Abstract
Context. There is contention over whether reported dose correlates with toxicity in paracetamol poisoning and risk assessment is currently based on serum paracetamol concentration compared to a nomogram, irrespective of reported dose. Objective. To determine if reported dose predicts the need for N-acetylcysteine (NAC). Methods. Data were taken from paracetamol overdoses presenting to a tertiary toxicology service. Age, sex, reported dose, ingestion time, timed paracetamol concentrations between 4 and 16 h, hepatotoxicity (peak alanine transaminase > 1000 U/L) and treatment (single dose-activated charcoal [SDAC] and NAC) were analysed. Data were analysed within a repeated measures logistic regression framework using NONMEM (ver 7.2). The primary outcome was administration of NAC, which was determined based on a serum paracetamol concentration greater than the nomogram line. Result.: There were 1571 admissions in 1303 patients, with a median age of 27 years (12–96 years) and 1140 (73%) were females. The median dose was 10 g (1–100 g). The paracetamol concentration was above the nomogram line in 337 of 1571 (22%) patients. Patients presenting later (first paracetamol concentration between 7 and 16 h post-overdose) compared to those presenting earlier (4–7 h post-overdose) were more likely to have hepatotoxicity (5.5% vs. 0.4%; p < 0.0001), have a toxic paracetamol concentration (34% vs. 18%; p < 0.0001) and receive NAC (48% vs. 23%; p < 0.0001). SDAC reduced the probability of the paracetamol concentration being above the nomogram. Based on SDAC not being administered there was a 5% probability of requiring NAC at a dose of 6–9 g, a 10% chance of requiring NAC at a dose of 13–16 g, a 50% chance of requiring NAC at a dose of 30–34 g and a 90% chance for needing NAC at 48–50 g. Conclusion. Reported dose was a good predictor of a toxic paracetamol concentration and SDAC reduced the probability of the concentration being above the nomogram. These predictions may assist in determining which patients could be started on NAC immediately.
Introduction
Paracetamol overdose remains one of the most important common poisonings in many parts of the worldCitation1,Citation2 and an increasing problem in many developing and resource poor nations.Citation3 N-acetylcysteine (NAC) is an effective treatment in the vast majority of cases if given early.Citation4–6 The decision to give NAC is usually based on a paracetamol concentration measured at least 4 h after ingestion or later if the patient presents to hospital after this time.Citation7 Although dose is recognised as a predictor of toxicity, reported dose is rarely used to define treatment if a paracetamol concentration is available.Citation8
It remains unclear what the toxic dose of paracetamol is and in the majority of cases patients will have a serum paracetamol concentration measured irrespective of the reported dose taken. Toxic dose is defined empirically as a dose that is known to cause toxicity. Various guidelines suggest different toxic doses. Australian and most international guidelines recommend 200 mg/kg or 10 g as a toxic dose.Citation1,Citation2 However, despite this being defined as a toxic dose the majority of patients will have a serum paracetamol concentration measured, even if they have ingested less than this amount. A comparison of the reported and toxic dose is only used in cases where a serum paracetamol concentration is not available (i.e. late presentations) or in resource poor settings where laboratory services are not available.Citation3 Although previous studies have shown that reported dose is an independent predictor of hepatotoxicity,Citation9 this has not influenced risk assessment in paracetamol poisoning.Citation8
If reported dose was a strong predictor of hepatotoxicity, then its use would potentially allow early initiation of NAC in large overdoses or conversely avoid waiting for paracetamol concentrations for non-toxic doses. This is already done in some cases with massive ingestions, but this is based on anecdotal evidence. Currently, the practice of a single serum paracetamol concentration being above the nomogram between 4 and 24 h from ingestion remains the gold standard for starting NAC therapy.Citation1,Citation6 Starting NAC prior to measuring a paracetamol concentration has been suggested as a potential approach in paracetamol poisoning because it allows for slower administration rates, that is the loading dose to be given over a longer duration.Citation10 However, it would be best if NAC was only given to patients with a high probability of requiring NAC. Therefore, predicting whether the paracetamol concentration will be above the nomogram line based on reported dose is of significance.
The primary aim of this study was to determine whether reported paracetamol dose was predictive of the need for NAC. In addition, we will investigate whether a minimal dose could be defined below which treatment may not be required (e.g. < 10%) and a maximum dose above which the probability of requiring NAC was greater than 90%.
Methods
This study included dosing and drug concentration data from single acute paracetamol overdoses recorded in a prospective database. The use of the database and de-identified patient information has been granted exemption as an audit by the local Human Research Ethics Committee.
Information for all presentations to a large regional toxicology centre was collected prospectively using a standardised data collection form that is completed at the time of presentation by the treating doctor. This information and any additional data from the medical record were entered into a relational database by trained research assistants. This included demographic information, details of the overdose/exposure (time of overdose and reported dose ingested), clinical information, laboratory investigations and treatment.
All paracetamol overdoses (single acute ingestions > 1 g) presenting to the toxicology service between January 1997 and December 2011 were extracted from the database. Cases were only included if there was a serum paracetamol concentration measured between 4 and 16 h after ingestion, and both the time of ingestion and the amount ingested were reported and recorded in the database. We used reported dose to represent the dose that the treating clinicians believe the patient took. The reported dose was based on the patient history which was confirmed on multiple occasions and cross checked with any collateral history from the family, friends or pre-hospital services (e.g. ambulance). Note the actual dose remains unknown and the perceived discrepancy between actual and reported dose is often perceived as the basis for uncertainty in the decision about treatment.
The following data were extracted from the database: age, sex, reported dose ingested, time of ingestion, paracetamol concentration and the time of the paracetamol concentration (between 4 and 16 h), peak alanine transaminase (ALT) and treatment (single dose activated-charcoal [SDAC] and NAC). Patients were further divided into two groups based on the time of their first paracetamol concentration. Early presenters were defined a priori as those that had their first paracetamol concentration between 4 and 7 h and could have NAC started within 8 h. Late presenters were defined as those that had their first level between 7 and 16 h post-overdose and were commenced on NAC on admission. Seven hours was chosen as the cut-off because this meant that NAC could be commenced within 8 h. Hepatotoxicity was defined as an ALT > 1000 U/L.
Data analysis
For ease of interpretation continuous variables are presented as medians with interquartile ranges (IQR). Dichotomous outcomes were compared using Fisher's exact test or Chi-square test.
Data extracted from the database were analysed within a repeated measures logistic regression framework using NONMEM (ver 7.2). The data were presented as 0 if the serum paracetamol concentration was below the line of the nomogram and hence no dose of NAC would usually be given and 1 if the concentration was above the nomogram. The nomogram used started from 150 mg/L (1000 μM) at 4 h and decreased with a half-life of 4 h. Factors that were considered were age, sex, reported paracetamol dose and SDAC. Model building was based on the likelihood ratio test where a decrease in the difference of two objective functions (proportional to twice the negative log-likehood) were assumed to be chi-squared distributed with the degrees of freedom equivalent to the difference in the number of parameter values for nested models. Interaction terms were also considered. The general form of the linear model was:
with additional terms being added for other effects (e.g. SDAC) as required. Following transformation, the probability that NAC would be administered:
The probability that the serum paracetamol concentration is greater than the value on the nomogram was the primary measure in the study but is considered to be a surrogate marker of the probability that NAC would have been administered.
Results
From a total of 2990 paracetamol poisoning admissions there were 1571 acute paracetamol overdoses with a reported dose, known time of ingestion and a paracetamol concentration performed between 4 and 16 h post-overdose. The reasons for exclusion were no known time of ingestion (385), no reported dose (94), dose less than 1 g (26) and no paracetamol concentration between 4 and 16 (914). The 1571 admissions were in 1303 patients, 1173 patients presented on one occasion and 130 on two or more occasions. The median age was 27 years (range: 12–96 years; IQR: 20–39 years) and 1140 (72.6%) were females. The median dose ingested was 10 g (range: 1–100 g; IQR: 6–16 g). The treatment and clinical outcome data for all admissions are included in . The paracetamol concentration was above the 150/1000 nomogram line in 337 of 1571 (21.5%) and 300 of these received NAC. An additional 143 patients received NAC who did not have a paracetamol concentration above the nomogram.
Table 1. Comparison of patients with an early (4–7 h) first paracetamol concentration to patients with a late (7–16 h) first paracetamol concentration.
compares patients who presented early (had a serum paracetamol concentration taken between 4 and 7 h post-overdose), to those who presented late (with a serum paracetamol concentration between 7 and 16 h). Patients who presented later had a higher rate of hepatotoxicity (5.5% vs. 0.4%; p < 0.0001), were more likely to have a paracetamol concentration above the nomogram (33.6% vs. 18.2%; p < 0.0001) and more likely to receive NAC (47.6% vs. 23.0%; p < 0.0001).
The final model included paracetamol reported dose, age, sex and use of SDAC. The data were not able to support a random effect on either the baseline or the influence of reported dose on the probability of the serum paracetamol concentration being above the nomogram line. There was no interaction between the use of SDAC and ingested dose, age or sex.
The probability of the paracetamol concentration being above the nomogram line based on the dose given is shown in for 30-year-old females and males. The probability of being above the nomogram line was lower for males at a similar dose compared to that for females. The influence of the ingested overdose dose on the adjusted probability of being above the nomogram line is given by the solid line. The probability based on dose is adjusted for the influence of SDAC (dashed line), age of the patient and sex of the patient. It is seen that SDAC reduces the probability of being above the nomogram line, shown by the dashed lines in . SDAC reduces the probability of NAC by up to 14% at 28 g and less than 10% at either doses lower than 19 g or doses greater than 37 g.
Fig. 1. Probability of the paracetamol concentration being above the nomogram line versus dose (a) for a 30-year-old female with (dashed line) and without (solid line) SDAC; and (b) for a 30-year- old male (solid line) compared to a 30-year-old female (dashed line).
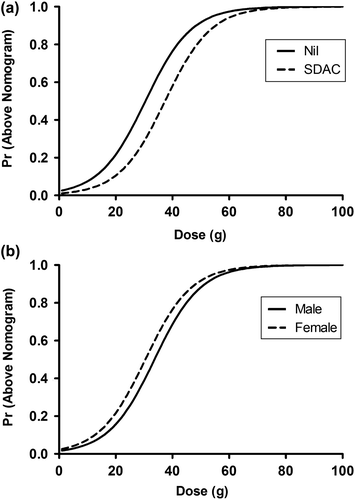
Based on SDAC not being administered then there was a 5% probability of being above the nomogram line at a dose of 6–9 g, a 10% probability of being above the nomogram line at a dose of 13–16 g, a 50% probability of being above the nomogram line at a dose of 30–34 g and a 90% probability of being above the nomogram line at 48–50 g.
Discussion
This study demonstrates that reported dose is a strong predictor of patients having paracetamol concentrations above the nomogram line. It has also shown that patient age, sex and the administration of SDAC influences this probability. The relationship between reported dose and toxic paracetamol concentration can potentially be used to allow early intervention in large overdoses. The study also confirmed that patients presenting 7 h or more after ingestion, who were therefore not administered NAC within 8 h, were more likely to receive NAC and develop an abnormal alanine transaminase. This supports current practice of commencing NAC prior to getting a paracetamol concentration in all patients presenting after 8 h and then ceasing it if the paracetamol concentration is below the nomogram line.
Most guidelines recommend that patients presenting within 8 h have a serum paracetamol concentration measured prior to commencing NAC – “wait and see approach”. The results of this study suggest that if the reported dose is greater than 50 g then more than 90% of patients require treatment so NAC could potentially be commenced earlier in these patients. An alternate approach has been to commence NAC early and stop it if the paracetamol concentration is non-toxic. For this approach, reported doses associated with a low probability of requiring NAC could be used to define a group of patients where NAC should not be commenced prior to a paracetamol concentration being determined. In other words, a reported dose cut-off could be used to determine patients where a “wait and see approach” is followed (< cut-off dose) or an approach to commence NAC prior to obtaining a paracetamol concentration (> cut-off dose) and then revisit the use of NAC once a paracetamol concentration is available. For instance, if a reported dose of 10 g was used this would prevent half of all paracetamol overdose patients (in this study) being started on NAC early (i.e. if all patients are initially commenced on NAC prior to a paracetamol concentration), with the associated small risk of adverse reactions. Patients ingesting 10 g only have a 5–7% probability of being above the nomogram line and therefore requiring NAC.
This study confirms a previous study (data prior to 1997) at the same toxicology unit which demonstrated that the administration of SDAC reduces the probability of requiring NAC. This would support the early administration of activated charcoal in cooperative patientsCitation11 to reduce the number of patients requiring NAC and therefore reduce the length of stay. The study suggests that there is the greatest benefit of SDAC for doses greater than 28 g. However, although SDAC is a low risk intervention it could not be given as a duty of care in these patients because NAC is an effective antidote.
There are a number of limitations to the study including the retrospective extraction and review of the data. However, this is unlikely to affect the primary aim of the study which was based on the reported dose and the measured serum paracetamol concentration. Previous studies have demonstrated that reported dose is a good estimate of the true ingested dose and that patient history is reliable in the majority of casesCitation12,Citation13 although this remains a point of controversy in the literature.Citation14–17 Another problem was that a large number of patients (47%) were excluded which may introduce selection bias. However, the majority (31%) were because a paracetamol concentration was not done between 4 and 16 h and includes late presenters, staggered, supratherapeutic and chronic paracetamol ingestions, where risk assessment is generally not based on a single paracetamol concentration plotted on the nomogram.
Another limitation was that SDAC was administered according to the emergency department doctor or treating clinical toxicologist and was not randomised. This may have meant that patients ingesting larger doses were more likely to receive SDAC. However, there was no interaction between dose and SDAC in the logistic regression model suggesting that this was unlikely to be the case.
There were a number of patients with paracetamol concentrations above the nomogram who did not receive NAC. This is most likely to be patients between the 150 and 200 mg/L nomogram lines who were not treated since this was prior to the change in Australia from the 200 mg/L nomogram line to the 150 mg/L nomogram line.
In addition to dose and SDAC, the final logistic regression model included both age and sex as significant covariates. shows that there was a greater probability of the serum paracetamol being above the line in females compared to males. This may be because females on average weigh less than males and weight was not included in the model because it was not available in the majority of patients.
The study shows that reported dose can be used as an early predictor of patients who require NAC. This may improve the risk assessment in patients with paracetamol poisoning allowing the earlier administration in large overdoses or more targeted use of NAC in early and late presenters.
Acknowledgements
We acknowledge the assistance of the medical and nursing staff who contribute to the Hunter Area Toxicology Service clinical database.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Funding
Geoff Isbister is funded by an NHMRC Clinical Career Development Award ID 605817.
References
- Daly FF, Fountain JS, Murray L, Graudins A, Buckley NA; Panel of Australian and New Zealand clinical toxicologists. Guidelines for the management of paracetamol poisoning in Australia and New Zealand–explanation and elaboration. A consensus statement from clinical toxicologists consulting to the Australasian poisons information centres. Med J Aust 2008; 188:296–301.
- Dart RC, Erdman AR, Olson KR, Christianson G, Manoguerra AS, Chyka PA, et al. Acetaminophen poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 2006; 44:1–18.
- Senarathna SM, Sri Ranganathan S, Buckley N, et al. A cost effectiveness analysis of the preferred antidotes for acute paracetamol poisoning patients in Sri Lanka. BMC Clin Pharmacol 2012; 12:6.
- Buckley NA, Whyte IM, O’Connell DL, Dawson AH. Oral or intravenous N-acetylcysteine: which is the treatment of choice for acetaminophen (paracetamol) poisoning?J Toxicol Clin Toxicol 1999; 37:759–767.
- Prescott LF, Illingworth RN, Critchley JA, Proudfoot AT. Intravenous N-acetylcysteine: the treatment of choice for paracetamol poisoning. Br Med J 1979; 2:1097–1100.
- Bateman DN. Controversies in the use of N-acetylcysteine as an antidote. J Toxicol Clin Toxicol 2003; 41:434–435.
- Buckley N, Eddleston M. Paracetamol (acetaminophen) poisoning. Clin Evid 2005; (14):1738–1744.
- Buckley NA, Whyte IM, O’Connell DL, Dawson AH. Activated charcoal reduces the need for N-acetylcysteine treatment after acetaminophen (paracetamol) overdose. J Toxicol Clin Toxicol 1999; 37:753–757.
- Schmidt LE, Dalhoff K, Poulsen HE. Acute versus chronic alcohol consumption in acetaminophen-induced hepatotoxicity. Hepatology 2002; 35:876–882.
- Shen F, Coulter CV, Isbister GK, Duffull SB. A dosing regimen for immediate N-acetylcysteine treatment for acute paracetamol overdose. Clin Toxicol (Phila) 2011; 49:643–647.
- Isbister GK, Kumar VV. Indications for single-dose activated charcoal administration in acute overdose. Curr Opin Crit Care 2011; 17: 351–357.
- Isbister GK. How do we use drug concentration data to improve the treatment of overdose patients?Ther Drug Monit 2010; 32: 300–304.
- Friberg LE, Isbister GK, Hackett LP, Duffull SB. The population pharmacokinetics of citalopram after deliberate self-poisoning: a bayesian approach. J Pharmacokinet Pharmacodyn 2005; 32: 571–605.
- Manini A, Smith S, Moskovitz J, Nelson L. In response to Isbister et al.: Application of pharmacokinetic-pharmacodynamic modeling in management of QT abnormalities after citalopram overdose. Intensive Care Med 2007; 33: 738; author reply 9.
- Lugassy DM, Hoffman RS, Chessex N. In response to van Gorp F. et al. Escitalopram overdose. Ann Emerg Med 2010; 55:128–129.
- Isbister GK, Friberg LE, Duffull SB. Application of pharmacokinetic-pharmacodynamic modelling in management of QT abnormalities after citalopram overdose. Intensive Care Med 2006; 32:1060–1065.
- van Gorp F, Whyte IM, Isbister GK. Clinical and ECG effects of escitalopram overdose. Ann Emerg Med 2009; 54:404–408.