate-13-acetate (10−7 M); MW, molecular weights; CHX, cycloheximide (10 μg/ml); and ATO, arsenic trioxide (5 μM).
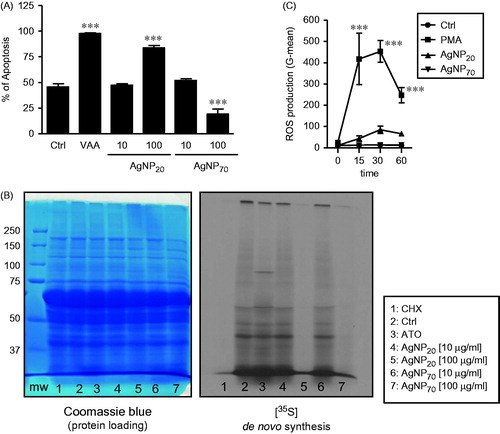
It was previously demonstrated that AgNP20 inhibited de novo protein synthesis. In verifying how AgNP70 would alter this response, unexpectedly it was seen that AgNP70 inhibited neo-synthesis of polypeptides in a manner similar to that of the potent protein synthesis inhibitor CHX and of AgNP20, resulting in the absence or reduced expression of proteins in the corresponding lanes (, right part, lanes 1, 5 and 7). Those results indicated that the ability of AgNP70 to delay PMN apoptosis did not involve de novo protein synthesis. Knowing that ROS are crucial in the execution of neutrophil apoptosis (Geering and Simon Citation2011) and since it had been observed that AgNP20 and AgNP70 had opposite effects on PMN apoptosis, ROS production was evaluated to further determine the modes of action for both AgNP. As illustrated in , both AgNP20 and AgNP70 did not significantly increase ROS production in the human PMN, even after 1 h of treatment. In these experiments, PMA, as expected, was found to rapidly induce ROS production by the cells.
High amounts of IL-8 are secreted by AgNP20- but not by AgNP70-induced PMN, but are not associated with pro-apoptotic activity
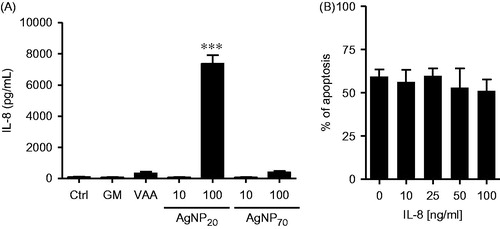
To further investigate how AgNP could alter the biology of human PMN, the possibility that AgNP20 and AgNP70 would induce secretion of IL-8 was investigated. As illustrated in , neither AgNP20 nor AgNP70 at 10 μg/ml caused significant increases above basal levels of IL-8 in the culture supernatants. However, at 100 μg/ml, AgNP20 – but not AgNP70 – caused a significant increase in cell IL-8 production (from ∼80 to > ∼7300 pg/ml). Because of this effect on IL-8 production induced by AgNP20, other sets of PMN were treated with different concentrations of IL-8 to establish whether or not this could be related to a change in their pro-apoptotic activity. As illustrated in , high concentrations of IL-8 (even at of 100 ng/ml) did not increase apoptotic rates. These results indicated to us that AgNP20 did not induce apoptosis via the release of large amounts of IL-8.
Figure 3. AgNP20 promote release of IL-8 at a concentration that does not induce neutrophil apoptosis when exogenously added to the cultures. (A) Freshly isolated human PMN were incubated for 24 h in the presence of buffer (control/Crtl), 65 ng/ml GM-SCF (GM), 1 μg/ml VAA-I (VAA), 10 or 100 μg/ml of AgNP70 or AgNP20 and the release of IL-8 into extracellular milieu was then quantified by ELISA. (B) Cells were incubated for 24 h in the presence of 0 (Ctrl), 10, 25, 50 or 100 ng/ml recombinant human IL-8 and apoptosis then determined by cytology (see Materials and methods). Results shown are means ± SEM (n = 3/treatment).
AgNP20, but not AgNP70, induce degranulation in human PMN
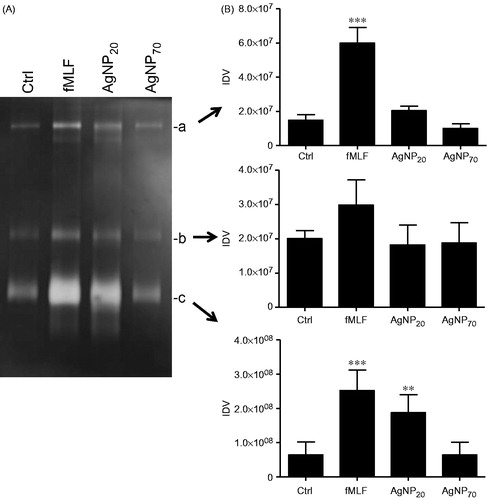
Based on a previous study indicating that other kind of NP, namely TiO2, ZnO and CeO2, could promote degranulation in human PMN (Babin et al. Citation2013) and knowing that AgNP could activate PMN (Poirier et al. Citation2014), the ability of AgNP20 and AgNP70 to exert degranulation was next determined in the human PMN. As illustrated in , exposure to AgNP20 induced marked and significant increases in the release of albumin after treatment for 30 and 60 min. The same results were observed with regard to MMP-9, but only after a 60-min treatment. Incubation of the cells with AgNP70 did not promote release of these two granule proteins (Borregaard and Cowland Citation1997). To further confirm that AgNP20 induced degranulation and release of MMP-9 and that the enzyme still maintained enzymatic activity, zymography was done. As illustrated in , increased gelatinase activity was detected in the supernatants of AgNP20-treated PMN (in comparison to values noted in samples from control cells), as judged by the increased signal detected in the characteristic of 92 kDa (zone c) zone, corresponding to pro-MMP-9 monomer (Achilli et al. Citation2011).
Figure 4. AgNP20 but not AgNP70 induce degranulation and release of albumin and MMP-9. Freshly isolated human PMN were incubated for 30 or 60 min with buffer (Ctrl), 10−8 M fMLF, 100 μg/ml AgNP20 or 100 μg/ml AgNP70 and the release of (A) albumin and (B) MMP-9 into the extracellular milieu was then detected by Western blot. Densitometry analysis (right part of each panel) was performed and results (means ± SEM, n ≥ 3/treatment) are expressed in arbitrary units derived from integrated density values (IDV). Left part: one representative experiments out of three (A) or four (B).
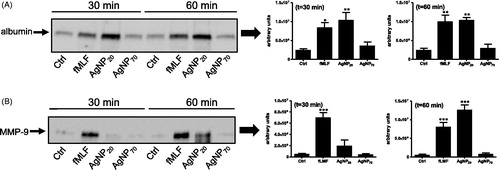
Figure 5. AgNP20 but not AgNP70 increase gelatinolytic activity in human PMN. Freshly isolated human PMN were isolated and incubated with the buffer (Ctrl), fMLF (10−8 M) or with 100 μg/ml of AgNP20 or AgNP70 for 60 min and the gelatinolytic activity of MMP-9 was then assessed by zymography. (A) Typical results where the enzymatic activity was observed by appearance of clear bands (a, b and c). (B) Densitometric analysis of corresponding bands, expressed as integrated density values (IDV). Results shown are means ± SEM (n = 3–5/treatment).
Discussion
In this study, how AgNP with two different sizes, i.e. AgNP20 and AgNP70, alter the biology of human neutrophils (PMN) was compared. Of greatest interest was to verify if AgNP70 would induce apoptosis and inhibit de novo protein synthesis, as previously demonstrated with AgNP20 (Poirier et al. Citation2014). In contrast to AgNP20, while AgNP70 were found to delay human PMN apoptosis, they also inhibited de novo protein synthesis. This was unexpected since, in general, PMN agonists that delay or suppress spontaneous apoptosis also increase de novo protein synthesis, as in the cases of GM-CSF, IL-4 and IL-15 (Brach et al. Citation1992; Girard et al. Citation1996, Citation1997), the chemicals sodium butyrate (Stringer et al. Citation1996) and dexamethasone (Cox and Austin Citation1997) and even zinc NP (Goncalves and Girard Citation2014). The only reported agent known to induce apoptosis as well as de novo protein synthesis is the anti-cancer drug arsenic trioxide (Binet et al. Citation2006).
Before the present study, only one compound, i.e. methylmercuric chloride, was found to delay human PMN apoptosis and to inhibit de novo protein synthesis (Moisan et al. Citation2003). Therefore, AgNP70 is one other agent with methylmercuric chloride altering the biology of PMN by a mechanism that is both unique and yet unclear for these two agents. Of note, it is plausible that other compounds will be found in future to act similarly to these. This remains to be determined. Preliminary experiments here failed to detect the presence of AgNP70 inside human PMN (as assessed by TEM); rather, a tendency to stay close to the cell membrane was noted (data not shown). However, it cannot be pretended that the NP have to be internalized to activate human PMN. In fact, the present data support this, i.e. AgNP70 can alter the biology of human PMN without penetrating the cells and can, at least, inhibit spontaneous apoptosis. Also, it cannot be ruled out that the different effects observed between AgNP20 and AgNP70 on PMN apoptotic rates were the result of release of silver ions. However, if this was the case, this could not explain how both types of AgNP inhibited de novo protein synthesis. This latter issue remains to be resolved. Also, we have to consider the possibility that different proteins can adsorb on AgNP70 vs AgNP20, a phenomenon known as the ‘protein corona’ associated with AgNP that could explain the different effects between both AgNP. This is particularly relevant since we added human serum to the cell cultures. In addition, a recent study reported that such a protein corona on AgNP20 mediates cellular toxicity via scavenger receptors, influencing the toxicity of the nanoparticles (Shanahan et al. Citation2015).
In contrast to the present data revealing absence of increased ROS production in AgNP-treated human PMN, a recent study performed with a human epithelial embryonic cell line reported an increase in ROS production after treatment with AgNP of a nominal diameter of 20 [± 5] nm (Rinna et al. Citation2015). The authors treated cells with 1, 5 or 25 μg/ml AgNP for 30 min after having incubated them for 40 min with 20 μM H2DCF-DA and, intriguingly, an increase of ROS production was observed only with the lowest tested concentration of AgNP. However, important variations from experiment to experiment were observed. One other study reported that 75 μg/ml of AgNP (size of 5–35 nm, as assessed by TEM) also promoted ROS production in primary cultures of cerebellar granule cells prepared from 7-day-old male and female Wistar rats (Zieminska et al. Citation2014). Indeed, the fluorescence intensity of H2DCFDA increased by ∼30% vs control levels after 30 min of incubation with AgNP. Although experimental conditions differed (here, 10 μg/ml of a chloromethyl derivative of H2DCFDA [CM- H2DCFDA] exhibiting a much better retention in live cells than H2DCFDA was used), PMN – known as potent ROS producers (Dupre-Crochet et al. Citation2013; Manda-Handzlik and Demkow Citation2015) – were highly responsive to PMA (used here as a positive control) (Karlsson et al. Citation2000).
The higher concentrations of IL-8 secreted by AgNP20-induced PMN here indicated these NP were potent activators of these cells. Curiously, although IL-8 is among one of the most studied and most potent PMN agonists, its direct role in modulating of PMN apoptosis is not well established. In one study, IL-8 was found to delay human PMN apoptosis after 12 h of treatment. Here, addition of exogenous IL-8 to the PMN cultures did not affect spontaneous apoptotic rates, even after 24 h. Several studies, however, reported that different compounds were found to delay or induce PMN apoptosis and to alter the IL-8 production. In our hands, no correlation could be observed between the level of IL-8 secretion and an effect on apoptosis.
Curcumin increased IL-8 production but induced PMN apoptosis (Antoine et al. Citation2013), whereas IL-4 inhibited apoptosis and also increased IL-8 production (Girard et al. Citation1997) – as did titanium dioxide (TiO2) NP (Goncalves et al. Citation2010). Other compounds, including dieldrin, increased IL-8 production, but did not alter spontaneous apoptotic rates in PMN (Pelletier et al. Citation2001). Works from others also demonstrated such lack of correlation. For example, fucoidan was found to delay PMN apoptosis and to increase IL-8 production (Jin and Yu Citation2015), whereas anti-double sDNA antibodies induced apoptosis and increased IL-8 secretion (Hsieh et al. Citation2001). Thus, no correlation can yet be made between the levels of secreted IL-8 and apoptotic rates; the results of the present study indicated this was also the case for both AgNP20 and AgNP70 that are known to induce (Poirier et al. Citation2014) and delay (current report) human PMN apoptosis.
The role of NP on human PMN degranulation is poorly documented. Our laboratory recently demonstrated that TiO2, cerium dioxide (CeO2) and zinc oxide NP promoted human PMN degranulation (Babin et al. Citation2013). Other investigators have reported that polymethyl-methacrylate NP also induces degranulation in these cells (Papatheofanis and Barmada Citation1991). To the best of our knowledge, the present study’s data demonstrating that AgNP20, but not AgNP70, promote degranulation in human PMN are the first to be reported in the literature. These results are in agreement with previous studies demonstrating AgNP could induce degranulation in rodent mast cells (Aldossari et al. Citation2015; Yang et al. Citation2010). Although not of human origin, these cells were found to degranulate in response to AgNP with a diameter of 20 nm (at 25 and 50 μg/ml), but not of 110 nm (Aldossari et al. Citation2015). Intriguingly, the basal level of degranulation was not increased using AgNP with larger sizes of 550 and 850 nm; this remains unexplained. In addition, the present study not only demonstrated that AgNP20 induced degranulation in human PMN, but it also showed that supernatants of cultures of AgNP20-induced cells preserved the enzymatic activity of released gelatinase, as determined by zymography.
Conclusions
Collectively, the results here indicate that both AgNP20 and AgNP70 act as potent inhibitors of de novo protein synthesis but have opposing effects on human PMN apoptosis with AgNP20 acting as inducer and AgNP70 acting as suppressor. Whether such an observation is strictly related to the initial size of the AgNP is not clear and remains to be determined. Both NP also did not alter ROS production by these cells. In addition to apoptosis, these two AgNP differentially altered the biology of PMN, as AgNP20 increased IL-8 production and induced degranulation, but AgNP70 did not. The current observations are consistent with the idea that a given NP could alter cellular processes differently, based on initial size. This has to be considered in future nanoimmunobiology studies, especially if the aim is to develop novel therapeutic strategies with AgNP to control inflammation, a process in which PMN are very active. In addition, it is important to mention that the present data originated from uncoupled ‘naked’ AgNP and that it is plausible that use of AgNP coupled to a given compound would also generate different results, reflecting further on the complex mode of action one faces with the use of NP.
Acknowledgements
The study was supported by grants from the Institut de Recherche Robert-Sauvé en santé et en sécurité du travail (IRSST).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abrikossova N, Skoglund C, Ahrén M, Bengtsson T, Uvdal K. 2012. Effects of gadolinium oxide nanoparticles on the oxidative burst from human neutrophil granulocytes. Nanotechnology. 23:275101.
- Achilli C, Ciana A, Balduini C, Risso A, Minetti G. 2011. Application of gelatin zymography for evaluating low levels of contaminating neutrophils in red blood cell samples. Anal Biochem. 409:296–297.
- Aldossari AA, Shannahan JH, Podila R, Brown JM. 2015. Influence of physicochemical properties of silver nanoparticles on mast cell activation and degranulation. Toxicol In Vitro. 29:195–203.
- Anderson DS, Silva RM, Lee D, Edwards PC, Sharmah A, Guo T, Pinkerton KE, Van Winkle LS. 2014. Persistence of silver nanoparticles in the rat lung: influence of dose, size, and chemical composition. Nanotoxicology. 9:591–602.
- Antoine F, Simard JC, Girard D 2013. Curcumin inhibits agent-induced human neutrophil functions in vitro and lipopolysaccharide-induced neutrophilic infiltration in vivo. Intl Immunopharmacol. 17:1101–1107.
- Arora S, Jain J, Rajwade JM, Paknikar KM. 2009. Interactions of silver nanoparticles with primary mouse fibroblasts and liver cells. Toxicol Appl Pharmacol. 236:310–318.
- Arora S, Rajwade JM, Paknikar KM. 2012. Nanotoxicology and in vitro studies: the need of the hour. Toxicol Appl Pharmacol. 258:151–165.
- Babin K, Antoine F, Goncalves DM, Girard D. 2013. TiO2, CeO2, and ZnO nanoparticles and modulation of the degranulation process in human neutrophils. Toxicol Lett. 221:57–63.
- Bartneck M, Keul HA, Zwadlo-Klarwasser G, Groll J. 2010. Phagocytosis independent extracellular nanoparticle clearance by human immune cells. NanoLetters. 10:59–63.
- Binet F, Cavalli H, Moisan E, Girard D. 2006. Arsenic trioxide (AT) is a novel human neutrophil pro-apoptotic agent: effects of catalase on AT-induced apoptosis, degradation of cytoskeletal proteins and de novo protein synthesis. Br J Haematol. 132:349–358.
- Borregaard N, Cowland JB. 1997. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89:3503–3521.
- Brach MA, deVos S, Gruss HJ, Herrmann F 1992. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood 80:2920–2924.
- Carvalho TC, Peters JI, Williams RO 3rd. 2011. Influence of particle size on regional lung deposition – what evidence is there? Intl J Pharm. 406:1–10.
- Chaloupka K, Malam Y, Seifalian AM. 2010. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 28:580–588.
- Chekanov AV, Baranova OA, Levin AD, Solov'eva ÉIu, Fedin AI, Kazarinov KD. 2013. Study of influence of gold nanoparticles on activation of human blood neutrophils. Biofizika. 58:495–500.
- Couto D, Freitas M, Vila-Boas V, Dias I, Porto G, Lopez-Quintela MA, Rivas J, Freitas P, Carvalho F, Fernandes E. 2014. Interaction of polyacrylic acid coated and non-coated iron oxide nanoparticles with human neutrophils. Toxicol Lett. 225:57–65.
- Cox G, Austin RC. 1997. Dexamethasone-induced suppression of apoptosis in human neutrophils requires continuous stimulation of new protein synthesis. J Leukocyte Biol. 61:224–230.
- Dupre-Crochet S, Erard M, Nubetae O. 2013. ROS production in phagocytes: why, when, and where? J Leukocyte Biol. 94:657–670.
- Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G, Galdiero M. 2015. Silver nanoparticles as potential anti-bacterial agents. Molecules 20:8856–8874.
- Geering B, Simon HU. 2011. Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ. 18:1457–1469.
- Girard D, Paquet ME, Paquin R, Beaulieu AD. 1996. Differential effects of IL-15 and IL-2 on human neutrophils: modulation of phagocytosis, cytoskeleton re-arrangement, gene expression, and apoptosis by IL-15. Blood 88:3176–3184.
- Girard D, Paquin R, Beaulieu AD. 1997. Responsiveness of human neutrophils to IL-4: induction of cytoskeletal re-arrangements, de novo protein synthesis, and delay of apoptosis. Biochem J. 325:147–153.
- Goncalves DM, Girard D. 2014. Zinc oxide nanoparticles delay human neutrophil apoptosis by a de novo protein synthesis–dependent and reactive oxygen species-independent mechanism. Toxicol In Vitro. 28:926–931.
- Goncalves DM, Chiasson S, Girard D. 2010. Activation of human neutrophils by titanium dioxide (TiO2) nanoparticles. Toxicol In Vitro. 24:1002–1008.
- Hsieh SC, Sun KH, Tsai CY, Tsai YY, Tsai ST, Huang DF, Han SH, Yu HS, Yu CL. 2001. Monoclonal anti-double stranded DNA antibody is a leucocyte-binding protein to up-regulate IL-8 gene expression and elicit apoptosis of normal human polymorphonuclear neutrophils. Rheumatology (Oxford). 40:851–858.
- Jin JO, Yu Q. 2015. Fucoidan delays apoptosis and induces pro-inflammatory cytokine production in human neutrophils. Intl J Biol Macromol. 73:65–71.
- Karlsson A, Nixon JB, McPhail LC. 2000. Phorbol myristate acetate induces neutrophil NADPH-oxidase activity by two separate signal transduction pathways: Dependent or independent of phosphatidylinositol 3-kinase. J Leukocyte Biol. 67:396–404.
- Manda-Handzlik A, Demkow U. 2015. Neutrophils: the role of oxidative and nitrosative stress in health and disease. Adv Exp Med Biol. 857:51–60.
- Moeller S, Kegler R, Sternberg K, Mundkowski RG. 2012. Influence of sirolimus-loaded nanoparticles on physiological functions of native human polymorphonuclear neutrophils. Nanomedicine. 8:1293–1300.
- Mohamud R, Xiang SD, Selomulya C, Rolland JM, O'Hehir RE, Hardy CL, Plebanski M. 2014. The effects of engineered nanoparticles on pulmonary immune homeostasis. Drug Metab Rev. 46:176–190.
- Moisan E, Kouassi E, Girard D. 2003. Mechanisms involved in methylmercuric chloride (MeHgCl)–induced suppression of human neutrophil apoptosis. Human Exp Toxicol. 22:629–637.
- Papatheofanis FJ, Barmada R. 1991. Polymorphonuclear leukocyte degranulation with exposure to polymethyl–methacrylate nanoparticles. J Biomed Mater Res. 25:761–771.
- Pelletier M, Roberge CJ, Gauthier M, Vandal K, Tessier PA, Girard D. 2001. Activation of human neutrophils in vitro and dieldrin-induced neutrophilic inflammation in vivo. J Leukocyte Biol. 70:367–373.
- Poirier M, Simard JC, Antoine F, Girard D. 2014. Interaction between silver nanoparticles of 20 nm (AgNP20) and human neutrophils: induction of apoptosis and inhibition of de novo protein synthesis by AgNP20 aggregates. J Appl Toxicol. 34:404–412.
- Pratsinis A, Hervella P, Leroux JC, Pratsinis SE, Sotiriou GA. 2013. Toxicity of silver nanoparticles in macrophages. Small. 9:2576–2584.
- Rinna A, Magdolenova Z, Hudecova A, Kruszewski M, Refsnes M, Dusinska M. 2015. Effect of silver nanoparticles on mitogen–activated protein kinases activation: role of reactive oxygen species and implication in DNA damage. Mutagenesis. 30:59–66.
- Rosas-Hernández H, Jiménez-Badillo S, Martínez-Cuevas PP, Gracia-Espino E, Terrones H, Terrones M, Hussain SM, Ali SF, González C. 2009. Effects of 45-nm silver nanoparticles on coronary endothelial cells and isolated rat aortic rings. Toxicol Lett. 191:305–313.
- Savoie A, Lavastre V, Pelletier M, Hajto T, Hostanska K, Girard D. 2000. Activation of human neutrophils by the plant lectin Viscum album agglutinin-I: modulation of de novo protein synthesis and evidence that caspases are involved in induction of apoptosis. J Leukocyte Biol. 68:845–853.
- Seiffert J, Hussain F, Wiegman C, Li F, Bey L, Baker W, Porter A, Ryan MP, Chang Y, Gow A, et al. 2015. Pulmonary toxicity of instilled silver nanoparticles: influence of size, coating, and rat strain. PLoS One. 10:e0119726.
- Shannahan JH, Podila R, Aldossari AA, Emerson H, Powell BA, Ke PC, Rao AM, Brown JM. 2015. Formation of a protein corona on silver nanoparticles mediates cellular toxicity via scavenger receptors. Toxicol Sci. 143:136–146.
- Simard JC, Simon MM, Tessier PA, Girard D. 2011. Damage-associated molecular pattern S100A9 increases bactericidal activity of human neutrophils by enhancing phagocytosis. J Immunol. 186:3622–3631.
- Sotiriou GA, Pratsinis SE. 2010. Anti-bacterial activity of nanosilver ions and particles. Environ Sci Technol. 44:5649–5654.
- Stringer RE, Hart CA, Edwards SW. 1996. Sodium butyrate delays neutrophil apoptosis: role of protein biosynthesis in neutrophil survival. Br J Haematol. 92:169–175.
- Wang Z, Xia T, Liu S. 2015. Mechanisms of nanosilver–induced toxicological effects: more attention should be paid to its sublethal effects. Nanoscale. 7:7470–7481.
- Yang W, Lee S, Lee J, Bae Y, Kim D. 2010. Silver nanoparticle-induced degranulation observed with quantitative phase microscopy. J Biomed Optics. 15:045005.
- Zieminska E, Stafiej A, Struzynska L. 2014. The role of the glutamatergic NMDA receptor in nanosilver–evoked neurotoxicity in primary cultures of cerebellar granule cells. Toxicology. 315:38–48.
