Abstract
Macrophages are effector cells in the innate and adaptive immune systems and in situ exist within three-dimensional (3-D) microenvironments. As there has been an increase in interest in the use of 3-D scaffolds to mimic natural microenvironments in vitro, this study examined the impact on cultured mice peritoneal macrophages using standard 2-D plates as compared to 3-D collagen-chitosan scaffolds. Here, 2-D and 3-D cultured macrophages were evaluated for responses to lipopolysaccharide (LPS), dexamethasone (Dex), BSA (bovine serum albumin), safranal (herbal component isolated from safranal [Saf]) and Alyssum homolocarpum mucilage (A. muc: mixed herbal components). After treatments, cultured macrophages were evaluated for viability, phagocytic activity and release of tumor necrosis factor (TNF)-α and interleukin (IL)-1β pro-inflammatory cytokines. Comparison of 2-D vs 3-D cultures showed that use of either system – with or without any exogenous agent – had no effect on cell viability. In the case of cell function, macrophages cultured on scaffolds had increases in phagocytic activity relative to that by cells on 2-D plates. In general, the test herbal components Saf and A. muc. had more impact than any of the other exogenous agents on nanoparticle uptake. With respect to production of TNFα and IL-1β, compared to the 2-D cells, scaffold cells tended to have significantly different levels of production of each cytokine, with the effect varying (higher or lower) depending on the test agent used. However, unlike with particle uptake, here, while Saf and A. muc. led to significantly greater levels of cytokine formation by the 3-D culture cells vs that by the 2-D plate cells, there was no net effect (stimulatory) vs control cultures. These results illustrated that collagen-chitosan scaffolds could provide a suitable 3-D microenvironment for macrophage phagocytosis and could also impact on the formation of pro-inflammatory cytokines.
Introduction
In the body, macrophages play significant roles in inflammation and host defense. These cells mediate inflammatory processes by contributing to the formation/circulating levels of nitric oxide (NO), prostaglandins, leukotrienes, reactive oxygen species, and cytokines (Fortin et al. Citation2010). This secretory function of macrophages leads to changes in diverse physiological functions; an over-active state of these cells is also known to contribute to many types of pathologies such as septic shock, organ destruction and autoimmune diseases (Rosário et al. Citation2013). In any studies to examine how macrophages function/respond to exogenous toxicants, consideration of the functional microenvironment is important. Since macrophages exist in natural 3-D microenvirons, culturing the cells in vitro (i.e. under 2-D conditions) may unintentionally skew outcomes and/or yield results that might not truly reflect how the cells would be acting/responding in situ.
In the body, all cells live and function in microenvironments shaped, in part, by extra-cellular matrices (ECM), but also influenced by interstitial matrices and basement membranes. Interstitial spaces are often filled with polysaccharides and fibrous proteins (collagen in particular) (Lee et al. Citation2008; Hayashi et al. Citation2012). These components interact with cells and maintain an appropriate buffering microenvironment in which the cells can function. Thus, in in vitro investigations that wish to mimic a natural ECM, polysaccharides and fibrous proteins should be utilized (Lee et al. Citation2008; Alberts et al. Citation2010). Among such types of proteins, collagen is a main structural protein of various connective tissues. In general, for use in generating de novo artificial systems, collagen is very ‘user-friendly’ in that it can bear either a positive or negative charge (depending on local pH) because of its many acid and basic amino acid residues (Wang et al. Citation2003; Hayashi et al. Citation2012).
Chitosan is a copolymer of D-glucosamine and N-acetylglucosamine derived from chitin harvested from marine crustacean exoskeletons (Wang et al. Citation2003). Previous studies reported on the beneficial use of collagen-chitosan scaffolds for in vitro cultures. Many types of cells have already been evaluated, including endothelial cells (Mori et al. Citation1998), fibroblasts (Mori et al. Citation1997), mesenchymal stem cells (Ragetly et al. Citation2010), nerve cells (Li et al. Citation2014) and chondrocytes (Lahiji et al. Citation2000). Although application of these materials/scaffolds has increased in medicine, their impact on the functionality of immune cells remains poorly studied.
Considering that macrophage functions within the microenvironment in situ are critical to an overall host response, evaluating if/whether use of de novo systems (i.e. 3-D cultures) affect how these cells function is a key first step in determining if such systems could have any future applicability in immunotoxicology studies. For example, if the systems impart effects that would amplify or negate effects of a given test agent, this would reduce their utility in such types of evaluations. Accordingly, this study was undertaken to evaluate the potential use of collagen-chitosan scaffolds for macrophage cultures. Here, the effects of various known stimulators and suppressors of macrophage function were evaluated and compared in macrophages cultured on novel 3-D collagen-chitosan scaffolds or on standard 2-D plates.
Methods and materials
Materials and herbal components
Chirosan (100–300 kDa; degree of deacetylation = 75–85%) and LPS (lipopolysaccharide, Type 0111:B4 from Escherichia coli), safranal (Saf), dexamethasone (DEX) and bovine serum albumin (BSA) were purchased from Sigma (St. Louis, MO). T-25 flasks and flat-bottomed plates were bought from Nunc (Kamstrup, Denmark). Mouse cytokine ELISA kits were obtained from eBiosciences (Frankfurt, Germany). Both RPMI-1640 and fetal bovine serum (FBS) were bought from Invitrogen/Gibco (Grand Island, NY). Type I collagen was isolated from fresh bovine tendons using trypsin digestion and acetic acid dissolution, as described in Ma et al. (Citation2003).
Alyssum homolocarpum seeds were collected from plants cultivated at the Center for Medicinal Plants Research (Tehran, Iran). The identity of the harvested materials was confirmed by the Center of Agricultural Research (Tehran, Iran). Extraction of seed mucilage was done using the method of Koocheki et al. (Citation2008), wherein seed gum was extracted using deionized water (40:1, water:seed, pH 4.5) at 35 °C and then the extract was treated with ethanol (97% ethanol:mixture ratio = 3:1). The routine yield was ≈14 g sieved seed mucilage/50 g starting material.
Animals
Balb/c mice (female, 8–10-weeks-of-age) were purchased from the Pasteur Institute of Iran (Tehran) to be the source of the macrophages for use in the study. Upon arrival, all mice were housed in pathogen-free facilities maintained at 22 °C with a 55% relative humidity and under a 12-h light:dark cycle. All mice had ad libitum access to standard mouse chow and filtered/sterilized water throughout the study. The University Animal Care and Use Committee approved all aspects of the study design.
Collagen-chitosan scaffolds preparations
Chitosan polymers and Type I collagen were each dissolved in 1% acetic acid solution to provide a 2% (w/v) solution of each. Thereafter, the chitosan solution was slowly dripped into the collagen suspension at a 1:1 (collagen:chitosan) ratio and then mixed slowly for 30 min to obtain a complete collagen-chitosan blend. This blend was then injected into a homemade mold (diameter = 15 mm, depth = 3 mm) and the sample then frozen at −20 °C for 1 h and then placed at −70 °C for 12 h. The frozen samples were then lyophilized using lyophilizer (Edwards Micro-Modulyo, Eastbourne, UK) until dry. To stabilize each lyophilized scaffold, a series of ethanol dilutions was used to crosslink the scaffolds polymers. In brief, each scaffold was immersed in 100, 70 and then 50% ethanol (for 1 h, 30 min and 30 min, respectively) and then equilibrated in phosphate-buffered saline (PBS, pH 7.2) for 1 h.
SEM analysis
Scaffold surface and pore size were evaluated using scanning electron microscopy (SEM). In brief, each collagen-chitosan scaffold to be analyzed was mounted onto an aluminum slab with double-sided carbon tape. The samples were then coated with a 10 nm-thick gold film (Micro-Teb, Tehran, Iran) using a sputter coater. Each coated sample was then examined in a DSM 940A Model scanning electron microscope (Zeiss, Hamburg, Germany) at an electron acceleration voltage of 20 KeV.
Macrophage cultures and treatments
Peritoneal macrophages were isolated by first euthanizing the naïve mice by CO2 asphyxiation and then immediately lavaging their peritoneal cavity with 10 ml cold RPMI 1640 (Sigma). The fluid was then centrifuged at 200 × g (4 °C, 10 min); the pelleted cells were then washed by centrifugation with RPMI. The final pellets of five mice were re-suspended, combined, pelleted and this stock of cells then re-suspended to 1.5 × 106 cells/ml in complete RPMI medium (RPMI supplemented with 11 mM sodium bicarbonate, 2 mM L-glutamine, 100 U penicillin/ml, 100 μg streptomycin/ml and 5% fetal bovine serum [FBS]) (all reagents purchased from Gibco). Samples, i.e. 5 ml, of the suspensions were then placed into T-25 flat-bottomed flasks and incubated for 4 h at 37 °C in a humidified atmosphere containing 5% CO2 to permit cell adherence. After this period, non-adherent cells were removed by gentle washes with PBS. Adherent macrophages were then harvested by Tripsin-EDTA. After washing by centrifugation, the cells were re-suspended in RPMI medium and then placed onto prepared scaffolds (1 ml) or into wells of a 24-well flat-bottomed plate (1 ml/well) at a density of 106 cells/ml complete RPMI.
After 20 min at 37 °C, the macrophages were stimulated by addition of 20 μl/culture of: LPS (10 μg LPS/ml, final concentration); BSA (20 μg/ml, final concentration); DEX suppressor (10 μg/ml, final concentration); safranal (Saf: 50 μg/ml, final concentration) or the crude herbal extract from A. homolocarpum seeds (mucilage; A. muc: 50 μg/mlm final concentration). After 48 h of culture at 37 °C, the treated macrophages were assayed for viability, cytokine release, and nanoparticle (NP) uptake activity. These agents were selected for their probable effects on macrophages since: LPS is a common stimulant and present during many types of infections; Dex is a commonly used macrophages suppressor and also employed routinely as a medicine; BSA is representative of a common group of proteins/antigens that macrophages encounter; safranal is a herbal component that is a part of several traditional medicines; and A. muc was assessed as it contains a mixture of herbal components also routinely encountered in several traditional medicines.
Macrophage viability
Macrophage viability at the end of the incubation was assessed using an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] reduction assay. In brief, after the 48-h culture and removal of culture supernatant, each well/scaffold system received fresh RPMI 1640 and then a 100 μl aliquot of freshly-prepared of MTT (5 mg/ml in PBS; Merck, Darmstadt, Germany) solution. The plate/scaffold system was then incubated for 4 h at 37 °C in the 5% CO2 system before the supernatants in each were removed and 100 μl acidified isopropanol (0.04 M HCl in isopropanol) was added to dissolve any formazan crystals that had formed in the cells. The absorbance (OD) in each well was then measured in a Multiskan micro-plate reader (Thermo Scientific Vantaa, Finland) at 540 nm. All results were expressed as Stimulation Index (SI), a value that is the measure of the OD540 of the test sample/OD540 of the negative (unstimulated) culture-condition control.
Assessment of nanoparticle uptake as phagocytosis activity
To evaluate the efficacy of uptake of chitosan nanoparticles (NP) by cultured macrophages, a nano-complex of fluorescein isothiocyanate (FITC)-labeled scrambled siRNA that was complexed with chitosan oligomers was constructed. To prepare the NP, low molecular weight chitosan oligomers were prepared by oxidative degradation of 1% (w/v) chitosan in 1% acetic acid (with 2 M NaNO2) at room temperature for 3 h. The chitosan oligomers (0.1% [w/v] in 0.1% [v/v] acetic acid) and FITC-labeled siRNA solution (Bioneer, Daejeon, South Korea; at 100 μg/ml in 0.1% acetic acid) were then separately pre-heated to 50–55 °C. Solutions in equal volumes were then quickly combined and vortexed for 15–30 s and then incubated at room temperature for 30 min. Samples of the NP were then evaluated using SEM to confirm size and uniformity (see above). These materials were then used for the assays with the treated cells.
To assess NP uptake, macrophages were exposed to the NP (with 10 μg siRNA/106 cells in 1 ml) for 6 h at 37 °C. Thereafter, the cells were washed and fluorescent microscopy evaluation and flow cytometric analyses then performed to determine the macrophage uptake capacity. For the microscopy, aliquots of cells were washed three times with PBS and then examined using a Eclipse TE2000 fluorescent microscope (Nikon, Tokyo, Japan). Images were captured using a Nikon digital camera and the numbers of cells bearing the labeled NP enumerated. Among these phagocytically-active cells, the total numbers of particles were determined; from this the macrophage phagocytic index was determined. In each case, a total of 500 cells per sample were quantified (in triplicate). To determine macrophages phagocytic capacity/activity, the cells were evaluated using a FACSVerse flow cytometer (BD Biosciences, San Jose, CA) and associated software. A minimum of 10 000 events/sample was acquired; each set of samples was evaluated five times. The numbers of FITC+ cells from among the whole set of cells analyzed was used to calculate capacity/activity values.
Cytokine analyses
Levels of TNFα and IL-1β in the culture supernatants were determined using commercially-available ELISA kits (eBiosciences, Frankfurt, Germany) according to manufacturer instructions. All samples were measured at least in duplicate. The sensitivity of the TNFα and IL-1β kits were, respectively, 3.7 pg TNFα/ml and 1.2 pg IL-1β/ml.
Statistical analysis
Results are expressed as mean ± SD. For multiple comparisons, data were analyzed using one-way analysis of variance (ANOVA) followed by an LSD test. A p value < 0.05 was considered significant. All analyses were performed using SPSS 15 software (SPSS Inc., Chicago, IL).
Results
Scaffolds and macrophage interactions properties and macrophages viability
Microscopic evaluations were performed to assess how the macrophages interacted with the scaffold systems. Light and electronic microscopy evaluations showed that the porous scaffolds provided surfaces suitable for cell integration and infiltration.
Macrophage viability in differing maintenance systems
Cytotoxicity evaluation () indicated that macrophages in all treatment groups were unaffected after the 48-h periods, regardless of whether the cells had been maintained in the 2-D plates or 3-D scaffolds systems.
Figure 1. Macrophage viability in terms of Stimulation Index (SI% = 100% × [OD test/OD control]). Viability of macrophages (MQ) were assessed after 48 h culture in various test systems and in the presence/absence of test (indicated) exogenous agents (n = 5 cultures/treatment). Data shown are means ± SD. LPS: Lipopolysaccharide; Dex: Dexamethasone; BSA: Bovine Serum Albumin; Saf: Safranal; A. muc: Alyssum mucilage.
![Figure 1. Macrophage viability in terms of Stimulation Index (SI% = 100% × [OD test/OD control]). Viability of macrophages (MQ) were assessed after 48 h culture in various test systems and in the presence/absence of test (indicated) exogenous agents (n = 5 cultures/treatment). Data shown are means ± SD. LPS: Lipopolysaccharide; Dex: Dexamethasone; BSA: Bovine Serum Albumin; Saf: Safranal; A. muc: Alyssum mucilage.](/cms/asset/55d0f657-ff4f-4c5d-a2e4-7864935bee29/iimt_a_1139642_f0001_c.jpg)
Macrophage NP uptake
Uptake of FITC-labeled chitosan NP was used to assess macrophages phagocytic activity. Fluorescent microscopy revealed the engulfment of the test NP by the macrophages after 6 h of incubation with the cells (). Flow cytometric evaluations () showed there was greater (p < 0.05) phagocytic activity by macrophages maintained on the 3-D collagen-chitosan scaffolds compared to that by cells held in the 2-D plate systems – in all test conditions (i.e. use of exogenous agents and even in the control culture scenario). With control cells, the net increase due to the test system itself was 18.9% (i.e. increase from 41.4% to 49.2%). Among the cells treated with the various exogenous agents, the percentage increases in uptake due to use of the 3-D system were consistent, ranging from a low of 9.6% with the LPS to a maximum of 54.1% in the case of Saf; increases for BSA, Dex and A. Muc were 13.7%, 24.4% and 36.1%, respectively.
Figure 2. Phagocytic activity. Macrophages (MQ) were cultured/treated using the 2-D and 3-D conditions and then phagocytic activity was evaluated via measures of phagocytosis of FITC-labeled NP after a 6 h period. (A) Representative light microscopic evaluation of cells in a 3-D system (NP only, no additional MQ treatments); Magnification 20×. (B) Corresponding representative fluorescent microscopic evaluation; Magnification 20×. (C) NP uptake using flow cytometry. Representative histograms derived from dot-plots show there an enhanced phagocytic activity among MQ held on collagen-chitosan scaffolds compared to by MQ in 2-D plates (with all exogenous treatments). Abbreviations are as in legend to . Relative percentages of NP+ cells (means ± SD; n = 5 replicates/analysis) for a given sample are in each histogram.
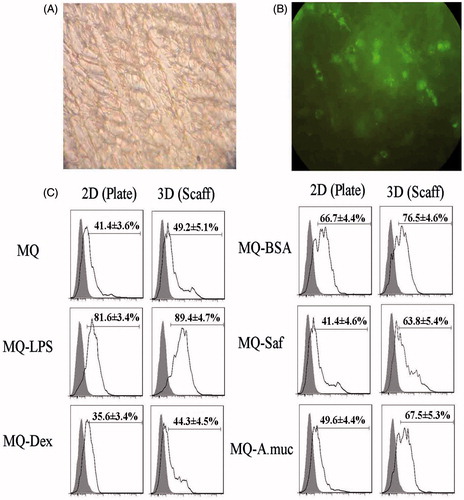
In each culture system – as expected – use of LPS and Dex led to increases and decreases (respectively) in NP uptake compared to control culture levels. In contrast, both Saf and A. muc had little impact on uptake in cells in the 2-D systems, but a very strong effect among cells on the scaffolds (increases of 29.7% and 37.2%, respectively, relative to 3-D control cell activity levels). Use of BSA resulted in net increases in activity roughly equivalent across the test systems (i.e. 55–60% above counterpart control culture values).
Macrophage cytokine production
Release of TNFα and IL-1β from macrophages was evaluated after 48 h of culture in the 2-D/3-D maintenance systems and under the influence of various exogenous treatments (). The production of both cytokines in each system displayed the same patterns of changes relative to production by control macrophages. For both systems, stimulation with LPS and BSA induced each cytokine to a level greater than that from the control cells. In the case of the LPS treatments, the effects were greater (p < 0.05) among cells held on the plates than among cells held in the scaffolds. With regard to the BSA-treated cells, in neither cytokine case were the effects from the maintenance system significantly different (i.e. all cells produced more than their control counterparts).
Figure 3. TNFα and IL-1β production. Effects of different treatment/maintenance systems on macrophage TNFα and IL-1β production over 48-h period are shown. Values are mean (pg/ml) ± SD from n = 5 replicates/regimen. *Value significantly different as a function of maintenance system for any given cell treatment is indicated (p < 0.05). Abbreviations are as in legend to . For each cytokine, treatment vs treatment analyses of significant differences within a defined maintenance system were not performed.
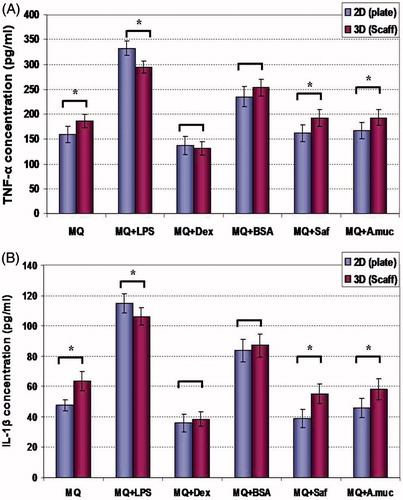
The impact of treatments with safranal or the mucilage on the macrophage formation of either cytokine was nominal (compared to that by control cells). Interestingly, as in the control populations, these latter sets of cultures consistently yielded formation values that were higher among cells held on the 3-D scaffolds in comparison to that by their 2-D culture counterparts (i.e. irrespective of complete lack of stimulation relative to control cell cytokine formation). Release of either cytokine after treatment of the cells with dexamethasone was reduced (relative to in control cells) in both maintenance systems. In this case, the effect within cells appeared to not differ as a result of the maintenance system being used.
Discussion
A variety of extracellular matrix components have been discovered in reticular fibers, including collagens I, III and IV, elastin, entactin, fibronectin, laminin-1, tenascin, vitronectin and heparan sulfate (Kaldjian et al. Citation2001). Therefore, use of these reticular glycoproteins in three-dimensional (3-D) platforms should enable these systems to serve as quite suitable forms for evaluating growth and function of a variety of cells, including, in the case of the immune system, T-cells and macrophages.
Porous 3-D collagen-chitosan scaffolds have been used extensively as biomaterials in the field of tissue engineering. As it is a significant constituent of extracellular matrices, the collagen can promote cell and tissue attachment and growth (O’Brien et al. Citation2005) Chitosan, an amino polysaccharide (poly-1,4-D-glucosamine), is non-toxic and has a more biocompatible nature (Shanmugasundaram et al. Citation2001); accordingly, it has been widely utilized in several biomedical applications, such as wound dressings and drug delivery systems.
In the present study, macrophages were cultured on routine 2-D plates and 3-D collagen-chitosan scaffolds and effects on macrophages viability, phagocytic capacity, and pro-inflammatory cytokine secretion was evaluated. Under each condition (2-D vs 3-D), the cultured macrophages were exposed to LPS (commonly-used macrophage stimulant), Dex (suppressor of macrophage functions), BSA (a common protein), safranal (a pure herbal component) or a crude mixture of herbal components (A. mucilage; A. muc). Microscopic evaluations demonstrated a porous surface on the scaffolds, with appropriate integration/infiltration of the macrophages into the scaffolds pores.
Analysis of cell viability showed that macrophages in different culture groups/treatments had the same viability as their controls. Thus, the scaffolds did not impart cytotoxic or adverse effects on the macrophages. A low toxicity among cells cultured on chitosan/collagen nano-scaffolds was a finding noted in other studies of cultured bone (Levengood & Zhang Citation2014), cartilage (Gong et al. Citation2010), skin (Sarkar et al. Citation2013), intestinal smooth muscle (Zakhem et al. Citation2012) and endothelial cells (Swarnalatha et al. Citation2013).
Differences in select cultured macrophage functions were also assessed here. The results showed that phagocytic activity in general was enhanced when cells were maintained on the collagen-chitosan scaffolds. Particle uptake was most strongly enhanced (relative to levels by 2-D plate cells) when the macrophages were treated with the safranal or A. muc. When cells were assessed for release of TNFα and IL-1β, as expected, under either culture condition both were induced by LPS and BSA and suppressed by dexamethasone in comparison to control group levels. Neither Saf nor A. muc caused changes in production above background. Nevertheless, in comparing the 2-D vs 3-D conditions, it was clear that cells on the scaffolds tended to have differences in levels of basal/inducible TNFα and IL-1β formation/release. Significant increases (3-D relative to 2-D) in production of these cytokines were only apparent among the control cells or when the cells were treated with safranal or A. muc; there was no net difference in effect in the studies with Dex of BSA. Oddly, in the studies with LPS, use of the 3-D system resulted in significant decreases in production of either cytokine relative to that by the 2-D cells. Thus, across the assays, it was apparent that collagen-chitosan scaffolds allowed the Saf and A. muc to impart their greatest effects – albeit that these effects were not always truly ‘stimulatory’ relative to the functions of control cell counterparts.
Previous studies have shown that chitosan stimulates macrophages to produce pro-inflammatory mediators such as IL-1, IL-6, TNFα, nitric oxide (NO) and granulocyte-macrophage colony stimulating factor (GM-CSF) (Seo et al. Citation2000; Yu et al. Citation2004). Wu and Tsai (Citation2007) showed that chitosan oligosaccharides could cause an increase in NO production via activation of nuclear factor (NF)-κB in RAW264.7 macrophages. Another study showed that chitosan could induce phospholipase A2 (PLA2) and enhance mobility in P388D1 macrophages (Bianco et al. Citation2000). Feng et al. (Citation2004) first demonstrated that oligo-chitosan had an in vitro stimulatory effect on the release of TNFα and IL-1β by macrophages; moreover, these authors showed that internalization of the oligo-chitosan was mediated by a lectin receptor with mannose specificity on the macrophage membrane.
In general, collagen has unique properties that help cause cell activation. It has been shown that 3-D collagen matrices facilitate cell function and migration (Rommerswinkel et al. Citation2014). There are some basic residues within collagen such as lysine and arginine and also specific cell adhesion sites such as arginine-glycine-aspartate (RGD) groups that actively induce cell adhesion by binding to integrin receptors; these interactions play an important role in cell growth, differentiation and overall regulation of cell functions (Quirk et al. Citation2001). Studies have specifically shown collagen VI to be critical for macrophage migration and polarization during peripheral nerve regeneration (Chen et al. Citation2015).
In spite of these reported stimulatory effects, there are some studies that have reported on anti-inflammatory effects of chitosan on macrophages. Yoon et al. (Citation2007) noted that chitosan oligosaccharides caused reduced TNFα and IL-6 production by LPS-stimulated RAW 264.7 macrophages. Oliveira et al. (Citation2012) showed that, after 10 days culture of macrophages on chitosan films, the cells displayed significantly down-regulated expression of pro-inflammatory surface markers (CD86 and MHC-II) and reduced production of pro-inflammatory TNFα, but a concurrent increase in the formation of anti-inflammatory IL-10 and TGF-β1. Such reductions in effect would appear to mimic the loss of effects from LPS upon the cells here that were cultured on the 3-D scaffolds (re: cytokine formation outcomes).
The present studies also evaluated the impact of the 2-D vs 3-D scenarios on how the macrophages would respond to a common stimulant, suppressor and protein found in many culture media. In the case of LPS, a typical activator of macrophages, while its stimulatory effects were predictable and verifiable with cells maintained in the 2-D plates, there was a lower efficacy noted among the cells maintained on the collagen-chitosan scaffolds. Such inhibitory effects of chitosan oligomers on the activating potential of LPS were akin to those reported (and noted above) by Yoon et al. (Citation2007). Results for cells exposed to BSA or Dex were also predictable – with BSA being taken up by and stimulating the macrophages, while dexamethasone was inhibitory. In comparing the two test systems, effects of BSA or Dex on macrophages functions were no different as a result of the 2-D vs 3-D culture conditions.
Although there are not specific studies in the literature reporting on comparison of effects from BSA [or Dex] on macrophages in 3-D vs 2-D culture conditions, Jacob and Sudhakaran (Citation2001) did show that macrophages maintained on collagen I (COL I) lattice scaffolds exhibited a higher rate of endocytosis of [125I]-acetyl-BSA than did cells grown on collagen-coated plastic plates. As both of those systems contained collagen and due to the above-mentioned activating effects from collagen, any impact from that enhanced uptake of BSA would most likely have been hard to discern (had such outcomes even been measured in that earlier study). Nonetheless, the lack of any ‘enhancing’ outcomes with BSA – or even increasingly ‘suppressive’ effects from Dex for the cells maintained on the 3-D system here illustrates the complications arising from collagen having a potential to enhance OR suppress the activation status of these cultured cells under given experimental conditions (such as use of a stimulant or a suppressant in the culture). Taking all the findings with LPS, BSA and Dex together, it would appear that the collagen is more suppressant than activator – at least in regards to the endpoints measured here. Clearly, further studies evaluating a wider array of endpoints are needed to better determine if the collagen in the collagen-chitosan system is more suppressant than stimulant for these cells.
With respect to effects from the tested herbal components, it was expected that the various metals (including Ni, Cr, Mn, Fe, Mg, Ca) found in the A. homolocarpum seed gum (Ghaderian et al. Citation2007; Centofanti et al. Citation2011) would result in anti-inflammatory effects/cytokine suppression (relative to in corresponding control cultures) in each test system. Similarly, safranal (2,6,6-trimethyl-1,3-cyclohexadiene-1-carboxaldehyde), an active ingredient in Crocus sativus (saffron) used in traditional medicines (Tarantilis et al. Citation1995), is known to cause decreases in inflammation, in part by reducing circulating levels of TNFα and IL-1β in treated hosts (Hazman & Ovali Citation2015). However, the study here showed that, even thought the macrophages cultured under the 3-D conditions had significantly higher phagocytic activity and higher levels of TNFα and IL-1β production in comparison to cells held in the 2-D systems, neither culture system ‘allowed’ for the suppressive effects of the herbal components to manifest.
It remains unclear to us why this was the case; if in fact the chitosan countered any suppressive effects from the herbal components, then this would run counter to the above-noted ‘suppressive’ effects of the chitosan in the LPS/Dex/BSA experiments. Such selectivity of effects would be hard to explain. Similarly, the 2-D plates lacking the chitosan have no basis for ‘mitigating’ the expected herbal suppressive effects. Thus, it is quite possible that the doses of the seed gum extract and of the safranal were insufficient to cause the expected inhibitory effects. Future experiments will utilize a range of higher (hopefully non-lethal) doses of each test agent in similar 2-D vs 3-D comparison studies.
Conclusions
This study evaluated several macrophage functional parameters as an outcome of being maintained on 2-D plates or 3-D collagen-chitosan scaffolds. Treatment of the cells with various stimulating/inhibiting agents in some cases yielded outcomes that differed as a result of the 2-D vs 3-D maintenance conditions. Considering the different components/factors that generate the microenvironments in which macrophages exist in situ, the 3-D scaffolds here seemed to provide a more favorable microenvironment for cell activation.
Acknowledgments
This work was support by a grant of Islamic Azad University, Sari Branch.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alberts B, Bray D, Hopkin K, Johnson A, Roberts K, Lewis J, Raff R, Walter P, editors. 2010. Essential cell biology. New York/London: Garland Science.
- Bianco ID, Balsinde J, Beltramo DM, Castagna LF, Landa CA, Dennis EA. 2000. Chitosan-induced phospholipase A2 activation and arachidonic acid mobilization in P388D1 macrophages. FEBS Lett. 466:292–294.
- Centofanti T, Tappero RV, Davis AP, Chaney RL. 2011. Chelator-buffered nutrient solution is ineffective in extracting Ni from seeds of Alyssum. Intl J Phytoremediation. 13:434–440.
- Chen P, Cescon M, Zuccolotto G, Nobbio L, Colombelli C, Filaferro M, Vitale G, Feltri ML, Bonaldo P. 2015. Collagen VI regulates peripheral nerve regeneration by modulating macrophage recruitment and polarization. Acta Neuropathol. 129:97–113.
- Feng J, Zhao L, Yu Q. 2004. Receptor-mediated stimulatory effect of oligochitosan in macrophages. Biochem Biophys Res Commun. 317:414–420.
- Fortin CF, McDonald PP, Fulop T, Lesur O. 2010. Sepsis, leukocytes, and nitric oxide (NO): An intricate affair. Shock. 33:344–352.
- Ghaderian SM, Mohtadi A, Rahiminejad MR, Baker AJ. 2007. Nickel and other metal uptake and accumulation by species of Alyssum (Brassicaceae) from the ultramafics of Iran. Environ Pollut. 145:293–298.
- Gong Z, Xiong H, Long X, Wei L, Li J, Wu Y, Lin Z. 2010. Use of synovium-derived stromal cells and chitosan/collagen Type I scaffolds for cartilage tissue engineering. Biomed Mater. 5:055005.
- Hayashi Y, Yamada S, Yanagi-Guchi K, Koyama Z, Ikeda T. 2012. Chitosan and fish collagen as biomaterials for regenerative medicine. Adv Food Nutr Res. 65:107–120.
- Hazman O, Ovali S. 2015. Investigation of the anti-inflammatory effects of safranal on high-fat diet and multiple low-dose streptozotocin-induced Type 2 diabetes rat model. Inflammation. 38:1012–1019.
- Jacob SS, Sudhakaran PR. 2001. Monocyte-macrophage differentiation in three dimensional collagen lattice. Biochim Biophys Acta. 1540:50–58.
- Kaldjian EP, Gretz JE, Anderson AO, Shi Y, Shaw S. 2001. Spatial and molecular organization of lymph node T-cell cortex: A labyrinthine cavity bounded by an epithelium-like monolayer of fibroblastic reticular cells anchored to basement membrane-like extra-cellular matrix. Intl Immunol. 13:1243–1253.
- Koocheki A, Mortazavi SA, Shahidi F, Razavi SM, Kadkhodaee R, Milani JM. 2008. Optimization of mucilage extraction from Qodumeshirazi seed (Alyssum homolocarpum) using response surface methodology. J Food Process Eng. 33:861–882.
- Lahiji A, Sohrabi A, Hungerford DS, Frondoza CG. 2000. Chitosan supports expression of extracellular matrix proteins in human osteoblasts and chondrocytes. J Biomed Mater Res. 51:586–595.
- Lee J, Cuddihy MJ, Kotov NA. 2008. Three-dimensional cell culture matrices: State of the art. Tissue Eng. 14:61–86.
- Levengood SL, Zhang M. 2014. Chitosan-based scaffolds for bone tissue engineering. J Mater Chem B Mater Biol Med. 2:3161–3184.
- Li G, Zhang L, Wang C, Zhao X, Zhu C, Zheng Y, Wang Y, Zhao Y, Yang Y. 2014. Effect of silanization on chitosan porous scaffolds for peripheral nerve regeneration. Carbohydrate Polymers. 101:718–726.
- Ma L, Gao C, Mao Z, Zhou J, Shen J, Hub X, Han C. 2003. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials. 24:4833–4841.
- Mori T, Irie Y, Nishimura SI, Tokura S, Matsuura M, Okumura M, Kadosawa T, Fujinaga T. 1998. Endothelial cell responses to chitin and its derivatives. J Biomed Mater Res. 43:469–472.
- Mori T, Okumura M, Matsuura M, Ueno K, Tokura S, Okamoto Y, Minami S, Fujinaga T. 1997. Effects of chitin and its derivatives on the proliferation and cytokine production of fibroblasts in vitro. Biomaterials. 18:947–951.
- O’Brien FJ, Harley BA, Yannas IV, Gibson L. 2005. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 26:433–441.
- Oliveira MI, Santos SG, Oliveira MJ, Torres AL, Barbosa MA. 2012. Chitosan drives anti-inflammatory macrophage polarization and pro-inflammatory dendritic cell stimulation. Eur Cell Mater. 24:136–152.
- Quirk RA, Chen WC, Davies MC, Tendler SJ. 2001. Poly-(L-lysine)–GRGDS as a biomimetic surface modifier for poly-(lactic acid). Biomaterials. 22:865–872.
- Ragetly G, Griffon DJ, Chung YS. 2010. Effect of Type II collagen coating of chitosan fibrous scaffolds on mesenchymal stem cell adhesion and chondrogenesis. ActaBiomater. 6:3988–3997.
- Rommerswinkel N, Niggemann B, Keil S, Zänker KS, Dittmar T. 2014. Analysis of cell migration within a three-dimensional collagen matrix. J Vis Exp. 92:e51963.
- Rosário C, Zandman-Goddard G, Meyron-Holtz EG, D'Cruz DP, Shoenfeld Y. 2013. Hyper-ferritinemic syndrome: Macrophage activation syndrome, Still's disease, septic shock, and catastrophic anti-phospholipid syndrome. BMC Med. 11:185.
- Sarkar SD, Farrugia BL, Dargaville TR, Dhara S. 2013. Chitosan-collagen scaffolds with nano-/micro-fibrous architecture for skin tissue engineering. J Biomed Mater Res. 101:3482–3492.
- Seo WG, Pae HO, Kim NY, Oh GS, Park IS, Kim YH, Kim YM, Lee YH, Jun CD, Chung HT. 2000. Synergistic cooperation between water-soluble chitosan oligomers and interferon-gamma for induction of nitric oxide synthesis and tumor-icidal activity in murine peritoneal macrophages. Cancer Lett. 159:189–195.
- Shanmugasundaram N, Ravichandran P, Neelakanta PR, Ramamurty N, Pal S, Rao KP. 2001. Collagen-chitosan polymeric scaffolds for in vitro culture of human epidermoid carcinoma cells. Biomaterials. 22:1943–1951.
- Swarnalatha B, Nair SL, Shalumon KT, Milbauer LC, Jayakumar R, Paul-Prasanth B, Menon KK, Hebbel RP, Somani A, Nair SV. 2013. Poly (lactic acid)-chitosan-collagen composite nanofibers as substrates for blood outgrowth endothelial cells. Intl J Biol Macromol. 58:220–224.
- Tarantilis PA, Tsoupras G, Polissiou M. 1995. Determination of saffron (Crocus sativus L.) components in crude plant extract using high performance liquid chromatography-UV-visible photodiode-array detection-mass spectrometry. J Chromatogr. 699:107–118.
- Wang XH, Li DP, Wang WJ, Feng QL, Cui FZ, Xu YX, Song XH, van der Werf M. 2003. Cross-linked collagen/chitosan matrix for artificial livers. Biomaterials. 24:3213–3220.
- Wu GJ, Tsai GJ. 2007.Chitooligosaccharides in combination with IFNγ increase nitric oxide production via NF-κB activation in murine RAW264.7 macrophages. Food Chem Toxicol. 45:250–258.
- Yoon HJ, Moon ME, Park HS, Kim SY, Kim YH. 2007. Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW 264.7 macrophage cells. Biochem Biophys Res Commun. 358:954–959.
- Yu Z, Zhao L, Ke H. 2004. Potential role of NF-κB in induction of nitric oxide and TNFα by oligochitosan in macrophages. Intl Immunopharmacol. 4:193–200.
- Zakhem E, Raghavan S, Gilmont RR, Bitar KN. 2012. Chitosan-based scaffolds for the support of smooth muscle constructs in intestinal tissue engineering. Biomaterials. 33:4810–4817.
