ABSTRACT
Background: This is the 31st Annual Report of the American Association of Poison Control Centers’ (AAPCC) National Poison Data System (NPDS). As of January 1, 2013, 57 of the nation's poison centers (PCs) uploaded case data automatically to NPDS. The upload interval was 8.08 [7.10, 11.63] (median [25%, 75%]) minutes, creating a near real-time national exposure and information database and surveillance system.
Methodology: We analyzed the case data tabulating specific indices from NPDS. The methodology was similar to that of previous years. Where changes were introduced, the differences are identified. Poison center (PC) cases with medical outcomes of death were evaluated by a team of 38 medical and clinical toxicologist reviewers using an ordinal scale of 1–6 to assess the Relative Contribution to Fatality (RCF) of the exposure to the death.
Results: In 2013, 3,060,122 closed encounters were logged by NPDS: 2,188,013 human exposures, 59,496 animal exposures, 806,347 information calls, 6,116 human-confirmed nonexposures, and 150 animal-confirmed nonexposures. Total encounters showed a 9.3% decline from 2012, while health care facility human exposure calls were essentially flat, decreasing by 0.1%.All information calls decreased 21.4% and health care facility (HCF) information calls decreased 8.5%, medication identification requests (drug ID) decreased 26.8%, and human exposures reported to US PCs decreased 3.8%. Human exposures with less serious outcomes have decreased 3.7% per year since 2008 while those with more serious outcomes (moderate, major or death) have increased by 4.7% per year since 2000.
The top five substance classes most frequently involved in all human exposures were analgesics (11.5%), cosmetics/personal care products (7.7%), household cleaning substances (7.6%), sedatives/hypnotics/antipsychotics (5.9%), and antidepressants (4.2%). Sedative/hypnotics/antipsychotics exposures as a class increased most rapidly (2,559 calls/year) over the last 13 years for cases showing more serious outcomes. The top five most common exposures in children of 5 years or less were cosmetics/personal care products (13.8%), household cleaning substances (10.4%), analgesics (9.8%), foreign bodies/toys/miscellaneous (6.9%), and topical preparations (6.1%). Drug identification requests comprised 50.7% of all information calls. NPDS documented 2,477 human exposures resulting in death with 2,113 human fatalities judged related (RCF of 1, undoubtedly responsible; 2, probably responsible; or 3, contributory).
Conclusions: These data support the continued value of PC expertise and need for specialized medical toxicology information to manage the more severe exposures, despite a decrease in calls involving less severe exposures. Unintentional and intentional exposures continue to be a significant cause of morbidity and mortality in the United States. The near real-time, always current status of NPDS represents a national public health resource to collect and monitor US exposure cases and information calls. The continuing mission of NPDS is to provide a nationwide infrastructure for public health surveillance for all types of exposures, public health event identification, resilience response and situational awareness tracking. NPDS is a model system for the nation and global public health.
WARNING: Comparison of exposure or outcome data from previous AAPCC Annual Reports is problematic. In particular, the identification of fatalities (attribution of a death to the exposure) differed from pre-2006 Annual Reports (see Fatality Case Review—Methods). Poison center death cases are described as all cases resulting in death and those determined to be exposure-related fatalities. Likewise, (Exposure Cases by Generic Category) since year 2006 restricts the breakdown including deaths to single-substance cases to improve precision and avoid misinterpretation.
Introduction
This is the 31st Annual Report of the American Association of Poison Control Centers’ (AAPCC; http://www.aapcc.org) National Poison Data System (NPDS).(1) On 1 January 2013, fifty-seven regional poison centers (PCs) serving the entire population of the 50 United States, American Samoa, District of Columbia, Federated States of Micronesia, Guam, Puerto Rico, and the US Virgin Islands submitted information and exposure case data collected during the course of providing telephonic patient-tailored exposure management and poison information.
NPDS is the data warehouse for the nation's 57 PCs. PCs place emphasis on exposure management, accurate data collection and coding, and responding to the continuing need for poison related public and professional education. The PC's health care professionals are available free of charge to users, 24-hours a day, every day of the year. PCs respond to questions from the public, health care professionals, and public health agencies. The continuous staff dedication at the PCs is manifest as the number of exposure, and information call encounters exceeds 3.0 million annually. PC encounters involve either an exposed human or animal (exposure call) or a request for information with no person or animal exposed to any foreign body, viral, bacterial, venomous, or chemical agent or commercial product (information call).
The NPDS Products Database
The NPDS products database contains over 400,000 products ranging from viral and bacterial agents to commercial chemical and drug products. The product database is maintained and continuously updated by data analysts at the Micromedex Poisindex®System (Micromedex Healthcare Series [Internet database]; Greenwood Village, CO: Truven Health Analytics). A robust generic coding system categorizes the products data into 1,081 generic codes. These generic codes collapse into Nonpharmaceutical (562) and Pharmaceutical (519) groups. These two groups are divided into Major (68) and Minor (172) categories. The generic coding schema undergoes continuous improvement through the work of the AAPCC—Micromedex Joint Coding Group. The group consists of AAPCC members and editorial and lexicon staff working to meet best terminology practices. The generic code system provides enhanced report granularity as reflected in . The following 30 generic codes were introduced in 2013:
Table: Generic Codes Added in 2013.
Because the new codes were added at different times during the year, the numbers in for these generic codes do not reflect the entire year. For completeness, certain codes of these categories require customized data retrieval until these categories have been in place for a year or more.
Methods
Characterization of Participating PCs and Population Served
Fifty-seven participating centers submitted data to AAPCC through 30 September, 2013, when one participating center closed with its calls picked up by another PC in its state, leaving 56 participating centers as of 31 December 2013.Fifty-four centers (95%) were accredited by AAPCC as of 1 July 2013. The entire population of the 50 states, American Samoa, the District of Columbia, Federated States of Micronesia, Guam, Puerto Rico, and the US Virgin Islands was served by the US PC network in 2013.(2,3,4,5).
The average number of human exposure cases managed per day by all US PCs was 5,995. Similar to other years, higher volumes were observed in the warmer months, with a mean of 6,365 cases per day in July compared with 5,424 per day in December. On average, US PCs received a call about an actual human exposure every 14.4 seconds.
Call Management—Specialized Poison Exposure Emergency Providers
Most PC operation management, clinical education, and instruction are directed by managing directors (most are PharmDs and RNs with American Board of Applied Toxicology [ABAT] board certification). Medical direction is provided by medical directors who are board-certified physician medical toxicologists. At some PCs, the managing and medical director positions are held by the same person.
Calls received at US PCs are managed by health care professionals who have received specialized training in toxicology and managing exposure emergencies. These providers include medical and clinical toxicologists, registered nurses, doctors of pharmacy, pharmacists, chemists, hazardous materials specialists, and epidemiologists. Specialists in Poison Information (SPIs) are primarily registered nurses, PharmDs, and pharmacists who direct the public to the most appropriate level of care while also providing the most up-to-date management recommendations to health care providers caring for exposed patients. They may work under the supervision of a Certified Specialist in Poison Information (CSPI). SPIs must log a minimum of 2,000 calls over a 12-month period to become eligible to take the CSPI examination for certification in poison information. Poison information providers (PIPs) are allied health care professionals. They manage information-type and low acuity (non-hospital) calls and work under the supervision of a CSPI. Of note is the fact that no nursing or pharmacy school offers a toxicology curriculum designed for PC work and SPIs must be trained in programs offered by their respective PC. PCs undergo a rigorous accreditation process administered by the AAPCC and must be reaccredited every 5 years.
NPDS—Near Real-time Data Capture
Launched on 12 April 2006, NPDS is the data repository for all of the US PCs. In 2013, all 57 US PCs uploaded case data automatically to NPDS. All PCs submitted data in near real-time, making NPDS one of the few operational systems of its kind. PC staff record calls contemporaneously in 1 of 4 case data management systems. Each PC uploads case data automatically. The time to upload data for all PCs is 8.08 [7.10, 11.63] (median [25%, 75%]) minutes creating a near real-time national exposure database and surveillance system.
The web-based NPDS software facilitates detection, analysis, and reporting of NPDS surveillance anomalies. System software offers a myriad of surveillance uses allowing AAPCC, its member centers, and public health agencies to utilize NPDS US exposure data. Users are able to access local and regional data for their own areas and view national aggregate data. Custom surveillance definitions are available along with ad hoc reporting tools. Information in the NPDS database is dynamic. Each year the database is locked prior to extraction of annual report data to prevent inadvertent changes and ensure consistent, reproducible reports. The 2013 database was locked on 27 October 2014 at 17:00 EDT.
Annual Report Case Inclusion Criteria
The information in this report reflects only those cases that are not duplicates and classified by the PC as CLOSED. A case is closed when the PC has determined that no further follow-up/recommendations are required or no further information is available. Exposure cases are followed to obtain the most precise medical outcome possible. Depending on the case specifics, most calls are “closed” within a few hours of the initial call. Some calls regarding complex hospitalized patients or cases resulting in death may remain open for weeks or months while data continue to be collected. Follow-up calls provide a proven mechanism for monitoring the appropriateness of management recommendations, augmenting patient guidelines and providing poison prevention education, enabling continual updates of case information as well as obtaining final/known medical outcome status to make the data collected as accurate and complete as possible.
Statistical Methods
All tables except and were generated directly by the NPDS web-based application and can thus be reproduced by each center. The figures and statistics in and were created using SAS JMP version 9.0.0 (SAS Institute, Cary, NC) on summary counts generated by the NPDS web-based application.
NPDS Surveillance
As previously noted, all of the active US PCs upload case data automatically to NPDS. This unique near real-time upload is the foundation of the NPDS surveillance system. This makes possible both spatial and temporal case volume and case based surveillance. NPDS software allows creation of volume and case-based definitions. Definitions can be applied to national, regional, state, or ZIP code coverage areas. Geocentric definitions can also be created. This functionality is available not only to the AAPCC surveillance team, but to every PC. PCs also have the ability to share NPDS real-time surveillance technology with external organizations such as their state and local health departments or other regulatory agencies. Another NPDS feature is the ability to generate system alerts on adverse drug events and other drug or commercial products of public health interest like contaminated food or product recalls. Thus, NPDS can provide real-time adverse event monitoring and surveillance of resilience response and situational awareness.
Surveillance definitions can be created to monitor a variety of volume parameters or case-based definitions on any desired substance or commercial product in the Micromedex Poisindex products database and/or set of clinical effects or other parameters. The products database contains over 400,000 entries. Surveillance definitions may be constructed using volume or case-based definitions with a variety of mathematical options and historical baseline periods from 1 to 13 years. NPDS surveillance tools include the following:
Volume Alert Surveillance Definitions
Total Call Volume
Human Exposure Call Volume
Animal Exposure Call Volume
Information Call Volume
Clinical Effects Volume (signs and symptoms, or laboratory abnormalities)
Case-Based Surveillance Definitions utilizing various NPDS data fields linked in Boolean expressions
Substance
Clinical Effects
Species
Medical Outcome and Others
Syndromic Surveillance Definitions allow Boolean-based definitions utilizing various NPDS data fields to be run based on historical trends for user-defined periods of interest.
Incoming data are monitored continuously and anomalous signals generate an automated email alert to the AAPCC's surveillance team or designated PC or public health agency staff. These anomaly alerts are reviewed daily by the AAPCC surveillance team, the PC, or the public health agency that created the surveillance definition. When reports of potential public health significance are detected, additional information is obtained via the NPDS surveillance correspondence system or phone as appropriate from reporting PCs. The PC then alerts their respective state or local health departments. Public health issues are brought to the attention of the Health Studies Branch, National Center for Environmental Health, Centers for Disease Control and Prevention (HSB/NCEH/CDC). This unique near real-time tracking ability is a unique feature offered by NPDS and the PCs.
Clinical and medical toxicologists of the AAPCC surveillance team review surveillance definitions on a regular basis to fine-tune the queries. CDC, as well as State and local health departments with NPDS access as granted by their respective PCs, also have the ability to create surveillance definitions for routine surveillance tasks or to respond to emerging public health events.
Fatality Case Review and Abstract Selection
NPDS fatality cases can be recorded as DEATH or DEATH (INDIRECT REPORT). Medical outcome of death is given by direct report. Deaths (indirect reports) are deaths that the PC acquired from medical examiners or media, but did not manage nor answer any questions related specifically to that death.
Although PCs may report death as an outcome, the death may not be the direct result of the exposure. We define exposure-related fatality as a death judged by the AAPCC Fatality Review Team to be at least contributory to the exposure. The definitions used for the Relative Contribution to Fatality (RCF) classification are given in Appendix B and the methods for selecting abstracts for publications are described in Appendix C. For details on the AAPCC fatality review process, see the 2008 annual report.(1)
Pediatric Fatality Case Review
A focused Pediatric Fatality Review team, comprised of 4 pediatric toxicologists, evaluated cases of patients of 19 years and under. The panel reviewed the documentation of all such cases, with specific focus on the conditions behind the poisoning exposure and on finding commonality which might inform efforts at prevention. The pediatric fatality cases reviewed exhibited a bimodal age distribution. Exposures causing death in children ≤ 5 years of age were mostly coded as “Unintentional-General”, while those in ages over 12 years were mostly as “Intentional”. Often the Reason Code did not capture the complexities of the case. For example, there were few mentions of details such as the involvement of law enforcement or child protective services. While there were some complete and informative reports, in many narratives the circumstances which preceded the exposure thought responsible for the death were unclear or absent. In response to these findings, the pediatric fatality review team developed and distributed Pediatric Narrative Guidelines, with specific attention to the root cause of these cases. PCs are requested to heed these guidelines and the need for a more in-depth investigation of “causality.”
Results
Information Calls to Poison Centers
Data from 806,347 information calls to PCs in 2013 () was transmitted to NPDS, including calls in optional reporting categories such as prevention/safety/education (24,249), administrative (25,878), and caller referral (47,682).
shows that all drug ID calls decreased dramatically in mid-2009, again in late 2010 and late 2011, and continue to decrease in 2012 and 2013. Law enforcement drug ID calls also showed a decline. The most frequent information call was for drug ID, comprising 408,711calls to PCs during the year. Of these, 239,364 (58.6%) were identified as drugs with known abuse potential; however, these cases were categorized based on the drug's abuse potential without any knowledge of whether abuse was actually intended.
While the number of drug information calls decreased 21.4% from 2012 (144,267 calls) to 2013 (113,378 calls), the distribution of these call types remained steady at 14.1% of all information request calls. The most common drug information requests were about drug–drug interactions, followed by other drug information, therapeutic use and indications, questions about dosage, and inquiries of adverse effects. Environmental inquiries comprised 2.3% of all information calls. Of these environmental inquiries, specific questions related to cleanup of mercury (thermometers and other) remained the most common followed by questions involving pesticides.
Of all the information calls, poison information comprised 7.0% of the requests with inquiries involving general toxicity the most common followed by questions involving food preparation practices, safe use of household products, and plant toxicity.
Exposure Calls to Poison Centers
In 2013, the participating PCs logged 3,060,122 total encounters including 2,188,013 closed human exposure cases (), 59,496 animal exposures (), 806,347 information calls (), 6,116 human confirmed non-exposures, and 150 animal confirmed non-exposures. An additional 570 calls were still open at the time of database lock. The cumulative AAPCC database now contains more than 60 million human exposure case records (). A total of 16,392,826 information calls have been logged by NPDS since the year 2000.
Table 1A. AAPCC Population Served and Reported Exposures (1983–2013).
Table 1B. Non-Human Exposures by Animal Type.
Table 1C. Distribution of Information Calls.
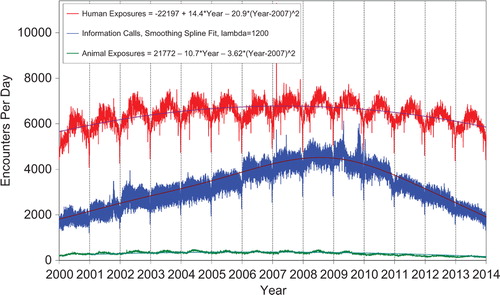
shows the human exposures, information calls and animal exposures by day since 1 January 2001. Second-order (quadratic) least squares regression of these data shows a statistically significant departure from linearity (declining rate of calls since mid-2007) for human exposure calls. Information calls are best described by a smoothing spline fit, and animal exposure calls have likewise been declining since mid-2005.
Figure 1. Human Exposure Calls, Information Calls and Animal Exposure Calls by Day since January 1, 2000. Both linear and second-order (quadratic) terms were statistically significant for least-squares second-order regressions of Human Exposures and Animal Exposures. Smoothing spline fit for Information calls has lambda = 1200, R-square = 0.832 (colour version of this figure can be found in the online version at www.informahealthcare.com/ctx).
A hallmark of PC case management is the use of follow-up calls to monitor case progress and medical outcome. US PCs made 2,515,811 follow-up calls in 2013. Follow-up calls were made in 46.1% of human exposure cases. One follow-up call was made in 22.0% of human exposure cases, and multiple follow-up calls (range, 2–121) were placed in 24.1% of cases.
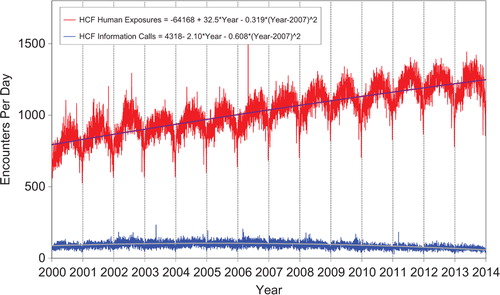
shows a graphic summary and analyses of Health Care Facility (HCF) exposure and HCF information calls. HCF exposure calls slightly departed from linearity but continued to increase at a steady rate, while the rate of HCF information calls has been declining since early 2005. This increasing use of the PCs for the more serious exposures (HCF calls) is important in the face of the decline in exposure and information calls. The 2 May 2006 exposure data spike on the figure was the result of 602 children in a Midwest school reporting a noxious odor which caused anxiety, but resolved without sequelae.
Figure 2. All Drug Identification and Law Enforcement Drug Identification Calls by day since January 1, 2000. Smoothing Spline Fits were better than second-order regressions, R-square = 0.933 for All Drug Identification Calls, R-square = 0.780 for Law Enforcement Drug ID Calls (colour version of this figure can be found in the online version at www.informahealthcare.com/ctx).
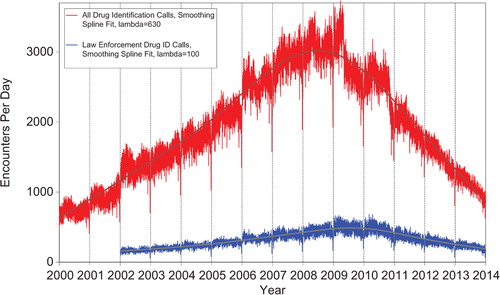
Figure 3. Health Care Facility (HCF) Exposure Calls and HCF Information Calls by day since January 1, 2000. Regression lines show least-squares second-order regressions for HCF Exposure and HCF Information Calls. All terms shown were statistically significant for each of the two regressions (colour version of this figure can be found in the online version at www.informahealthcare.com/ctx).
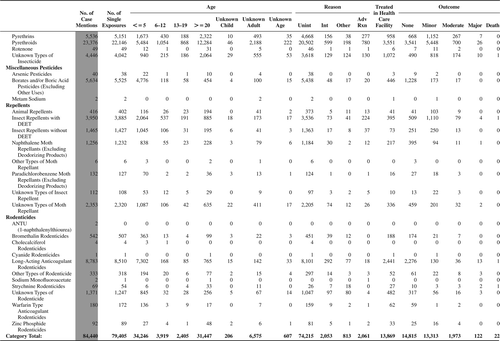
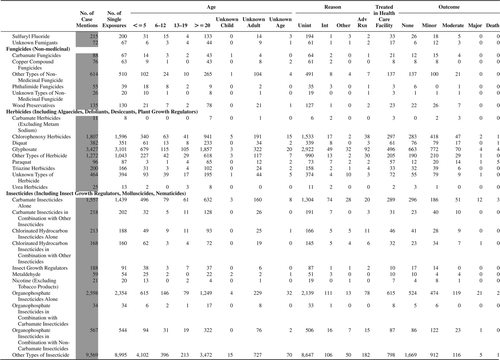
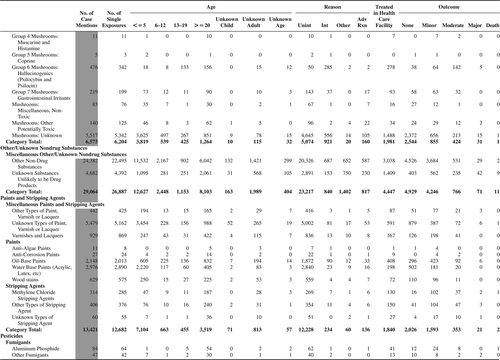
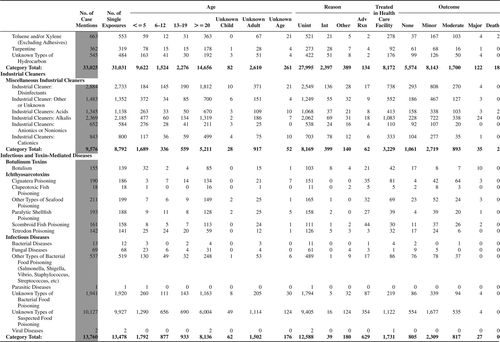
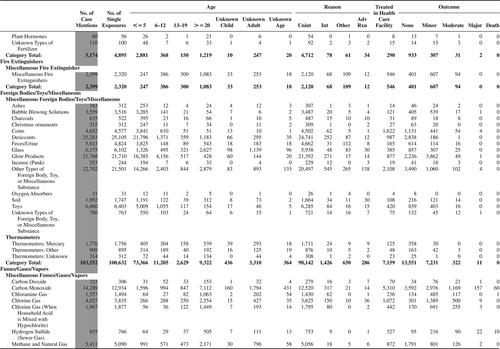
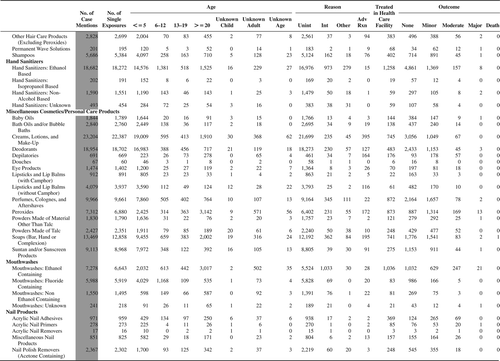
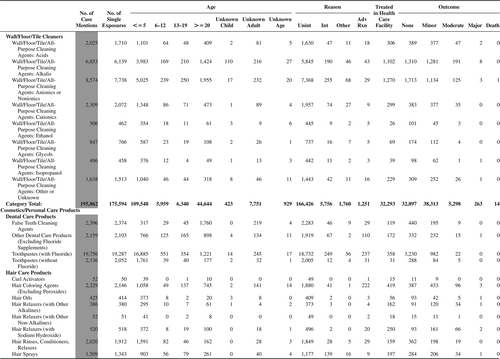
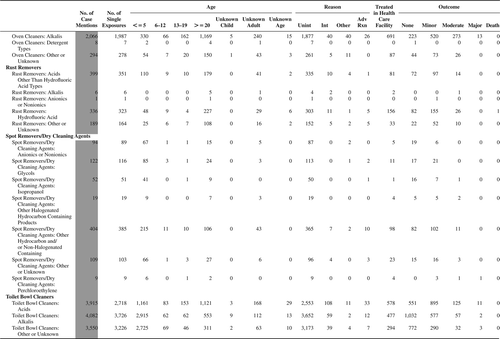
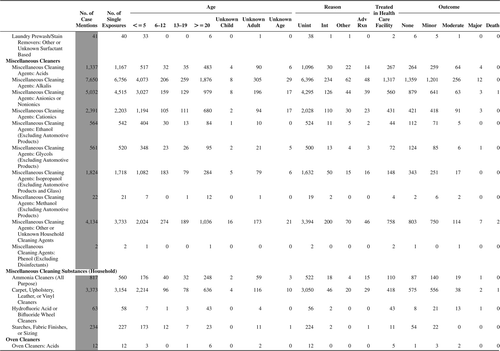
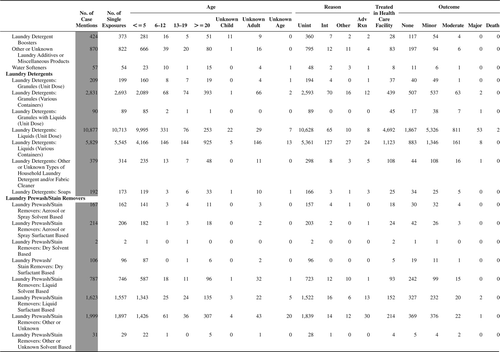
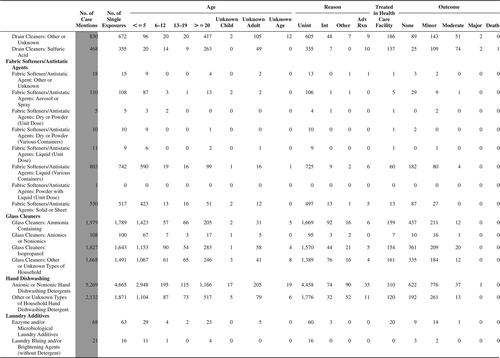
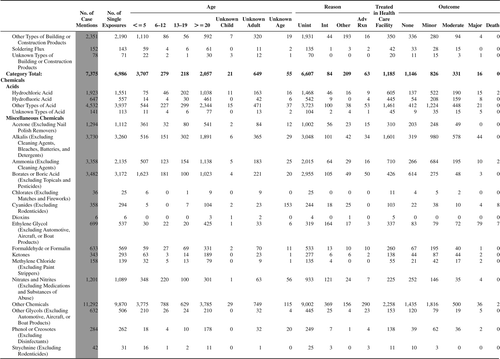
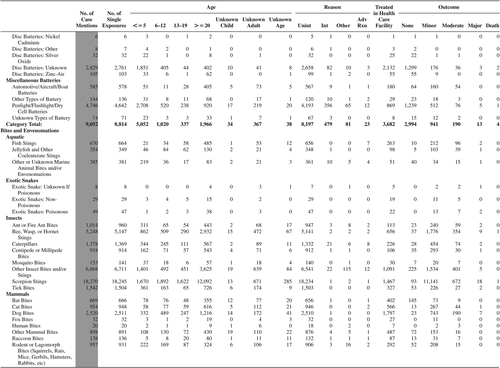
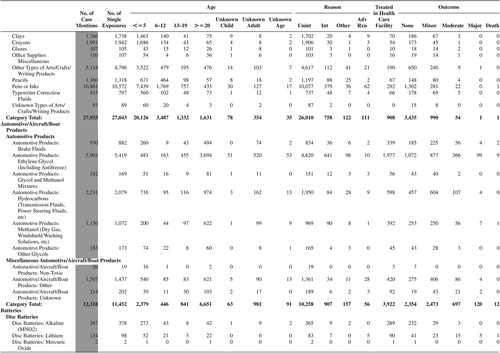
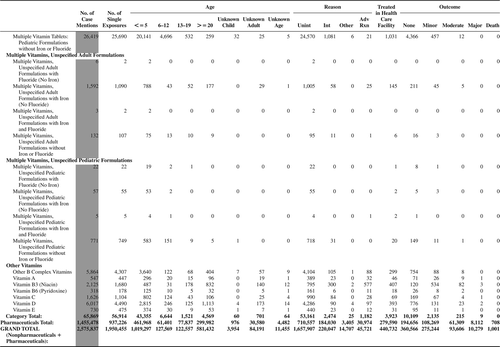
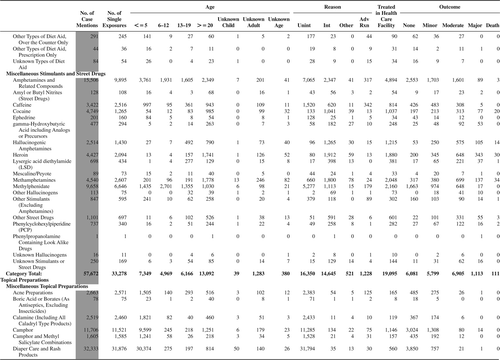
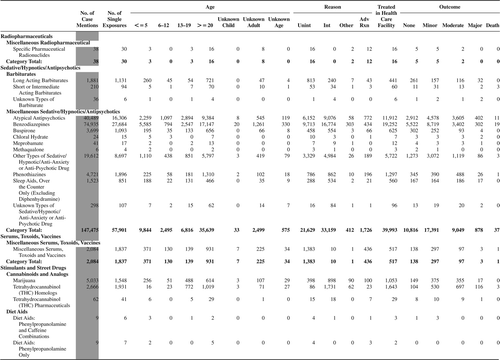
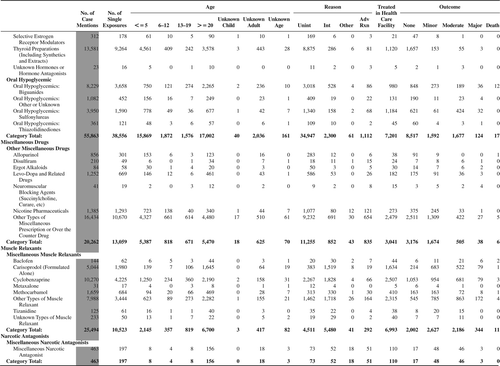
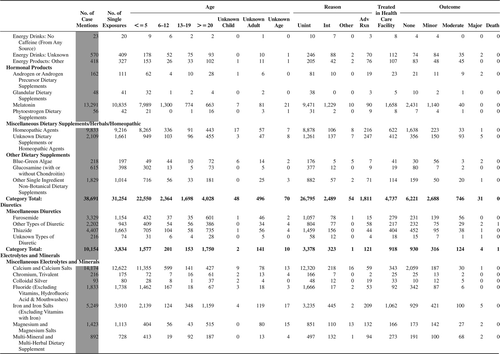
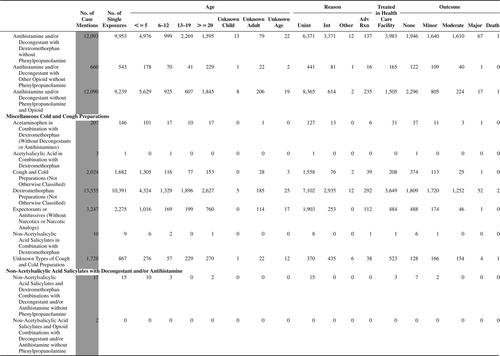
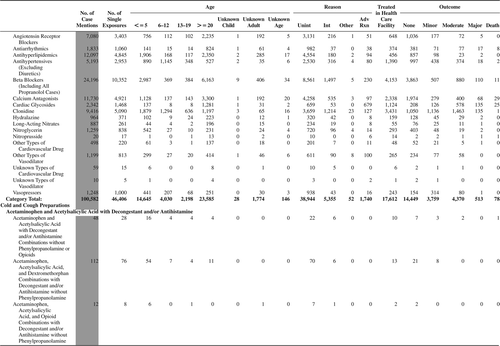
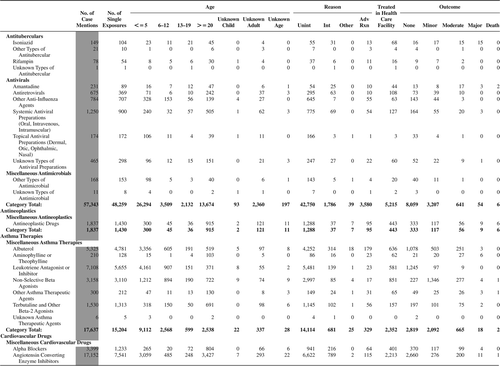
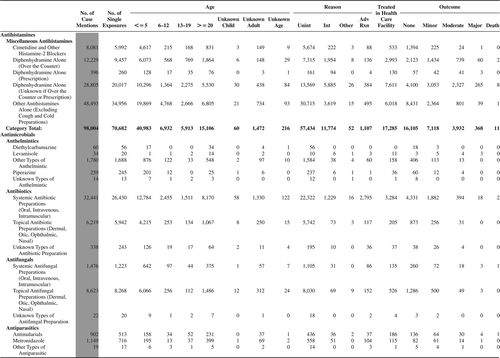
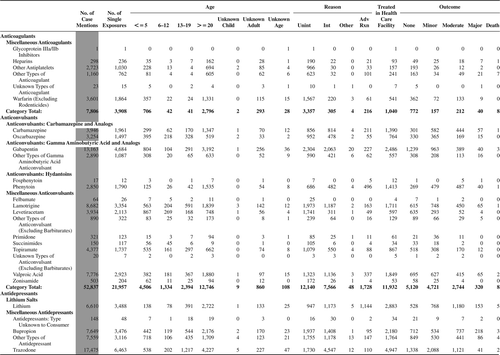
(Nonpharmaceuticals) and (Pharmaceuticals) provide summary demographic data on patient age, reason for exposure, medical outcome, and use of a health care facility for all 2,188,013 human exposure cases, presented by substance categories. The Pharmaceuticals category includes both licit and illicit drugs.
Column 1: Name of the major, minor generic categories and their associated generic codes.
Column 2: Number of Case Mentions (All Exposures) in grey shading, displays the number of times the specific generic code was reported in all human exposure cases. If a human exposure case has multiple instances of a specific generic code, it is counted only once.
Column 3: Single Substance Exposures; this column was previously named “No. of Single Exposures” and was renamed in the 2009 report for clarity. This column displays the number of human exposure cases that identified only one substance (one case, one substance).
The succeeding columns (Age, Reason, Treatment Site, And Outcome) show selected detail from these single-substance exposure cases. Death cases include both cases that have the outcomes of Death or Death (indirect report).These death cases are not limited by the relative contribution to fatality.
and restrict the breakdown columns to single-substance cases. Prior to 2007, when multisubstance exposures were included, a relatively innocuous substance could be mentioned in a death column when, for example, the death was attributed to an antidepressant, opioid, or cyanide. This subtlety was not always appreciated by the user of this table. The restriction of the breakdowns to single-substance exposures should increase precision and reduce misrepresentation of the results in this unique by-substance table. Single-substance cases reflect the majority (89.1%) of all exposures. In contrast, only 44.2% of fatalities are single substance exposures ().
and tabulate 2,575,837 substance exposures, of which 1,950,455 were single-substance exposures, including1,013,229 (52.0%) nonpharmaceuticals and 937,226 (48.0%) pharmaceuticals. In 19.6% of single- substance exposures that involved pharmaceutical substances, the reason for exposure was intentional, compared with only 3.6% that involved a nonpharmaceutical substance. Correspondingly, treatment in a health care facility was provided in a higher percentage of exposures that involved pharmaceutical substances (29.8%) compared with that of nonpharmaceutical substances (15.9%). Exposures to pharmaceuticals also had more severe outcomes. Of single-substance exposure-related fatal cases, 708 (70.7%) were pharmaceuticals compared with 293 (29.3%) nonpharmaceutical.
Age and Gender Distributions
The age and gender distribution of human exposures is outlined in . Children younger than 3 years were involved in 35.5% of exposures and children younger than 6 years accounted for approximately half of all human exposures (48.0%). Male predominance was found among cases involving children younger than 13 years, but this gender distribution was reversed in teenagers and adults, with females comprising the majority of reported exposures.
Caller Site and Exposure Site
As shown in , of the 2,188,013 human exposures reported, 71.8% of calls originated from a residence (own or other) but 93.5% actually occurred at a residence (own or other). Another 20.3% of calls were made from a HCF. Beyond residences, exposures occurred in the workplace in 1.6% of cases, schools (1.3%), health care facilities (0.3%), and restaurants or food services (0.2%).
Table 2. Site of Call and Site of Exposure, Human Exposure Cases.
Table 3A. Age and Gender Distribution of Human Exposures.
Table 3B. Population-Adjusted Exposures by Age Group.
Exposures in Pregnancy
Exposure during pregnancy occurred in 7,384 women (0.3% of all human exposures). Of those with known pregnancy duration (n = 6,830), 31.5% occurred in the first trimester, 37.0% in the second trimester, and 31.5% in the third trimester. Most (73.9%) were unintentional exposures and 19.6% were intentional exposures. There was one death of a pregnant woman in 2013.
Chronicity
Most human exposures, 1,922,316 (87.9%), were acute cases (single, repeated, or continuous exposure occurring over 8 hours or less) compared with 1,328 acute cases of 2,477 fatalities (53.6%). Chronic exposures (continuous or repeated exposures occurring over > 8 hours) comprised 2.1% (46,900) of all human exposures. Acute-on-chronic exposures (single exposure that was preceded by a continuous, repeated, or intermittent exposure occurring over a period of > 8 hours) numbered 188,899 (8.6%).
Reason for Exposure
The reason for most human exposures was unintentional (79.9%) with unintentional general (54.2%), therapeutic error (12.5%), and unintentional misuse (5.6%) of all exposures ().
Table 4. Distribution of Agea and Gender for Fatalitiesb.
Table 5. Number of Substances Involved in Human Exposure Cases.
Table 6A. Reason for Human Exposure Cases.
Scenarios
Of the total 289,699 therapeutic errors, the most common scenarios for all ages included: inadvertent double dosing (28.2%), wrong medication taken or given (16.2%), other incorrect dose (13.6%), doses given/taken too close together (10.3%), and inadvertent exposure to someone else's medication (8.0%). The types of therapeutic errors observed are different for each age group and are summarized in .
Table 6B. Scenarios for Therapeutic Errorsa by Ageb.
Reason by Age
Intentional exposures accounted for 16.2% of human exposures. Suicidal intent was suspected in 10.5% of cases, intentional misuse in 2.5%, and intentional abuse in 2.2%. Unintentional exposures outnumbered intentional exposures in all age groups with the exception of ages 13–19 years (). Intentional exposures were more frequently reported than unintentional exposures in patients aged 13–19 years. In contrast, of the 1,218 reported fatalities with RCF 1–3, the major reason reported for children ≤ 5 years was unintentional while most fatalities in adults (> 20 years) were intentional ().
Table 7. Distribution of Reason for Exposure by Age.
Table 8. Distribution of Reason for Exposure and Age for Fatalitiesa.
Route of Exposure
Ingestion was the route of exposure in 83.4% of cases (), followed in frequency by dermal (7.0%), inhalation/nasal (6.1%), and ocular routes (4.3%). For the 1,218 exposure-related fatalities, ingestion (80.9%), inhalation/nasal (10.2%), unknown (8.9%), and parenteral (5.1%) were the predominant exposure routes. Each exposure case may have more than one route.
Table 9. Route of Exposure for Human Exposure Cases.
Clinical Effects
The NPDS database allows for the coding of up to 131 individual clinical effects (signs, symptoms, or laboratory abnormalities) for each case. Each clinical effect can be further defined as related, not related, or unknown if related. Clinical effects were coded in 810,259 (37.0%) cases (17.8% had 1 effect, 9.5% had 2 effects, 5.1% had 3 effects, 2.2% had 4 effects, 1.0% had 5 effects, and 1.4% had > 5 effects coded). Of the clinical effects coded, 77.8% were deemed related to the exposure, 9.9% were considered not related, and 12.3% were coded as unknown if related.
Case Management Site
The majority of cases reported to PCs were managed in a non-HCF (68.7%), usually at the site of exposure, primarily the patient's own residence (); 1.5% of cases were referred to a HCF but they refused referral. Treatment in a HCF was rendered in 27.5% of cases.
Table 10. Management Site of Human Exposures.
Of the 601,642 cases managed in a HCF, 286,690(47.7%) were treated and released, 99,117(16.5%) were admitted for critical care, and 67,114(11.2%) were admitted to a noncritical unit.
The percentage of patients treated in a HCF varied considerably with age. Only 11.8% of children ≤ 5 years and only 14.7% of children between 6 and 12 years were managed in a HCF compared with 54.1% of teenagers (13–19 years) and 41.7% of adults (age, ≥ 20 years).
Medical Outcome
displays the medical outcome of human exposure cases distributed by age. Older age groups exhibit a greater number of severe medical outcomes. compares medical outcome and reason for exposure, and shows a greater frequency of serious outcomes in intentional exposures.
Table 11. Medical Outcome of Human Exposure Cases by Patient Agea.
Table 12. Medical Outcome by Reason for Exposure in Human Exposuresa.
The duration of effect is required for all cases which report at least one clinical effect and have a medical outcome of minor, moderate, or major effect (n = 503,501; 23.0% of exposures). demonstrates an increasing duration of the clinical effects observed with more severe outcomes.
Table 13. Duration of Clinical Effects by Medical Outcome.
Decontamination Procedures and Specific Antidotes
and outline the use of decontamination procedures, specific physiological antagonists (antidotes), and measures to enhance elimination in the treatment of patients reported in the NPDS database. These should be interpreted as minimum frequencies because of the limitations of telephone data gathering.
Table 14. Decontamination and Therapeutic Interventions.
Table 15. Therapy Provided in Human Exposures by Age.
Ipecac-induced emesis for poisoning continues to decline as shown in and . Ipecac was administered in only 42 (0.0%) of pediatric exposures in 2013. The continued decrease in ipecac syrup use over the last 2 decades is likely a result of ipecac use guidelines issued in 1997 by the American Academy of Clinical Toxicology and the European Association of Poisons Centres and Clinical Toxicologists and updated in 2004.(6,7) In a separate report, the American Academy of Pediatrics not only concluded that ipecac should no longer be used routinely as a home treatment strategy, but also recommended disposal of home ipecac stocks.(8) A decline was also observed since the early 1990s for reported use of activated charcoal. While not as dramatic as the decline in use of ipecac, reported use of activated charcoal decreased from 3.7% of pediatric cases in 1993 to just 0.9% in 2013.
Table 16A. Decontamination Trends (1985–2013).
Table 16B. Decontamination Trends: Total Human and Pediatric Exposures < = 5 Yearsa.
Top Substances in Human Exposures
presents the most common 25 substance categories, listed by frequency of human exposure for cases with more serious outcomes (moderate, severe, and death). This ranking provides an indication where prevention efforts might be focused, as well as the types of serious exposures PCs regularly manage. It is relevant to know whether exposures to these substances are increasing or decreasing.
Table 17A. Substance Categories Most Frequently Involved in Human Exposures (Top 25).
To better understand these relationships, we examined exposures with more serious outcomes per year over the last 13 years for the change over time for each of the 68 major generic categories via least-square linear regression. The serious outcome exposure calls per year over this period were increasing for 39 and decreasing for 29, respectively, of the 68 categories. The change over time for the 13 yearly values was statistically significant (p < 0.05) for 45 of the 68 categories. shows the 25 categories which were increasing most rapidly. Statistical significance of the linear regressions can be verified by noting the 95% confidence interval on the rate of increase excluding 0 for all, but 3 of the 25 categories. shows the linear regressions for the top 4 increasing categories in .
Table 17B. Substance Categories with the Greatest Rate of More Serious Exposure Increase (Top 25).
and present exposure results for children and adults, respectively, and show the differences between substance categories involved in pediatric and adult exposures.
Table 17C. Substance Categories Most Frequently Involved in Pediatric (≤ 5 years) Exposures (Top 25)a.
Table 17D. Substance Categories Most Frequently Involved in Adult (≥ 20 years) Exposures (Top 25)a.
reports the 25 categories of substances most frequently involved in pediatric (≤ 5 years) fatalities in 2013.
Table 17E. Substance Categories Most Frequently Involved in Pediatric (≤ 5 years) Deathsa.
reports the 25 drug ID categories most frequently queried in 2013, highlighting the value of drug ID information to the AAPCC, public health, public safety, and regulatory agencies. Internet-based resources do not afford the caller the option to speak with a health care professional if needed. Proper resources to continue this vital public service are essential, especially since the top 10 substance categories include antibiotics as well as drugs with widespread use and abuse potential such as opioids and benzodiazepines.
Table 17F. Substance Categories Most Frequently Identified in Drug Identification Calls (Top 25).
reports the 25 substance categories most frequently reported in exposures involving pregnant patients.
Table 17G. Substance Categories Most Frequently Involved in Pregnant Exposuresa (Top 25).
Changes Over Time
Total encounters peaked in 2008 at 4,333,012 calls with 2,491,049 human exposure calls and 1,703,762 information calls. Total encounters decreased 9.3% from 3,373,025 in 2012 to 3,060,122 in 2013. Information calls decreased by 21.4% from 1,025,547 calls in 2012 to 806,347 in 2013, with a 26.8% decrease in drug identification calls and a 8.5 % decrease in HCF information calls. Human exposures decreased by 3.8% from 2,275,141 to 2,188,013 cases.
shows the year-to-year change since 2000 as a percentage of year 2000 for human exposure calls broken down into cases with more serious outcomes (death, major effect, and moderate effect) and less serious outcomes [minor effect, no effect, not followed (non-toxic), not followed (minimal toxicity possible), unable to follow (potentially toxic), and unrelated effect]. Since 2000, cases with more serious outcomes have increased by 4.5% [95% CI (4.0%, 4.9%)] per year from 108,148 cases in 2000 to 171,583 cases in 2013. However, cases with less serious outcomes have consistently decreased since 2008 by 3.7% [95% CI (−4.4%, −3.1%)] per year from 2,339,460 in 2008 to 2,015,505 cases in 2013. This decrease in less serious exposures has driven the overall decrease in human exposures since 2008.
Figure 4. Change in encounters by outcome from 2000. The figure shows the percent change from baseline for Human Exposure Calls divided among the 10 Medical Outcomes. The More Serious Exposures (Major, Moderate, and Death) increased. The Less Serious Exposures (no effect, minor effect, not followed (non-toxic), not followed (minimal toxicity possible), unable to follow (potentially toxic), and unrelated effect) decreased after 2008. Solid lines show least-squares linear regressions for the change in More Serious Exposures per year (□) and Less Serious Exposures (○). Broken lines show 95% confidence interval on the regression (colour version of this figure can be found in the online version at www.informahealthcare.com/ctx).
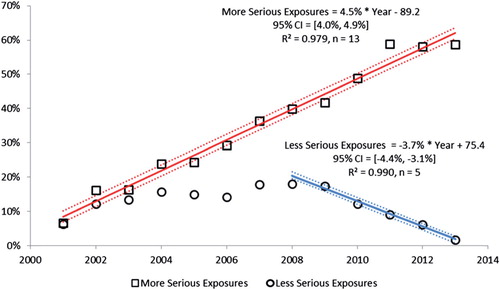
Likewise, we see a consistent increase in exposure calls from HCFs () and for the more severe exposures (), despite a decrease in calls involving less severe exposures.
Distribution of Suicides
shows the modest variation in the distribution of suicides and pediatric deaths over the past 2 decades as reported to the NPDS national database. Within the last decade, the percentage of exposures determined to be suspected suicides ranged from 30.3%% to 53.9%, and the percentage of pediatric cases has ranged from 1.5% to 3.2%.The relatively large change seen for 2011 and 2012 reflects the large increase in indirect death reports in those years. Analyses of suicides and pediatric deaths for direct and indirect reports are shown in .
Plant Exposures
provides the number of times the specific plant was reported to NPDS (n = 46,376). The 25 most commonly involved plant species and categories account for 39.7% of all plant exposures reported. The top 3 categories in the table are essentially synonymous for unknown plant and comprise 12.8% (5,955/46,376) of all plant exposures. For several reasons, it was not possible to make a precise identification in these three groups. The top most frequent plant exposures where a positive plant identification was made were the following (descending order): Phytolacca americana (L.) (Botanic name), Spathiphyllum species (Botanic name), Cherry (Species unspecified), Ilex species (Botanic name), Philodendron (Species unspecified), Caladium species (Botanic name of all species of the genus caladium) and Malus species (Botanic name)
Deaths and Exposure-related Fatalities
A list of cases () and summary of cases (, , , , , and ) are provided for fatal cases for which there exists reasonable confidence that the death was a result of that exposure (exposure-related fatalities). , , and list all deaths, irrespective of the RCF. Beginning in 2010, cases with outcome of Death, Indirect Report were not further reviewed by the AAPCC fatality review team, and the RCF was determined by the individual PC review team.
Table 18. Categories Associated with Largest Number of Fatalities (Top 25)a.
Table 19A. Comparisons of Death Data (1985–2013)a.
Table 19B. Comparisons of Direct and Indirect Death Data (2000–2013)a.
Table 20. Frequency of Plant Exposures (Top 25)a.
Table 21. Listing of Fatal Nonpharmaceutical and Pharmaceutical Exposures.
Table 22A. Demographic profile of SINGLE SUBSTANCE Nonpharmaceuticals exposure cases by generic category.
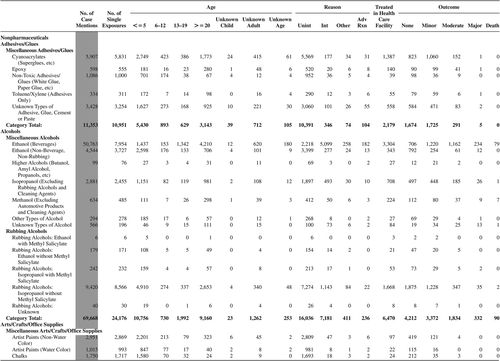
Table 22B. Demographic profile of SINGLE SUBSTANCE Pharmaceuticals exposure cases by generic category.
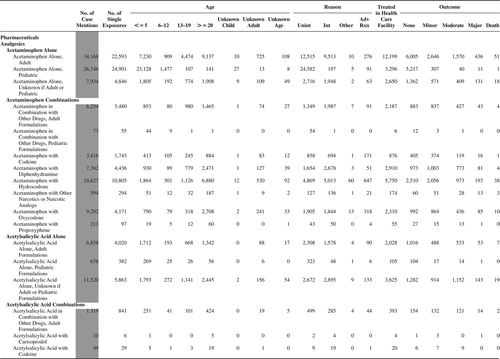
There were 925 deaths (indirect) and 1,552 deaths. Of these 2,477 cases, 2,113 were judged to be exposure-related fatalities (RCF = 1-Undoubtedly responsible, 2-Probably responsible, or 3-Contributory). The remaining 361 cases were judged as follows: 84 as RCF—probably not responsible; 34 as 5-clearly not responsible; and 246 as 6-unknown.
Deaths are sorted in according to the category, substance deemed most likely responsible for the death (Cause Rank), and then patient age. The Cause Rank permits the PC to judge 2 or more substances as indistinguishable in terms of cause, for example, 2 substances which appear equally likely to have caused the death could have Substance Rank of 1, 2 and Cause Rank of 1, 1. Additional agents implicated are listed below the primary agent in the order of their contribution to the fatality.
As shown in , a single substance was implicated in 89.1% of reported human exposures, and 10.9% of patients were exposed to 2 or more drugs or products. The exposure-related fatalities involved a single substance in 538 cases (44.2%), 2 substances in 295 cases (24.2%), 3 in 152 cases (12.5%), and 4 or more in the balance of the cases.
In , the Annual Report ID number [bracketed] indicates that the abstract for that case is included in Appendix C. The letters following the Annual Report ID number indicate: i = Death, Indirect report (occurred in 895, 42.4% of cases), p = prehospital cardiac and/or respiratory arrest (occurred in 462 of 2,113, 21.9% of cases), h = hospital records reviewed (occurred in 497, 23.5% of cases), and a = autopsy report reviewed (occurred in 1,230, 58.2% of cases). The distribution of NPDS RCF was as follows: 1 = Undoubtedly responsible in 572 cases (27.1%), 2 = Probably responsible in 1,344 cases (63.6%), and 3 = Contributory in 197 cases (9.3%). The denominator for these percentages is 2,113.
All fatalities—all ages
presents the age and gender distribution for these 1,218 exposure-related fatalities (excluding death, indirect). The age distribution of reported fatalities shows an increase in deaths in children (< 20 years old) compared with that of the past years, with 99 cases representing 8.1% of fatalities, an absolute increase of 26 child fatalities and a 35.6% increase in that age group. The age distribution of reported fatalities in adults (age, ≥ 20 years) is similar to that of prior years with 1,115 of 1,218 (91.5%) fatal cases occurring in that age group and 4 (0.3%) of fatalities occurring in unknown age patients. While children ≤ 5 years were involved in the majority of exposures, the 29 deaths in this group comprised just 2.4% of the exposure-related fatalities. However, it is noted that this represented a 38% increase in fatalities over 2012. While most (67.2%) of the fatalities occurred in 20- to 59-year-old individuals, the percentage is slightly decreased from prior years.
lists each of the 2,113 human fatalities (including death, indirect report) along with all of the substances involved for each case. Please note that the substance listed in column 3 of (alternate name) was chosen to be the most specific generic name based upon the Micromedex Poisindex product name and generic code selected for that substance. Alternate names are maintained in the NPDS for each substance involved in a fatality. The cross-references at the end of each major category section in list all cases that identify this substance as other than the primary substance. This alternate name may not agree with the AAPCC generic categories used in the summary tables (including ).
lists the top 25 minor generic substance categories associated with reported fatalities and the number of single substance exposure fatalities for that category—miscellaneous sedative/hypnotics/antipsychotics, miscellaneous cardiovascular drugs, opioids, and miscellaneous stimulants and street drugs lead this list followed by miscellaneous alcohols, acetaminophen combinations, acetaminophen alone, selective serotonin reuptake inhibitors, and miscellaneous fumes/gases/vapors. Note that is sorted by all substances to which a patient was exposed (i.e., a patient exposed to an opioid may have also been exposed to 1 or more other products) and shows single-substance exposures in the right-hand column.
The first-ranked substance () was a pharmaceutical in 1,710 (80.9%) of the 2,113 fatalities. These 1,710 first-ranked pharmaceuticals included:
690 analgesics (110 acetaminophen/hydrocodone, 109 methadone,106 acetaminophen, 98 oxycodone, 58 morphine, 34 salicylate, 26 fentanyl, 23 tramadol, and 20 opioid)
414 stimulants/street drugs [255 heroin, 56 methamphetamine, 52 cocaine, and 15 amphetamines (hallucinogenic)]
174 cardiovascular drugs (30 verapamil, 28 amlodipine, 18 cardiac glycoside, 15 diltiazem, 16 metoprolol, 11 carvedilol, and 11 propranolol)
133 antidepressants (34 amitriptyline, 20 bupropion, 14 venlafaxine, 10 doxepin, 10 citalopram, and 8 lithium)
100 sedative/hypnotic/antipsychotics (23 alprazolam, 20 quetiapine, 7 zolpidem, 6 benzodiazepine, and 5 diazepam)
The exposure was acute in 1,183 (56.0%), A/C = acute on chronic in 282 (13.3%), C = chronic exposure in 98 (4.6%), and U = unknown in 550 (26.0%).
A total of 1,204 tissue concentrations for 1 or more related analytes were reported in 582 cases. Most of these (1,197) involved fatalities with RCF = 1–3, and are listed in , while all tissue concentrations are available to the member centers through the NPDS Enterprise Reports. These 128 analytes included the following: 234 acetaminophen, 94 ethanol, 73 salicylate, 52 carboxyhemoglobin, 34 morphine, 27 alprazolam, 26 digoxin, 25 diphenhydramine, 25 oxycodone, 22 hydrocodone, 22 lithium, 22 methadone, 19 benzoylecgonine, and 19 morphine (free).
Route of exposure was as follows: ingestion only in 1,322 cases (62.6%), inhalation/nasal in 135 cases (6.4%) and parenteral in 78 cases (3.7%). Most other routes were combination routes or unknown.
The intentional exposure reason was: abuse in 863 cases (40.8%), suspected suicide in 691 cases (32.7%), and misuse in 48 cases (2.3%). Unintentional exposure reason was: environmental in 90 cases (4.3%), therapeutic error in 37 cases (1.8%), and misuse in 6 cases (0.3%). Adverse drug reaction was the reason in 47 (2.2%).
Pediatric fatalities—age ≤ 5 years
Although children younger than 6 years were involved in the majority of exposures, they comprised 51 of 2,477 (2.1%) of fatalities. These numbers are similar to those reported since 1985 (, all RCFs and includes indirect deaths). (RCF 1–3, excludes indirect deaths) shows the percentage fatalities in children ≤ 5 years related to total pediatric exposures was 29/1,049,475 = 0.00276%. By comparison, 1,115/833,563 = 0.13% of all adult exposures involved a fatality. Of these 29 pediatric fatalities, 24 (82.8%) were reported as unintentional and 3 (10.3%) were coded as resulting from malicious intent ().
The 33 fatalities in children ≤ 5 years in (includes death, indirect reports, and RCF 1–3) included 14 pharmaceuticals and 19 nonpharmaceuticals. The first-ranked substances associated with these fatalities included smoke (9), disc battery (2), hydromorphone (2), methadone (2), amitriptyline (2), and 16 other substances (1 each).
Pediatric fatalities—ages 6–12 years
In the age range 6–12 years, there were 6 reported fatalities, 4 of which were unintentional environmental, 1 was intentional suspected suicide, and 1 was intentional abuse (). The 11 fatalities listed in (includes death, indirect reports, and RCF 1–3) included 7 smoke, 2 carbon monoxide, 1 freon, and 1 methadone.
Adolescent fatalities—ages 13–19 years
In the age range of 13–19 years, there were 64 reported fatalities, an increase of 19 (42%) and included 57 intentional, 3 unintentional, 2 adverse reaction, and 2 unknown reason (). The 78 fatalities listed in (includes death, indirect reports and RCF 1–3) included 67 pharmaceuticals and 11 nonpharmaceuticals. The first-ranked pharmaceuticals associated with these fatalities included heroin (4), acetaminophen (3), methadone (3), oxycodone (3), drug, unknown (3), acetaminophen/hydrocodone (2), diphenhydramine (2), metformin (2), alprazolam (2), quetiapine (2), amphetamine (hallucinogenic), 2C-E (2), methamphetamine (2), methylenedioxymethamphetamine (MDMA) (2), THC homolog (2), 4-acetoxy-N,N-dimethyltryptamine (2), amphetamine (2), amphetamine (hallucinogenic) (2) and the remainder with1 substance each. The first ranked nonpharmaceutical associated with these fatalities included: cyanide (3), carbon monoxide (2),ethanol (1), methanol (1), freon (1), substance (non-drug) unknown (1), aldicarb (1), and dinitrophenol (1).
Pregnancy and Fatalities
A total of 31deaths of pregnant women have been reported from the years 2000 through 2013. The majority (27 of 31) were intentional exposures (misuse, abuse, or suspected suicide). There was 1 death in pregnant women reported to NPDS in 2013.
AAPCC Surveillance Results
A key component of the NPDS surveillance system is the variety of monitoring tools available to the NPDS user community. In addition to AAPCC national surveillance definitions, 35 PCs utilize NPDS as part of their surveillance programs. The Centers for Disease Control and Prevention (CDC), 6 state health departments and 1 state police department run surveillance definitions in NPDS. Since Surveillance Anomaly 1, generated at 2:00 pm EDT on 17 September 2006, over 230,000 anomalies have been detected. More than 1,500 were confirmed as being of public health significance with PCs working collaboratively with their local and state health departments and in some instances the CDC on the public health issues identified.
At the time of this report, 353 surveillance definitions run continuously, monitoring case and clinical effects volume and a variety of case-based definitions from food poisoning to nerve agents. These definitions represent the surveillance work by many PCs, state health departments, the AAPCC, and the Health Studies Branch, Division of Environmental Hazards and Health Effects, National Center for Environmental Health, Centers for Disease Control and Prevention (CDC).
Automated surveillance continues to remain controversial as a viable methodology to detect the index case of a public health event. Uniform evaluation algorithms are not available to determine the optimal methodologies.(9) Less controversial is the benefit to situational awareness that NPDS can provide.(10) Typical NPDS surveillance data detects a response to an event rather than an event prediction. This aids in situational awareness and resilience during and after a public health event.
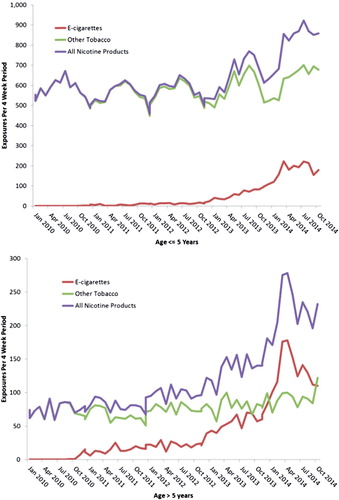
A current example of the involvement of the PC system and NPDS can be seen in the following. In January 2010, the AAPCC introduced two generic codes for electronic cigarettes (e-cigarettes): one for the e-cigarette delivery system and one for the liquid nicotine refills. As the amount of nicotine in e-cigarettes and their refills were not initially regulated by the Food and Drug Administration or any states, they could represent a unique poisoning hazard. As the refills were not required to be sold in child resistant containers, the potentially large amount of nicotine in these products (some containing over 100 mg/ml) could potentially produce serious toxicity in both adults and children, if inhaled, swallowed or spilled on the skin. And although flavored cigarettes have been banned by the FDA since September 2009, there were no restriction on e-cigarette flavorings. Flavors such as black cherry, café mocha, peanut butter cup, and ice cream potentially represent an additional attraction to children.
The first exposure to an e-cigarette product was noted in September 2010, with the first child exposure in November 2010. A gradual increase in the number of exposures occurred until the beginning of 2013 when a dramatic increase in the number of exposures to e-cigarettes and their refills was seen (). The total number of nonpharmaceutical nicotine exposures has increased, driven primarily by exposures to e-cigarette products. E-cigarette exposure calls peaked in April 2014 and comprised 35% of all nicotine-related single exposure calls. In children, e-cigarettes now account for roughly 25% of exposures, while in other age groups, e-cigarettes exposures have surpassed other tobacco products and account for as many as 65% of exposures. E-cigarette exposures in children under age 5 have serious outcomes in only 1.9% of cases compared with 5.3% in other ages. A decline in exposures has been seen since April 2014, possibly reflecting increased scrutiny on e-cigarettes and increased state and local regulation. Please note that the data for 2014 are considered preliminary since the 2014 database is not locked.
Figure 5. Substance Categories with the Greatest Rate of More Serious Exposure Increase (Top 4). Solid lines show least-squares linear regressions for More Serious Human Exposure Calls per year for that category (![]()
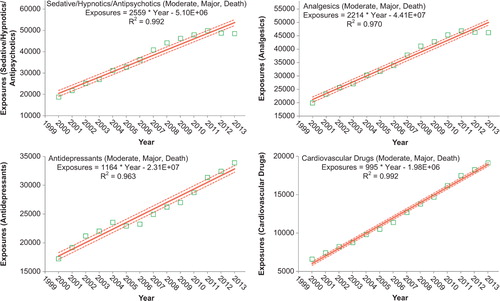
Figure 6. E-cigarette product exposures, January 2010–October 2014. The figures show the number of calls received per 4-week period by age group for single-substance human poison exposure calls to an e-cigarette device or refill (![]()
Discussion
The exposure cases and information requests reported by PCs in 2013 do not reflect the full extent of PC efforts which also include poison prevention activities and public and health care professional education programs.
NPDS exposure data may be considered as providing “numerator data”, in the absence of a true denominator; that is, we do not know the number of actual exposures that occur in the population. NPDS data include only those exposures which are reported to PCs.
NPDS 2000–2013 call volume data clearly demonstrate a continuing decrease in total exposure calls. This decline has been apparent and increasing since mid-2007, and reflects the decreasing use of the PC for less severe exposures. However, in contrast, during this same period, exposures with a more severe outcome (death, major, moderate) and HCF calls have continued a consistent increase. Possible contributors to the declining PC access include declining US birth rates (especially since exposure rates are much higher in children ≤ 5 years of age), increasing use of text rather than voice communication, and increased use of and reliance on internet search engines and web resources. To meet our public health goals, PCs will need to understand and meet the public's 21st-century communication preferences. We are concerned that failure to respond to these changes may result in a retro-shift with more people seeking medical care for exposures that could have been managed at home by a PC. Likewise, minor exposures may progress to more severe morbidity and mortality because of incorrect internet information or no PC management. The net effect could be more severe poisoning outcomes because fewer people took advantage of PC services, with a resultant increased burden on the national health care infrastructure as may be reflected in the increased number of cases managed in a health care facility this year.
NPDS statistical analyses indicate that all analgesic exposures including opioids and sedatives are increasing year over year. This trend is shown in and . NPDS data mirror CDC data that demonstrates similar findings.(10) Thus, NPDS provides a real-time view of these public health issues without the need for data source extrapolations.
One of the limitations of NPDS data has been the perceived lack of fatality case volume compared with that of other reporting sources. However, when change over time is studied, NPDS is clearly consistent with other public health fatality analyses. One of the issues leading to this concern is the fact that medical record systems seldom have common output streams. This is particularly apparent with the various electronic medical record systems available. It is important to build a federated approach similar to the one modeled by NPDS to allow data sharing, for example, between hospital emergency departments and other medical record systems including medical examiner offices nationwide. Enhancements to NPDS can promote interoperability between NPDS and electronic medical records systems to better trend poison-related morbidity and mortality in the United States and internationally.
Summary
Unintentional and intentional exposures continue to be a significant cause of morbidity and mortality in the United States. The near real-time, always current status of NPDS represents a national public health resource to collect and monitor US exposure cases and information calls.
Changes in encounters in 2013 shown in , , and include the following:
total encounters (all exposure and information calls) decreased by 9.3%;
all information calls decreased 21.4%, drug ID calls decreased 26.8%, and human exposures decreased 3.8%;
HCF information calls decreased 8.5% and HCF exposures decreased 0.1% notwithstanding an overall steady increase since 2000;
human exposures with less serious outcomes decreased 4.1%, while those with more serious outcomes (minor, moderate, major or death) increased 0.4% notwithstanding an overall 4.5% yearly increase since 2000;
The categories of substance exposures in cases with more serious outcomes increasing most rapidly are as follows: sedative/hypnotics/antipsychotics, followed by analgesics, antidepressants, and cardiovascular drugs.
These data support the continued value of PC expertise and the need for specialized medical toxicology information to manage the more severe exposures, despite a decrease in calls involving less severe exposures. PCs must consider newer communication approaches that match current public communication patterns in addition to the traditional telephone calls.
The continuing mission of NPDS is to provide a nationwide infrastructure for public health surveillance for all types of exposures, public health event identification, resilience response, and situational awareness tracking. NPDS is a model system for the nation and global public health.
Disclaimer
The American Association of Poison Control Centers (AAPCC; http://www.aapcc.org) maintains the national database of information logged by the country’s regional poison centers (PCs) serving all 50 United States, Puerto Rico, and the District of Columbia. Case records in this database are from self-reported calls: they reflect only information provided when the public or health care professionals report an actual or potential exposure to a substance (e.g., an ingestion, inhalation, or topical exposure), or request information/educational materials. Exposures do not necessarily represent a poisoning or overdose. The AAPCC is not able to completely verify the accuracy of every report made to member centers. Additional exposures may go unreported to PCs and data referenced from the AAPCC should not be construed to represent the complete incidence of national exposures to any substance(s).
References
- National Poison Data System: Annual reports 1983-2012[Internet]. Alexandria (VA): American Association of Poison Control Centers;. Available from: http://www.aapcc.org/annual-reports/
- US Census Bureau. Table 1.Annual Estimates of the Resident Population for the United States, Regions, States, and Puerto Rico: April 1, 2010 to July 1, 2012 (NST-EST2012-01)[downloaded 2013 Oct 23] http://www.census.gov/popest/data/state/totals/2012/index.html
- US Census Bureau: International Data Base (IDB) Demographic Indicators for: American Samoa, Federated States of Micronesia, Guam, Virgin Islands, [downloaded 2012 Oct 26]: http://www.census.gov/population/international/data/idb/region.php
- US Census Bureau: State Characteristics Datasets: Annual Estimates of the Civilian Population by Single Year of Age and Sex for the United States and States: April 1, 2010 to July 1, 2012[downloaded 2013Oct 23]: http://www.census.gov/popest/data/state/asrh/2012/SC-EST2012-AGESEX-CIV.html
- US Census Bureau Population Estimates Downloadable Datasets: Annual Estimates of the Resident Population by Single Year of Age and Sex for the United States, States, and Puerto Rico Commonwealth: April 1, 2010 to July 1, 2013, Data [downloaded 2014 Nov 4]: http://www.census.gov/popest/data/puerto_rico/asrh/2013/index.html
- Position statement: ipecac syrup. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1997;35:699–709.
- American Academy of Clinical Toxicology European Association of Poisons Centres and Clinical Toxicologists. Position Paper: Ipecac Syrup. J Toxicol Clin Toxicol 2004; 42: 133–143.
- American Academy of Pediatrics Policy Statement. Poison treatment in the home. Pediatrics 2003; 112:1182–1185.
- Savel TG, Bronstein A, Duck, M, Rhodes MB, Lee, B, Stinn J, Worthen, K. Using Secure Web Services to Visualize Poison Center Data for Nationwide Biosurveillance: A Case Study [Internet]. Online Journal of Public Health Informatics 2010; 2:1–9; [downloaded 2012Oct 30] http://ojphi.org/htbin/cgiwrap/bin/ojs/index.php/ojphi/article/view/2920/2505
- Centers for Disease Control and Prevention. QuickStats: Number of Poisoning Deaths* Involving Opioid Analgesics and Other Drugs or Substances --- United States, 1999—2007. MMWR Morb Mortal Wkly Rep. 2010; 59:1026
- McGraw-Hill's AccessMedicine, Laboratory Values of Clinical Importance (Appendix), Harrison's Principles of Internal Medicine 17e. McGraw-Hill Professional, 2008[cited 2010 Nov 1]. Available from: http://www.accessmedicine.com/.
- Goldfrank'sToxicologic Emergencies, Ninth Edition, McGraw-Hill Companies, 2010.
- Dart RC, editor. Medical Toxicology, Third Edition. Philadelphia, Lippincott, Williams & Wilkins, 2004.
Appendix A – Acknowledgments
The compilation of the data presented in this report was supported in part through the US Centers for Disease Control and Prevention AAPCC Contract 200-2011-41767.
The authors wish to express their profound appreciation to the following individuals who assisted in the preparation of the manuscript: Katherine W. Worthen and Laura J. Rivers.
The authors express their sincere gratitude to the staff at the AAPCC Central Office for their support during the preparation of the manuscript: Stephen Kaminski, JD, Executive Director, Beth Copes and the entire staff.
Poison Centers (PCs)
We gratefully acknowledge the extensive contributions of each participating PC and the assistance provided by the many health care providers who provided comprehensive data to the PCs for inclusion in this database. We especially acknowledge the dedicated efforts of the specialists in poison information (SPIs) who meticulously coded 3,060,122 calls made to US PCs in 2013.
As in previous years, the initial review of reported fatalities and development of the abstracts and case data for NPDS was the responsibility of the staff at the 57 participating PCs. Many individuals at each center participated in the fatality case preparation. These toxicology professionals and their centers are:
Alabama Poison Center
Perry Lovely, MD, ACMT
John Fisher, PharmD, DABAT, FAACT
Lois Dorough BSN, RN, CSPI
Arizona Poison and Drug Information Center
Keith Boesen, PharmD, CSPI
F. Mazda Shirazi, MS, MD, PhD, FACEP, FAMCT
Arkansas Poison & Drug Information Center
Henry F. Simmons, Jr., MD
Pamala R. Rossi, PharmD
Howell Foster, PharmD, DABAT
Banner Good Samaritan Poison and Drug Information Center
Daniel Brooks, MD
Frank LoVecchio, DO, MPH
Jane Klemens, RN, CSPI
Sharyn Welch, RN
Rebecca Hilder, RN, CSPI
Diane Glogan, RN
Blue Ridge Poison Center
Christopher P. Holstege, MD
Nathan P. Charlton, MD
William Rushton, MD
Luke Hardison, MD
California Poison Control System—Fresno/Madera Division
Richard J. Geller, MD, MPH
California Poison Control System—Sacramento Division
Timothy Albertson, MD, PhD
Justin Lewis, PharmD, CSPI
California Poison Control System—San Diego Division
Richard F. Clark, MD
Lee Cantrell, PharmD
Alicia B. Minns, MD
Janna Villano, MD
Charles O’Connell, MD
California Poison Control System—San Francisco
Suad A. Al-Abri, MD
Ilene Anderson, PharmD
Jo Ellen Dyer, PharmD
Hallam Gugelmann, MD
Sandra Hayashi, PharmD
Raymond Ho, PharmD
Susan Kim-Katz, PharmD
Beth Manning, PharmD
Kathryn Meier, PharmD
Kent R. Olson, MD
Freda Rowley, PharmD
Ben Tsutaoka, PharmD
Carolinas Poison Center
Michael C. Beuhler, MD
Anna Rouse Dulaney, PharmD
Christine M. Murphy, MD
William Kerns II, MD
Central Ohio Poison Center
Hannah Hays, MD
Marcel J. Casavant, MD, FACEP, FACMT
Henry Spiller, MS, DABAT, FAACT
Jason Russell, DO
Devin Wiles DO
Kaitlyn Day
Central Texas Poison Center
Ryan Morrissey, MD
S. David Baker, PharmD, DABAT
Children's Hospital of MI Regional Poison Center
Cynthia Aaron, MD
Lydia Baltarowich, MD
Aimee Nefcy, MD
Bram Dolcourt, MD
Susan C. Smolinske, PharmD
Matthew Hedge, MD
Andrew King, MD
Keenan Bora, MD
Cincinnati Drug and Poison Information Center
Shan Yin, MD, MPH
Sara Pinkston, RN
Connecticut Poison Center
Charles McKay, MD, ABMT
Mary Kay Balboni, RN, CSPI
Bernard C. Sangalli, MS, DABAT
Florida/USVI Poison Information Center—Jacksonville
Thomas Kunisaki, MD, FACEP, ACMT
Florida Poison Information Center—Miami
Jeffrey N. Bernstein, MD
Richard S. Weisman, PharmD
Florida Poison Information Center—Tampa
TamasPeredy, MD, FAACT
Charisse Webb, RN, CSPI
Aryne Patterson, RN, CSPI
Judy Turner, RN, CSPI
Pamela Eubank, RN, CSPI
Georgia Poison Center
Robert J. Geller, MD
Brent W. Morgan, MD
Ziad Kazzi, MD
Stella Wong, DO
Gaylord P. Lopez, PharmD
Stephanie Hon, PharmD
Adam Pomerleau, MD
Justin Arnold, DO
Alaina Steck, MD
Melissa Halliday, MD
Molly Boyd, MD
Hennepin Regional Poison Center
Deborah L. Anderson, PharmD
Jon B. Cole, MD
Katherine Katzung, MD
JoAn Laes, MD
Benjamin S. Orozco, MD
David J. Roberts, MD
Laurie Willhite, PharmD, CSPI
Illinois Poison Center
Michael Wahl, MD
Sean Bryant, MD
Indiana Poison Center
James B. Mowry, PharmD
Gwenn Christianson, MSN, CSPI
R. Brent Furbee, MD
Iowa Poison Control Center
Sue Ringling, RN
Linda B. Kalin, RN
Edward Bottei, MD
Kentucky Regional Poison Control Center
George M. Bosse, MD
Ashley N. Webb, MSc, PharmD, DABAT
Louisiana Poison Center
Mark Ryan, PharmD
Thomas Arnold, MD
Maryland Poison Center
Suzanne Doyon, MD, FACMT
Mingzohn (Ellen) Tsay, PharmD
Mississippi Poison Control Center
Robert Cox MD, PhD, DABT, FACMT
Christina Parker, RN, CSPI
Missouri Poison Center at SSM Cardinal Glennon
Children's Medical Center
Rebecca Tominack, MD
Shelly Enders, PharmD, CSPI
National Capital Poison Center
Cathleen Clancy, MD, FACMT
Nicole Reid, RN, BA, BSN, MEd, CSPI
Nebraska Regional Poison Center
Prashant Joshi, MD
Ronald I. Kirschner, MD
New Jersey Poison Information and Education System
Steven M. Marcus, MD
Bruce Ruck, PharmD
New Mexico Poison and Drug Information Center
Steven A. Seifert, MD, FAACT, FACMT
Brandon Warrick, MD
Susan Smolinske, PharmD, DABAT
New York City Poison Control Center
Maria Mercurio-Zappala, MS, RPh
Robert S. Hoffman, MD
Lewis Nelson, MD
Rana Biary, MD
Nicholas Connors, MD
Mai Takematsu, MD
Betty Chen, MD
Lauren Shawn, MD
Hong Kim, MD
North Texas Poison Center
Brett Roth MD, ACMT, FACMT
Melody Gardner, RN, MSN, MHA, CCRN
Northern Ohio Poison Center
Lawrence S. Quang, MD
Adrianne Grendzynski, RN, BSN, CSPI
Danielle Richardson, RN, BSN, CSPI
Susan Scruton, RN, BSN, CSPI
Northern New England Poison Center
Karen E. Simone, PharmD, DABAT, FAACT
Oklahoma Poison Control Center
William Banner, Jr., MD, PhD, ABMT
Scott Schaeffer, RPh, DABAT
Oregon Poison Center
Zane Horowitz, MD
Sandra L. Giffin, RN, MS
Palmetto Poison Center
William H. Richardson, MD
Jill E. Michels, PharmD
Pittsburgh Poison Center
Michael Lynch, MD
Rita Mrvos, BSN
Puerto Rico Poison Center
José Eric Dîaz-Alcalá, MD
Andrés Britt, MD
Elba Hernández, RN
Regional Center for Poison Control and Prevention Serving Massachusetts and Rhode Island
Michele M. Burns, MD, MPH
Dennis Wigandt, PharmD
Rebecca Bruccoleri, MD
Diana Felton, MD
Regional Poison Control Center—Children's of Alabama
Erica Liebelt, MD, FACMT
Michele Nichols, MD
Sherrel Kirkland, RN, CSPI
Ann Slattery DrPH DABAT
Diane Smith, RN, CSPI
Rocky Mountain Poison & Drug Center
Alvin C. Bronstein MD, FACEP
Beau Braden, DO, MPH
Dazhe Cao, MD
Janetta L. Iwanicki, MD
Eric J. Lavonas, MD
Sam Wang, MD
Shireen Banerjji, PharmD, DABAT
Carol Hesse RN, CSPI
Regina R. Padilla
South Texas Poison Center
Cynthia Abbott-Teter, PharmD
Douglas Cobb, RPh
Miguel C. Fernandez, MD
George Layton, MD
C. Lizette Villarreal, MA
Southeast Texas Poison Center
Wayne R. Snodgrass, MD, PhD, FACMT
Jon D. Thompson, MS, DABAT
Jean L. Cleary, PharmD, CSPI
Tennessee Poison Center
John G. Benitez, MD, MPH
Donna Seger, MD
Texas Panhandle Poison Center
Shu Shum, MD
Jeanie E. Jaramillo, PharmD
Cristie Johnston, RN, CSPI
The Poison Control Center at the Children's Hospital of Philadelphia
Fred Henretig, MD
Kevin Osterhoudt, MD
Jeanette Trella, PharmD
University of Kansas Hospital Poison Control Center
Tama Sawyer, PharmD, DABAT
Stephen Thornton, MD
Upstate NY Poison Center
Jeanna M. Marraffa, PharmD
Nicholas Nacca, MD
Rachel Schult, Pharm.D.
Christine M. Stork, PharmD
Ross Sullivan, MD
Timothy Wiegand, MD
Utah Poison Control Center
B. Zane Horowitz, MD
Virginia Poison Center
Rutherfoord Rose, PharmD
Kirk Cumpston, DO
Brandon Wills, DO
Michelle Troendle, MD
Washington Poison Center
William T. Hurley, MD, FACEP, FACMT
Curtis Elko, PharmD
David Serafin, CPIP
Tom Martin, MD, MPH, FACEP
West Texas Regional Poison Center
Hector L. Rivera, RPh, CSPI
Stephen W. Borron, MD, MS, FACEP, FACMT
Salvador H. Baeza, PharmD, DABAT
West Virginia Poison Center
Elizabeth J. Scharman, PharmD, DABAT, BCPS, FAACT
Anthony F. Pizon, MD, ABMT
Wisconsin Poison Center
David D. Gummin, MD
Amy E. Zosel, MD
AAPCC Fatality Review Team
The Lead and Peer review of the 2013 fatalities was carried out by the 39 individuals listed here including four who reviewed the pediatric cases [Peds]. The authors and the AAPCC wish to express our appreciation for their volunteerism, dedication, hard work, and good will in completing this task in a limited time.
Alfred Aleguas Jr, PharmD, DABAT, Florida Poison Information Center- Tampa
Andy King, MD, Children’s Hospital of Michigan RPCC, Detroit
Amy Zosel, MD, Wisconsin Poison Center
Anna Rouse Dulaney*, PharmD, DABAT, Carolinas Poison Center
Ann-Jeannette Geib, MD, FACEP, FACMT, Assistant Professor of Emergency Medicine, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ
Ashley Webb, MSc, PharmD, DABAT, Director, Kentucky Regional PCC
Bernard C Sangalli*, MS, DABAT, Connecticut Poison Center
Charles McKay, MD, Associate Medical Director, Connecticut Poison Control Center, University of Connecticut School of Medicine
Curtis Elko*, PharmD, CSPI, Washington Poison Center, Seattle
Cynthia Lewis-Younger, MD, MPH, Vancouver, Washington
Daniel E Brooks*, MD, Banner Good Samaritan Medical Center, Phoenix
David D Gummin, MD, Wisconsin Poison Center
Diane Calello, MD, FAAP, FACMT, New Jersey Poison Information and Education System [Peds]
Elizabeth J Scharman, PharmD, DABAT, BCPS, FAACT, West Virginia Poison Center
Frank LoVecchio, DO, Banner Poison and Drug and Information Center, Phoenix, AZ
Gar Chan, MD, FACEM, Launceston General Hospital, Tasmania, Australia
Hannah Hays, MD, Central Ohio Poison Center, Columbus, OH
Henry Spiller*, MS, DABAT, FAACT, Central Ohio Poison Center, Columbus OH
Jan Scaglione*, PharmD, DABAT, Cincinnati Drug and Poison Information Center
Jeffrey S Fine, MD, NYU School of Medicine/Bellevue Hospital [Peds]
Jennifer Lowry, MD, Division of Clinical Pharmacology, Toxicology, and Therapeutic Innovations, Children’s Mercy Hospital, Kansas City, MO [Peds]
Jill E Michels, PharmD, DABAT, Managing Director, Palmetto Poison Center, SC
John McDonagh, MD, Hartford, CT
Kathy Hart, MD, Connecticut Poison Control Center
L Keith French, MD, Oregon Poison Center
Maria Mercurio-Zappala, RPh, MS, DABAT, FAACT, NYC PCC
Mark Su, MD, MPH, FACEP, FACMT, Director, New York City Poison Control Center, New York, NY
Mike Levine*, MD, Banner Good Samaritan Medical Center, Phoenix
Nathanael McKeown*, DO, Oregon Poison Center
Rachel Gorodetsky, PharmD, D’Youville College School of Pharmacy, University of Rochester Medical Center
Rais Vohra, MD, California Poison Control System, Fresno/Madera
Robert Goetz*, PharmD, DABAT, Cincinnati Drug and Poison Information Center
Shana Kusin, MD, Department of Emergency Medicine, OHSU
Sophia Sheikh MD, Department of Emergency Medicine, University of Florida College of Medicine-Jacksonville
Steven M Marcus*, MD, NJ Poison Information and Education System, NJ Medical School, of the School of Biomedical and Health Sciences of Rutgers University, The State University of NJ [Peds]
Susan Smolinske, PharmD, Children’s Hospital of Michigan RPCC, Detroit
Timothy Wiegand, MD, Director of Toxicology, University of Rochester, Medical Center and Strong Memorial Hospital; Consultant Toxicologist, SUNY Upstate Poison Center
Tom Martin, MD, Medical Director, Utah Poison Control Center
William Hurley, MD, Washington Poison Center, Seattle
* These reviewers further volunteered to read the top ranked 200 abstracts and judged to publish or omit each.
AAPCC Micromedex Joint Coding Group
Chair: Elizabeth J. Scharman, Pharm.D., DABAT, BCPS, FAACT
Alvin C. Bronstein, MD, FACEP, FACMT
Rick Caldwell
Christina Davis, PharmD
Sandy Giffin, RN, MS
Kendra Grande, RPh
Katherine M. Hurlbut, MD
Wendy Klein-Schwartz, PharmD, MPH
Fiona McNaughton
Susan C. Smolinske, PharmD
AAPCC Rapid Coding Team
Chair: Alvin C. Bronstein, MD, FACEP, FACMT
Elizabeth J. Scharman, Pharm.D., DABAT, BCPS, FAACT
Jay L. Schauben, PharmD, DABAT, FAACT
Susan C. Smolinske, PharmD
AAPCC Surveillance Team
NPDS surveillance anomalies are analyzed daily by a team of 9 medical and clinical toxicologists working across the country in a distributed system. These dedicated professionals interface with the Health Studies Branch, National Center for Environmental Health, Centers for Disease Control and Prevention (HSB/NCEH/CDC) and the PCs on a regular basis to identify anomalies of public health significance and improve NPDS surveillance systems:
Alvin C. Bronstein, MD, FACEP, FACMT - Director
Alfred Aleguas, Pharm D, DABAT
S. David Baker, PharmD, DABAT
Douglas J. Borys, PharmD, DABAT
John Fisher, PharmD, DABAT, FAACT
Jeanna M. Marraffa, PharmD, DABAT
Maria Mercurio-Zappala, RPH, MS, DABAT, FAACT
Henry A. Spiller, MS, DABAT, FAACT
Richard G. Thomas, Pharm D, DABAT
Regional Poison Center (PC) Fatality Awards
Each year the AAPCC and the Fatality Review team recognized several regional PCs for their extra effort in their preparation of fatality reports and prompt responses to reviewer queries during the review process. The awards were presented at the October 2014, North American Congress of Clinical Toxicology meeting in New Orleans, LA.
First Center to Complete all Cases (30-Dec 2013, last of their 17 cases)
West Virginia Poison Center (Charleston)
Largest Number with Autopsy Reports (44 of 73 cases) Carolinas Poison Center (Charlotte)
Highest Percentage with Autopsy Reports (88% of 8 cases) Oklahoma Poison Control Center (Oklahoma City)
Largest Number of INDIRECT cases (507 of 925 total cases reported for 2013)
Maryland Poison Center (Baltimore)
Highest Overall Quality of Reports (12.0 of possible 22 for 1 case)
Texas Panhandle Poison Center (Amarillo)
Greatest improvement in Overall Quality of Reports (7.67 increase from last year)
Texas Panhandle Poison Center (Amarillo)
Most Abstracts Published in last year's Annual report (12 of the 70 published narratives)
Carolinas Poison Center (Charlotte)
Most Helpful Regional Poison Center Staff (based on survey of AAPCC review team)
Carolinas Poison Center (Charlotte)
- - - Honorable Mention
Banner Poison Drug and information Center (Dan Brooks)
Appendix B—Data Definitions
Reason for Exposure
NPDS classifies all calls as either EXPOSURE (concern about an exposure to a substance) or INFORMATION (non-exposed human or animal). A call may provide information about one or more exposed person or animal (receptors).
Specialists in poison information (SPIs) coded the reasons for exposure reported by callers to PCs according to the following definitions:
Unintentional general: All unintentional exposures not otherwise defined below.
Environmental: Any passive, non-occupational exposure that results from contamination of air, water, or soil. Environmental exposures are usually caused by manmade contaminants.
Occupational: An exposure that occurs as a direct result of the person being on the job or in the workplace.
Therapeutic error: An unintentional deviation from a proper therapeutic regimen that results in the wrong dose, incorrect route of administration, administration to the wrong person, or administration of the wrong substance. Only exposures to medications or products used as medications are included. Drug interactions resulting from unintentional administration of drugs or foods which are known to interact are also included.
Unintentional misuse: Unintentional improper or incorrect use of a nonpharmaceutical substance. Unintentional misuse differs from intentional misuse in that the exposure was unplanned or not foreseen by the patient.
Bite/sting: All animal bites and stings, with or without envenomation, are included.
Food poisoning: Suspected or confirmed food poisoning; ingestion of food contaminated with microorganisms is included.
Unintentional unknown: An exposure determined to be unintentional, but the exact reason is unknown.
Suspected suicidal: An exposure resulting from the inappropriate use of a substance for reasons that are suspected to be self-destructive or manipulative.
Intentional misuse: An exposure resulting from the intentional improper or incorrect use.
Contaminant/tampering: The patient is an unintentional victim of a substance that has been adulterated (either maliciously or unintentionally) by the introduction of an undesirable substance.
Malicious: Patients who are victims of another person’s intent to harm them.
Withdrawal: Inquiry about or experiencing of symptoms from a decline in blood concentration of a pharmaceutical or other substance after discontinuing therapeutic use or abuse of that substance.
Adverse Reaction Drug: Unwanted effects due to an allergic, hypersensitivity, or idiosyncratic response to the active ingredient(s), inactive ingredient(s) or excipient of a drug, chemical, or other drug substance when the exposure involves the normal, prescribed, labeled or recommended use of the substance.
Adverse Reaction Food: Unwanted effects due to an allergic, hypersensitivity, or idiosyncratic response to a food substance.
Adverse Reaction Other: Unwanted effects due to an allergic, hypersensitivity, or idiosyncratic response to a substance other than drug or food.
Unknown Reason: Reason for the exposure cannot be determined or no other category is appropriate.
Medical Outcome
No effect: The patient did not develop any signs or symptoms as a result of the exposure.
Minor effect: The patient developed some signs or symptoms as a result of the exposure, but they were minimally bothersome and generally resolved rapidly with no residual disability or disfigurement. A minor effect is often limited to the skin or mucus membranes (e.g., self-limited gastrointestinal symptoms, drowsiness, skin irritation, first-degree dermal burn, sinus tachycardia without hypotension, and transient cough).
Moderate effect: The patient exhibited signs or symptoms as a result of the exposure that were more pronounced, more prolonged, or more systemic in nature than minor symptoms. Usually, some form of treatment is indicated. Symptoms were not life-threatening, and the patient had no residual disability or disfigurement (e.g., corneal abrasion, acid-base disturbance, high fever, disorientation, hypotension that is rapidly responsive to treatment, and isolated brief seizures that respond readily to treatment).
Major effect: The patient exhibited signs or symptoms as a result of the exposure that were life-threatening or resulted in significant residual disability or disfigurement (e.g., repeated seizures or status epilepticus, respiratory compromise requiring intubation, ventricular tachycardia with hypotension, cardiac or respiratory arrest, esophageal stricture, and disseminated intravascular coagulation).
Death: The patient died as a result of the exposure or as a direct complication of the exposure.
Not followed, judged as a nontoxic exposure: No follow-up calls were made to determine the outcome of the exposure because the substance implicated was nontoxic, the amount implicated was insignificant, or the route of exposure was unlikely to result in a clinical effect.
Not followed, minimal clinical effects possible: No follow-up calls were made to determine the patient's outcome because the exposure was likely to result in only minimal toxicity of a trivial nature. (The patient was expected to experience no more than a minor effect.)
Unable to follow, judged as a potentially toxic exposure: The patient was lost to follow-up, refused follow-up, or was not followed, but the exposure was significant and may have resulted in a moderate, major, or fatal outcome. Unrelated effect: the exposure was probably not responsible for the effect.
Confirmed nonexposure: this outcome option was coded to designate cases where there was reliable and objective evidence that an exposure initially believed to have occurred, but actually never occurred (e.g., all missing pills are later located). All cases coded as confirmed nonexposure are excluded from this report.
Death, indirect report: Death, indirect report are deaths that the poison center acquired from medical examiner or media, but did not manage nor answer any questions about the death.
Relative Contribution to Fatality (RCF)
The definitions used for the Relative Contribution to Fatality (RCF) classification by the Case Review Team (CRT) were as follows:
Undoubtedly responsible—In the opinion of the CRT, the clinical case evidence establishes beyond a reasonable doubt that the substances actually caused the death.
Probably responsible—In the opinion of the CRT, the clinical case evidence suggests that the substances caused the death, but some reasonable doubt remained.
Contributory —In the opinion of the CRT, the clinical case evidence establishes that the substances contributed to the death, but did not solely cause the death. That is, the substances alone would not have caused the death, but combined with other factors, were partially responsible for the death.
Probably not responsible—In the opinion of the CRT, the clinical case evidence establishes to a reasonable probability, but not conclusively, that the substances associated with the death did not cause the death
Clearly not responsible—In the opinion of the CRT, the clinical case evidence establishes beyond a reasonable doubt that the substances”did not cause this death.
Unknown—In the opinion of the CRT, the clinical case evidence is insufficient to impute or refute a causative relationship for the substances in this death.
Appendix C—Abstracts of Selected Cases
Selection of Abstracts for Publication
The abstracts included in Appendix C were selected for publication in a three-stage process consisting of qualifying, ranking, and reading. Qualifying was based on the RCF:—only RCF = 1—Undoubtedly Responsible; 2—Probably Responsible; or 3—Contributory were eligible for publication. Fatalities by indirect report were excluded beginning with the 2008 annual report. Ranking was based on the number of substances (1/N) and weighted case score. The case weighting factors were the averages chosen based on review team recommendations in 2006. Each case score was multiplied by the respective factors to obtain a weighted publication score: Hospital records * 8.8 + Postmortem * 15.2 + Blood levels * 6.9 + Quality/Completeness * 6.4 + Novelty/Educational value * 13.2. Scores were normalized (z-score) within each reviewer before the final weighting: 25% for Age Z-Score + 25% for Freq Z-Score of 1st cause rank substance + 25% for weighted case scores + 25% for 1/N + 10 for pregnant patient + 10 for patient under 3 years old.
The top-ranked abstracts (200 + ties) were each read by individual reviewers (see Appendix A) and the 2 managers (Cantilena and Spyker). Each reader judged each abstract as “publish” or “omit,” and all abstracts receiving 7 or more of 12 publish votes were selected, further edited and cross-reviewed by the two managers.
Abstracts
Abstracts of the cases were selected (see Selection of Abstracts for Publication, above) from the human fatalities judged related to an exposure as reported to US PCs in 2013. A structured format for abstracts was required in the PC preparation of the abstracts and was used in the abstracts presented. Abbreviations, units, and normal ranges omitted from the abstracts are given at the end of this appendix.
Case 1. Acute methanol ingestion: undoubtedly responsible.
Scenario/Substances: A 17-year-old (y/o) female with no significant past medical history presented to a community hospital with shortness of breath preceded by fatigue. She developed status epilepticus unresponsive to midazolam and required endotracheal intubation. She was transferred to a tertiary care hospital.
Physical Exam: BP 127/78, HR 113, RR 56, T 36°C, O2 sat 95% on room air. She was alert and interactive, appeared dehydrated and cachectic. Severely tachypneic. Globally weak. Otherwise remainder of examination was unremarkable.
Laboratory Data: pH 6.8 / pCO2 10 / HCO3 2

lactate 2.2 mmol/L, WBC 18, Hgb 17, platelets 345. Non-contrast head CT mild cerebral edema. Ammonia 163 mcmol/L, AST, ALT, and bilirubin normal. Serum valproate not detected. Methanol 45 mg/dL, 20 h after presentation and after 3.5 h of CRRT. Lumbar puncture was unrevealing.
Clinical Course: Patient became progressively more hypotensive despite IV fluid resuscitation, sodium bicarbonate infusion and three vasopressors. ECMO and CRRT were initiated. Metabolic service was consulted for persistent hyperammonemia and initiated a workup for late presenting inborn error of metabolism. Patient was given cobalamin, thiamine, biotin, levocarnitine, and riboflavin. Toxicology service was then consulted for unresolving metabolic acidosis despite resuscitation and bicarbonate infusion. Patient was given fomepizole. Metabolic acidosis resolved with CRRT. However, the patient's cerebral edema worsened, progressing to uncial herniation. Based on the prognosis, the family opted for institution of comfort measures and she expired. Following her death, police investigation revealed that the patient had conducted internet search on methanol poisoning. Multiple empty bottles of windshield wiper fluid containing methanol were found at the patient's home and car.
Autopsy Findings: Numerous linear scars on the body were consistent with self-destructive behavior. Other gross and microscopic pathology results were unremarkable. Cause of death was methanol intoxication. Manner of death was suicide.
Case 148. Acute ethylene glycol (antifreeze) per feeding tube: undoubtedly responsible.
Scenario/Substances: A 66 y/o male reportedly instilled 100 mL of antifreeze into his GI tract via tube feeding port ∼2 h prior to arrival in ED.
Past Medical History: Throat cancer, human immunodeficiency virus infection.
Laboratory Data: Venous blood gases upon arrival in ED pH 7.42/pCO2 31/pO2 35/HCO3 20/BE -4. Hour 5: Na 147, Cl 107, CO2 20, Glu 153, BUN 17, Cr 0.7, anion gap 23, lactate 1.73 mmol/L, HCO3 17, BE 4, O2 sat 70%. Hour 18: pH 7.55 / pCO2 16 / HCO3 14, anion gap 19, salicylate not detected.
Clinical Course: Upon arrival in ED, he was tachypneic (RR 40), BP 146/84, HR 113. Fomepizole therapy was initiated, and thiamine was administered. The patient was admitted to the ICU Hour 5. Based on the prognosis and prior history, the family opted for institution of comfort measures. Fomepizole therapy was discontinued and he expired on Day 4.
Autopsy Findings: Hour 6 hospital blood ethylene glycol was 1,200 mg/dL. An autopsy was not performed. Probably cause of death: ethylene glycol toxicity due to antifreeze ingestion. Manner of death: Suicide.
Case 153. Acute disc battery and acetaminophen ingestion: undoubtedly responsible.
Scenario/Substances: A 16 m/o male was brought to the ED after a week of cough. Supratherapeutic doses of acetaminophen may have been given. An X-ray showed a 20-mm coin cell-shaped foreign body in the esophagus.
Past Medical History: Previously healthy.
Clinical Course: The child was transferred to a tertiary care hospital for endoscopic removal. The battery was successfully removed, and the child was admitted to the ICU. The child developed a massive GI bleed, liver failure, acidosis, and renal failure. He was intubated, sedated, and ventilated; N-acetylcysteine and blood products were administered. The child was taken to the OR where he arrested during exploratory laparotomy. CPR was initially successful, but the child remained hypoxic and hypotensive and died.
Autopsy Findings: Not available.
Case 154. Acute scorpion sting: undoubtedly responsible.
Scenario/Substances: A 3 y/o boy awoke at home, crying and complaining of ear pain, and was brought to the ED.
Laboratory Data: Initial labs at transferred hospital in PICU,
Clinical Course: Patient arrived at ED talking and answering questions, but rapidly developed a grade IV scorpion envenomation with crying, excessive secretions, opsoclonus, writhing, and tachycardia. He was receiving sedatives and analgesia when he developed respiratory distress and arrested. He was intubated and treated with atropine, epinephrine, flumazenil, bicarbonate. He received five vials of scorpion antivenin post code, was intubated, transferred to a tertiary care hospital, and admitted to the PICU. Lungs were clear and he exhibited posturing. Na 146 Cl, 114, lactate 2.9, AST 221 ALT 81, CK 922, ABG (capillary)-pH 7.48/pCO2 26.7/pO2 61.0/HCO3 19.9/BE -4.0. Repeat Venous BG-pH 7.29/pCO2 36/pO2 49/HCO3 17/BE -10.0 on FIO2 45%. CxR “normal.” He was given naloxone to rule out over sedation. Pupils were fixed and dilated, and no response panting between ventilator breaths. No other medical or genetic abnormalities were found. Patient expired on Day 2 of suspected cerebral edema.
Autopsy Findings: “Complications of probable scorpion sting.” Femoral blood: tryptase 3.6 ng/mL.
Case 155. Acute crotalid envenomation: undoubtedly responsible.
Scenario/Substances: A 53 y/o 57 kg male was bitten while attempting to cut the rattle off a rattlesnake, which he presumed was dead. He developed an anaphylactic reaction with cardiopulmonary arrest. He was unresponsive to CPR measures including cardioversion, was intubated, and ventilated.
Physical Exam: After resuscitation HR 110, BP 94/50, he had an edematous right hand with three puncture marks.
Laboratory Data: 5 h post bite: Na 145, K 4.1, CO2 17, Glu 41, WBC 37, Hgb 19.4, Hct 58, platelets 268, CK 9,196, Cr 1.6, BUN 7, AST 2,018, ALT 1,031, Alk phos 225, troponin 4.3, albumin 2.8 g/dL, D-dimer > 20. 6.5 h post exposure, Glu 109, fibrinogen 30 mg/dL, INR 2.1, PTT 47, CK 5,000. Day 2: WBC 23.7, Hgb 15. platelets 131, INR 2.8, PTT 56.3, fibrinogen 104 mg/dL, Cr 3.8, AST 2,275, ALT 800. Day 3: WBC 18, Platelets 58, Hgb 13.9, Hct 40.8, Cr 3.2, BUN 32, INR 1.9, PTT 44, CK 5176, fibrinogen 367 mg/dL. Day 4: WBC 4.7, Platelets 42, Hgb 13.6, Hct 38.7, Cr 3.3, BUN 31, INR 1.4, PTT149, fibrinogen 564 mg/dL, AST 2,797, ALT 1,972.
Clinical Course: He was given dopamine, 6 vials of antivenin (Fab fragment), tetanus toxoid, epinephrine, methylprednisolone, and diphenhydramine. He was transferred to a tertiary care hospital and admitted to the ICU 3 h post exposure. He was ventilated with FiO2 100% + PEEP 5 with no pupil response. Bite site slightly swollen with no apparent progression. At 20 h post bite (14 vials of antivenin) he remained on the ventilator, receiving norepinephrine IV. Pupils were pinpoint and nonreactive. The affected hand measured 19.5 cm, was ecchymotic and blistering. By 24 h post bite (26 vials antivenin), HR 123 and BP 115/63, a femoral catheter was placed and dialysis started for acute kidney injury. On Day 3 (34 vials of antivenin), there were no neurological changes. On Day 4, his entire body was mottled, and he was purple from his nipple line up. The affected arm was ecchymotic and blistered up to his bicep. Right pupil was 3 mm and left pupil 4 mm and non-reactive. EEG showed “severe brain damage”, gag reflex was absent, and he had negative dolls eye reflex. He was receiving multiple vasopressors and IV NS. On Day 5, based on the prognosis, the family opted for institution of comfort measures and he expired later that day.
Autopsy Findings: Not performed.
Case 161. Acute cyanide exposure: undoubtedly responsible.
Scenario/Substances: A 19 y/o male purchased several grams of NaCN and KCN salts online, collapsed at home, EMS intubated, and was transported to the ED.
Past Medical History: Asperger's syndrome, depression, previous suicide attempt with chloroform.
Laboratory Data: ABG-pH 6.91/pCO2 38/pO2 153/HCO3 7/BE 26, WBC 20.5, Hgb 20.4, Hct 63.4, platelets 314
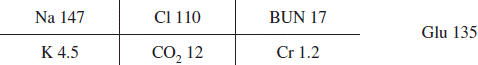
anion gap 25, INR 1.48, lactate 20, serum acetaminophen and salicylate not detected, lithium 0.2 mmol/L, digoxin 0.2 ng/mL, UDS negative. Serum CN ∼10 mg/L (potentially toxic > 0.5 mg/L), 1.3 mg/L (thought drawn after first dose of hydroxocobalamin).
Clinical Course: On arrival in the ED, he was unresponsive, GCS 3, pupils midrange and fixed. He was reintubated, remained profoundly tachycardic and hypotensive despite maximum doses of norepinephrine and dopamine. Further history from family disclosed that patient's access to cyanide salts. Initial labs were notable for profound metabolic acidosis with markedly elevated lactate. ECG showed nonspecific intra ventricular conduction delay with QRS 120 which was improved to 94–100 after sodium bicarbonate. He received hydroxocobalamin 5g x3 doses total, with repeat BP improved from systolic 40 to 60 to 70-80 then to 180–200 after third dose. HR increased to 180s after 3rd dose of hydroxocobalamin. Repeat labs showed slight improvement in acidosis and lactate; however hypotension recurred requiring a 4th dose of hydroxocobalamin with minimal improvement. Head CT showed diffuse subarachnoid hemorrhage, poorly differentiated gray-white matter with global effacement consistent with anoxic encephalopathy, and hypoxic ischemic injury. Based on the prognosis, the family opted for institution of comfort measures and he expired on Day 1.
Autopsy Findings: External exam and laboratory evaluation performed only due to family's religious wishes. Lumbar tap with bloody CSF with RBCs settling and residual maroon CSF. Ante mortem blood prior to hydroxocobalamin treatment screened positive for CN (∼10 mcg/mL, reporting limit 0.3 mcg/mL). Cause of death: hypoxic encephalopathy and possible subarachnoid hemorrhage complicating acute cyanide toxicity. The manner of death was suicide.
Case 171. Acute ammonia inhalation and ocular: contributory.
Scenario/Substances: A 45 y/o male was driving a semi-truck carrying anhydrous ammonia that collided with a train. There was no damage to the cab and he was alert, but soon experienced difficulty breathing. EMS found him in respiratory distress with confusion, intubated him, noted vocal cord edema, and transported him to the ED.
Physical Exam: In the ED, bilateral scleral and conjunctival injection, erythematous eyelids, pupils equal and reactive to light, moist oral mucosa, diminished lung sounds in right base with occasional expiratory wheezes, extremities: 1–2 + edema of right lower extremity with trace lower extremity edema on the left. BP 135/63, O2 sat 98% on 100% FiO2, T 36°C.
Laboratory Data: ABG-pH7.11 / pCO2 82 / pO2 299 / HCO3 26.5, WBC 22.3, CO2 19.6
Clinical Course: He was admitted to the ICU, eyes copiously irrigated, and ophthalmology examination completed. He was maintained on mechanical ventilation, and CxR showed bibasilar infiltrates; he received prophylactic antibiotics for presumed aspiration pneumonia. Respiratory status improved, and he was weaned from ventilator on the morning of Day 5. Later on that day, he developed increasing dyspnea, bradycardia with a decline in O2 sats that were unresponsive to supplemental O2. A code was called, the patient re-intubated, but had ventilator asynchrony and was difficult to ventilate. He became tachycardic, was on maximal IV propofol and midazolam when he had a pulmonary embolism and was suspected despite prophylactic heparin administration. Prior to obtaining a CT of the chest, he had a bradycardic episode, unresponsive to atropine, which quickly became a PEA arrest. He underwent ACLS resuscitation for 40 minutes without return of circulation. He expired on Day 6.
Autopsy Findings: Not performed per family.
Case 185. Acute cyanide ingestion: undoubtedly responsible.
Scenario/Substances: A 73 y/o male jeweler presented to the ED with his wife via private vehicle.
Past Medical History: CAD, s/p CABG and pacemaker placement.
Laboratory Data: ABG-pH 7.32 / pCO2 18 / pO2 453 / HCO3 17 / BE 8, Na 148, K 3.8, Cl 115, CO2 17, anion gap 16, BUN 23, Glu 94 ALT 19, AST 75, serum ethanol not detected.
Clinical Course: Patient was acting normally in the ED waiting room. The patient’s wife reported that he left the waiting room, telling her that he was going to get some apple juice. Upon return, he sat down and slumped over in his chair. ED staff found the patient to be apneic and pulseless and began resuscitation. He was taken to a room where standard resuscitative measures were instituted, including IV access, chest compressions, endotracheal intubation, placement on a ventilator and provision of oxygen. Initial rhythm on the monitor was VT. Return of spontaneous circulation was established. He was tremulous, unresponsive, “posturing”, skin clean and dry, gag reflex and corneal reflexes absent, pupils 5–6 mm and nonreactive A dopamine infusion was started. Inspection of his person revealed a small vial of potassium cyanide in his pocket and a suicide note around his neck stating he wanted “no code.” Further history at that time revealed that he was in need of another “cardiac surgery” and was “just done with it.” The patient received sodium nitrite and sodium thiosulfate in standard doses. Computed tomography of the brain revealed “global infarcts” and “subarachnoid hemorrhage”. The patient was admitted to the ICU where he was declared that his brain was dead the next day, and life support was withdrawn.
Autopsy Findings: Autopsy included hemorrhagic gastritis, marked cerebral edema, cerebellar tonsillar herniation and infarct, cerebral venous sinus thrombosis. Postmortem specimens of heart blood were negative for amphetamines, barbiturates, carisoprodol, cocaine, opiates, and THC metabolite. Hospital blood lidocaine was > 1, 000 mg/mL, believed secondary to use lidocaine during ACLS resuscitation. Premortem blood from the hospital was positive for cyanide (qualitative). Urine specimen and postmortem blood specimens were negative for cyanide. Cause of death: cyanide intoxication. Manner of death: suicide.
Case 186. Acute potassium aluminum sulfate parenteral: undoubtedly responsible.
Scenario/Substances: A 78 y/o 88 kg male received 10 g potassium aluminum sulphate in 1 L D5W IV instead of per urethral catheter. He received 600 ml of the solution IV in 3–4 h after which patient felt cold and became tachycardic and dyspneic.
Past Medical History: Hematuria, prostate cancer.
Physical Exam: BP 132/82, HR 114, RR 18, T 97.3F, Urine cherry in color, urine output total volume 600 ml.
Laboratory Data: ABG-pH 7.54 / pCO2 30 / pO2 359, O2 sat 100% on ventilator. Na 136, K 4.0, BUN 9-17, Cr 0.89-1.26, Hgb 10.1, Hct 28, platelets 222, INR 2.2-2.8.
Clinical Course: CxR showed pulmonary embolism. He was twice successfully resuscitated following cardiac arrest. He intubated and sedated in the ICU, completed first dose of IV deferoxamine 1g in 1 L at 15 mg/kg/hr and hemodialysis. He received a second dialysis and deferoxamine treatment on Day 2. Attempts were made to wean patient off sedation on Day 3, but he became agitated and sedation was restarted. His BP became labile and norepinephrine was started. He was found to have blood clots in his urinary catheter. Based on the prognosis, the family opted for institution of comfort measures and he expired on Day 3
Autopsy Findings: Not available
Case 199. Acute hypochlorite parenteral: probably responsible.
Scenario/Substances: This 63 y/o male had just completed a hemodialysis run on his home dialysis machine. He forgot to disconnect himself from the machine before putting bleach into the machine to clean it and infused ∼60 ml of sodium hypochlorite bleach into his dialysis catheter. He “felt funny” and called EMS. He had a cardiac and respiratory arrest during transport, CPR was begun, intubation was attempted, and he was transported to the ED. He received multiple rounds of epinephrine and atropine enroute to the ED.
Past Medical History: Multiple surgical procedures, including right and left nephrectomies, partial ureterectomy, adrenalectomy, parathyroidectomy, arteriovenous fistula, autogenous arteriovenous fistula, and insertion of a tunneled centrally inserted central venous catheter. He had seasonal allergies, smoked cigarettes daily, used alcohol 1–2 times a month.
Physical Exam: The patient was unresponsive. His skin was cool. No detectable BP or HR.
Laboratory Data: ABG-pH 7.20/pCO2 73/pO2 11/HCO3 28.2/BE -1, O2 sat 8%, Na 141, K 5.7, Glu 139, Ca (ionized) 1.06, total CO2 30.
Clinical Course: In the ED, he was in PEA: CPR was resumed at 15 min post-arrest. He was intubated and a femoral line was placed. He received IV fluids, epinephrine (7 mg total), calcium, and sodium bicarbonate. He expired ∼1 hour after the accidental bleach exposure occurred.
Autopsy Findings: Not performed.
Case 206. Acute laundry detergent (pod) ingestion: undoubtedly responsible.
Scenario/Substances: A 7 m/o male bit into a laundry detergent pod and the contents entered his mouth. The child was crying with occasional cough and became somnolent. EMS was notified and transported the child. Vomiting occurred en route to the ED.
Past Medical History: Recent upper respiratory tract and urinary tract infections treated with cefdinir, but did not complete the course because of runny red stools.
Physical Exam: Somnolent with upper airway wheezing and retractions; moderate respiratory distress. HR 170, RR 30, T 37°C, O2 sats in the 80s% on RA and improved with supplemental oxygen. His palate and pharyngeal cavity had visible red spots.
Laboratory Data: ABG-pH 6.50 / pCO2 70.5 / pO2 27, Na 156, K 2.8, Cl 126. CxR right upper lobe infiltrate.
Clinical Course: During transfer preparations in the ED, the patient experienced a seizure. He was more lethargic with agonal breathing in the 50's. An interosseus catheter was placed, and he was endotracheally intubated; 3 h after exposure, the patient experienced a cardiac arrest and could not be resuscitated.
Autopsy Findings: Mild hyperemia of the oropharynx and tracheal without evident burns or ulcerations. There was a small amount green brown gastric content. There was significant asymmetric pulmonary congestion on right and some cerebral edema. UDS was negative. Central post- mortem blood propylene glycol of 33 mg/dl; gastric contents: propylene glycol of 370 mg/dL. No ethylene or diethylene glycol detected. The death was determined to be accidental exposure to laundry soap detergent.
Case 209. Acute magnets and carbaryl ingestion: undoubtedly responsible.
Scenario/Substances: A 19 m/o female was examined in the ED for complaints of vomiting and diarrhea, instructions for supportive care were given, and the patient was released. The next day she was found unresponsive by her mother. EMS and police were called, bystander CPR was performed and she was transported to the ED.
Past Medical History: Good general health
Clinical Course: On arrival to the ED, the patient had expired, but PALS was performed. Blood was noted in the nose and mouth, but no other signs of trauma were noted. ABG-pH 6.50/pCO2 46/pO2 36, Na 155, K 6.2, Glu 20 Hgb 3.6. Skeletal survey to rule out abuse was performed post-mortem in the ED did not reveal any acute or healing fractures. Portal venous gas and pneumatosis intestinalis was noted. Seven small metallic spherical radio dense foreign bodies were present within the posterior medial aspect of the left abdomen in a linear fashion. EMS and police reported that the child's room was covered in a while powder. The mother stated that the powder was carbaryl insecticide, which had been placed in the room at an unknown time.
Autopsy Findings: Cause of death was listed as ischemic bowel due to spherical magnets found in the small intestine, causing pressure necrosis when the magnets presumably adhered to one another with a portion of small bowel between them. Other conditions related to the death were bed sharing and unsafe sleep surface. No evidence of serious trauma was noted externally. Internal examination revealed the seven above-mentioned magnets to be within the bowel in a linear formation. The stomach and esophagus were normal, while the small bowel proximal to the magnets was hyperemic. Small bowel distal to the magnets was normal in appearance.
Femoral blood was drawn and analyzed. Carbaryl was NOT detected in blood. Ketamine was detected at 7.0 mcg/mL, but this was administered in the ED during intubation. Nor-ketamine was not detected. Heart blood was negative for ethanol. Vitreous electrolytes: Na, 140; K, 18; Cl, 131; Ca, 1.6; Mg, 0.92; Glu, 78; lactate, 21 mmol/L; urea nitrogen, 10; Cr 0.8.
Powder samples × 3 were assessed: all 3 samples were positive for carbaryl and 1-naphthalenol.
Case 224. Acute carbon monoxide inhalation: undoubtedly responsible.
Scenario/Substances: An 11 y/o male was found dead in bed in pool of emesis in a hotel room. His mother was found on the bathroom floor, unconscious suffering from severe CO toxicity. The source was determined to be a retrofitted swimming pool heater that vented very close to the window with a faulty exhaust line that leaked into the room as well. Very high levels of CO were noted when the pool heater was turned on later. Two deaths occurred in the same hotel room 2 months earlier, initially attributed to “heart attacks”, but were later determined to be due to carbon monoxide.
Laboratory Data: Postmortem COHb level from aortic blood was reported as > 60%.
Autopsy Findings: Autopsy demonstrated pulmonary edema and congestion. Petechiae were distributed over head and neck. Cause of death was carbon monoxide toxicity, with the manner being accidental.
Case 283. Acute hydrogen sulfide inhalation: undoubtedly responsible.
Scenario/Substances: A 53 y/o male collapsed inside an asphalt truck container and was pulled out by his son. His son also experienced symptoms. The tank was believed to contain hydrogen sulfide. EMS found that the patient had agonal breathing, intubated him with a laryngeal tube, and removed his clothing prior to transport to the ED.
Past Medical History: Hypertension.
Physical Exam: Upon arrival to the ED, the patient was unconscious with seizure-like movements. The laryngeal tube was exchanged for endotracheal intubation during which a large amount of emesis occurred resulting in aspiration. He was given hydroxocobalamin. On arrival, BP 130/80, HR 87, and O2 sat 82% on 100% FiO2. The urine was found to be in deep purple after the hydroxocobalamin treatment.
Laboratory Data: Initial ABG-pH 7.07 / pCO2 58.0 / pO2 60 / HCO3 10.0, K 3.4, Cl 108, CO2 18, BUN 16, Cr 1.4, Glu 146, Ca 8.1, AST 108, ALT 65. CK 807, INR 1.1, troponin I 0.5, and methemoglobin 0.8%.
Clinical Course: The patient was sedated using propofol, midazolam and fentanyl, and mechanically ventilated. He was given IV fluids and antibiotics. On hour 12, the patient became hypotensive, tachycardic, developed ECG changes consistent with an anterior wall myocardial infarction, and developed a PEA arrest. He was resuscitated with CPR and epinephrine, sodium bicarbonate, and calcium gluconate. He required post-arrest epinephrine and norepinephrine infusions. Post-arrest: pH 7.11, lactate 14.7, troponin I 3.5. He developed a T 38.7°C. The patient had a second cardiac arrest at Hour 21 and could not be resuscitated.
Autopsy Findings: Left ventricular hypertrophy and nephrosclerosis. No drug or chemical levels detected. The death was determined to be from an accidental exposure to hydrogen sulfide.
Case 316. Acute carbon monoxide inhalation: undoubtedly responsible.
Scenario/Substances: A 72-year-old female was found unresponsive and on respiratory arrest in her hotel room bed by housekeeping. CPR was initiated. She was intubated and taken to the local ED. Resuscitation attempts were unsuccessful and she expired. Her husband was found dead in the bathtub. The hotel room was not assessed for the presence of any gases.
Past Medical History: hypertension and atrial fibrillation.
Autopsy Findings: The ME initially assumed the patient and her husband died of overdoses. An autopsy showed pulmonary edema and mild cardiomegaly. Toxicology revealed a COHb of > 60%. Results were finalized 6 weeks after the deaths, and 1 week prior to an 11-year-old male dying of carbon monoxide toxicity in the same hotel room. An investigation determined the heater for the hotel's indoor pool was below the hotel room where all 3 deaths occurred and the heater exhaust was not functioning properly.
Case 318. Acute carbon monoxide inhalation: undoubtedly responsible.
Scenario/Substances: A 73-year-old male was found dead in the bathtub of his hotel room by housekeeping. CPR was initiated, but he was pronounced dead at the scene. His wife was found unresponsive in the bed. The hotel room was not assessed for the presence of any gases.
Autopsy Findings: Pulmonary edema, severe atherosclerosis, and cardiomegaly. The ME initially assumed that the patient and his wife died of overdoses. Toxicology revealed a COHb of > 60%. Results were finalized 6 weeks after the death, and 1 week prior to an 11-year-old dying of carbon monoxide toxicity in the same hotel room. An investigation determined the heater for the hotel's indoor pool was below the hotel room where all 3 deaths occurred and the heater exhaust was not functioning properly.
Case 342. Lead and ethanol ingestion: undoubtedly responsible.
Scenario/Substances: A 73 y/o male made and drank his own moonshine, and developed altered mental status the evening before presentation, and began having seizures at home. EMS intubated him, gave several doses of benzodiazepines, and transported him to the ED.
Past Medical History: His wife had been recently hospitalized and intubated secondary to lead encephalopathy thought to be caused by drinking homemade moonshine. She recovered with chelation to near baseline. She and the entire family were counseled to discontinue the use of this moonshine.
Physical Exam: In the ED, he was in status epilepticus, intubated, sedated. He was afebrile, BP 127/98, HR 80.
Laboratory Data: ABG-pH 7.36 / pCO2 33 / pO2 153 / HCO3 19,
Bilirubin 0.8, AST 35, ALT 23, Alk phos 41, blood lead > 160 mcg/dL.
Clinical Course: The patient was sedated, placed on high dose antiepileptic agents and started on dimercaprol followed by Ca disodium EDTA. Despite maximal therapy, the patient remained in status epilepticus, and was treated with phenytoin, levetiracetam, propofol, midazolam, and phenobarbital. He continued to have subtle twitching during the hospitalization and seizure activity on his EEG. Repeat blood lead: 95 mcg/dL at 48 h after the initiation of chelation and 60 mcg/dL at 96 h. Despite continued therapy, the patient made no neurologic recovery. When propofol sedation was reduced, the patient would again start to seize. On Day 7, he became hemodynamically unstable with hypotension and bradycardia. Based on the prognosis, the family opted for institution of comfort measures and he expired on Day 9.
Autopsy Findings: Not available.
Case 355. Chronic freon inhalation: undoubtedly responsible.
Scenario/Substances: A 33 y/o male was huffing compressed Freon in the woods throughout the day with frequent loss of consciousness. He was found passed out in the woods and brought to the ED by EMS.
Past Medical History: Chronic back pain, reconstructive surgery following a motor vehicle accident, anxiety and depression. History of huffing including a case of pneumonitis 1 year earlier resulting from chronic huffing of compressed air.
Laboratory Data: Na 137, Cl 97, CO2 17, anion gap 23, BUN 23, Cr 1.5, Glu 220, AST 53, CK 1,000, troponin 0.24, Ca 5.1, Ca (ionized) 0.6, WBC 20.
Clinical Course: On ED arrival, the patient was agitated, HR in the 140s. He was dehydrated but afebrile. He was given IV fluids, lorazepam, and promethazine. Within 2 h of arrival in the ED, he lost consciousness and began to seize. He developed VT and was electrically cardioverted to a sinus rhythm with HR 110. Calcium was administered. Labs showed albumin 3.8, ALT 22, Mg 1.0, CKMB 20.9, and Phos 1.7. Repeat Ca 5.3, repeat CK 2,245. The patient had another seizure ∼3 h later and developed VF, received defibrillation twice, was then intubated and transferred to the ICU. At that time he remained tachycardic, HR 106, BP 96/69, RR 20. Propofol infusion was started and he received electrolyte replacement. The patient expired ∼9 h post ED arrival.
Autopsy Findings: No autopsy was performed. Coroner concluded the death was due to fatal cardiac arrhythmias as a result of prolonged huffing of fluorinated hydrocarbons.
Case 367. Acute lamp oil ingestion/aspiration: probably responsible.
Scenario/Substances: A 15 m/o 12-kg male ingested/ aspirated torch fuel at home. EMS transported the patient to the ED.
Clinical Course: In the ED, the patient required oral intubation, was placed on oscillator ventilation, and arrangements were made for transfer for ECMO. Initial BP was “unstable”, pH 6.8, “CO2 in the 100's”, ABG-pH 7.183 / pO2 64 / CO2 57.9 / HCO3 21.3 / BE 7. His status deteriorated during transfer to the tertiary care hospital. On arrival in the PICU, O2 sats 50–60%, O2 sat100% after ECMO. BP 94/42, HR “140's”, T 37.6°C. EEG showed no activity. After aggressive treatment over a course of 4 days, an EEG was done and showed no activity. Brain death was declared Day 4.
Autopsy Findings: Not available.
Case 368. Acute gasoline ingestion/aspiration: undoubtedly responsible.
Scenario/Substances: A 17-month-old male ingested gasoline, choked, vomited, and rapidly developed severe respiratory distress. EMS found him coughing, tachypnea and dyspneic and transported him to the ED. Supplemental oxygen was provided in ambulance, O2 sat 90%, but the child deteriorated and required intubation by EMS en route.
Laboratory Data: CxR showed “white-out” of lungs.
Clinical Course: In the ED O2 sat fell to 70%, and PEEP was added; he was transferred by air to a tertiary care hospital where he suffered a bradycardic arrest ∼7 h after ingestion initially responsive to atropine, epinephrine, and sodium bicarbonate. He arrested again a short time later and could not be resuscitated.
Autopsy Findings: Not available.
Case 369. Acute hydrofluoric acid ingestion: undoubtedly responsible.
Scenario/Substances: A 2 y/o male presented to the ED 30 min after ingesting a mouthful of automotive wheel cleaner. The substance had been stored in a water bottle, and was given to him by his grandmother, who thought she was giving the child a bottle of water.
Physical Exam: He presented awake and alert, but was drooling.
Laboratory Data: Initial laboratory work included a Ca, 8.1; K, 3.0; and venous pH, 7.21. Several h later Ca 2.6.
Clinical Course: Initial treatment consisted of IV calcium gluconate. Approximately 3 h after ED arrival, the patient had a cardiac arrest. He was resuscitated and given additional calcium. He was transferred to a tertiary children's hospital where he was aggressively treated with IV calcium, and suffered a terminal cardiac arrest ∼7 h after ingestion.
Autopsy Findings: Not performed.
Case 377. Acute dinitrophenol ingestion: undoubtedly responsible.
Scenario/Substances: A 19 y/o male purchased dinitrophenol on the internet as a weight loss supplement, took 1 dose (quantity unknown) in the morning, and began feeling unwell late that day and sought care at the ED.
Past Medical History: No reported serious, chronic medical problems. No psychiatric history.
Laboratory Data: ABG-pH 7.46, Cr 1.4, Phos 6, other electrolytes unremarkable, lactate 2.9 mmol/L, salicylates 27, serum acetaminophen and ethanol not detected.
Clinical Course: Upon arrival to the ED, the patient was awake and conversant, HR 120–140, and hypertensive. He was given IV fluids and lorazepam. Mental status declined over the following 2 h, HR increased to 170s, systolic BP 100, T 38.1°C, RR 45, and O2 sat 99% on room air. He received additional IV fluids and IV lorazepam. Methemoglobin was not detected: respiratory and mental status continued to worsen requiring intubation and external cooling measures which were initiated. The patient suffered an asystolic cardiac arrest, ACLS was initiated, but resuscitation was unsuccessful. During the resuscitation T was > 42.71°C (the upper limit on the thermometer).
Autopsy Findings: not available.
Case 380. Acute-on-chronic dinitrophenol and diphenhydramine ingestion: probably responsible.
Scenario/Substances: A 28 y/o male was using dinitrophenol 200 mg a day for weight loss, ingested 4 g in a suicide attempt.
Past Medical History: Obesity
Physical Exam: Awake but “groggy” and diaphoretic on presentation, BP 156/74, HR 174, T 37.9°C, RR 40.
Laboratory Data: None provided
Clinical Course: The patient was given lorazepam IV for agitation. Due to the expected high lethality of DNP, lipid emulsion infusion was given. Prior to transfer to a transferred to a tertiary care hospital, HR 184, BP 163/62, and T 38.4°C. The patient was extremely agitated during transport and 7 hospital personal were required to manage him. He had a cardiac arrest soon after arrival at the tertiary care hospital from which he could not be resuscitated.
Autopsy Findings: Post mortem blood was negative for cocaine, amphetamines, THC and toxic alcohols. 2, 4-dinitrophenol was not detected (specific HP-TLC assay). Trace amounts of diphenhydramine (within the therapeutic concentration) were found. ME final diagnosis: death probably due to 2, 4-dinitrophenol toxicity.
Case 384. Acute DEET (insect repellent) ingestion: undoubtedly responsible.
Scenario/Substances: A 37 y/o male obtained and ingested a 6 ounce bottle of DEET insect repellant. Patient had a witnessed seizure and EMS was summoned. Patient had a VT cardiac arrest enroute to hospital. He received 20 min of CPR and received epinephrine, sodium bicarbonate, dextrose, naloxone and atropine with return of spontaneous circulation. He was intubated and given oxygen prior to arrival at the ED.
Past Medical History: Developmental delay (profound, lived in a group home), PICA, and cardiomegaly.
Physical Exam: BP 84/60, HR 96, RR 18, O2 sat 100% on 100% FiO2, T33.5°C. Head atraumatic, pupils fixed and dilated at 8 mm, oroendotracheal tube in place, multiple abrasions on anterior chest with some oozing of blood, no bowel sounds, and urinary catheter in place with grossly bloody urine without clots.
Laboratory Data: ABG-pH 7.15/pCO2 42.1/pO2 172/HCO3 13.9, lactate 9.1, PT 22.9, INR 2, AST 404, ALT 397

serum acetaminophen, ethanol and salicylate not detected, UDS negative, ECG (initial): sinus tachycardia with intraventricular conduction delay, no ST/T wave changes, QTc 507, ECG #2: sinus rhythm at ventricular rate, normal axis, QTc 537.
Clinical Course: Patient was placed on a hypothermia protocol, given NS 2 L bolus and admitted to the ICU where a norepinephrine infusion was started. Over the following 48 h hypothermia and tachycardia resolved and BP was stabilized with pressors but patient remained completely unresponsive. Cerebral flow study demonstrated no flow, EEG demonstrated diffuse background with little appreciable brain activity, and non-contrast brain MRI showed cerebral edema, transtentorial and tonsillar herniations. On Day 3, the patient was declared brain dead.
Autopsy Findings: Not performed.
Case 389. Acute malathion ingestion: undoubtedly responsible.
Scenario/Substances: A 49 y/o man intentionally drank a bottle of malathion. EMS was called and transported the patient to the ED.
Past Medical History: Alcoholism, COPD, hypertension and depression.
Physical Exam: Upon arrival to the ED, the patient was unresponsive with posturing movements, lungs clear, bowel sounds normal, pupils 2 mm and reactive. BP 246/112, HR157, RR 30, O2 sat 92%, T (oral) 36°C.
Laboratory Data: Upon transfer to the referral hospital: ABG-pH 7.26 / pCO2 33 / pO2 495 / HCO3 15.0, Na 142, K 3.3, Cl 107, CO2 16, BUN 3, Cr 1.2. On Days 3, 4, and 5: Cr 1.1, 1.7 and 3.9 respectively. WBC peaked on Day 4 at 26.
Clinical Course: He was endotracheally intubated, sedated, and mechanically ventilated using midazolam, fentanyl, and propofol. Diarrhea was treated with a total of 7 mg of atropine and pralidoxime (2 g of IV push and an infusion at 8 mg/kg/hr). All body fluids had a strong chemical odor. On Day 2, the patient had no spontaneous neurological activity despite being weaned from all sedation. Bronchoscopy showed aspiration pneumonia. The patient developed progressive hypotension and tachycardia requiring vasopressors, became acidotic and anuric. He died on Day 5.
Autopsy Findings: Bronchopneumonia, left ventricular hypertrophy, liver steatosis, BPH, and diverticulosis coli. Antemortem blood: malathion concentration of 0.12 mg/L and a naloxone concentration of 0.14 mg/L, no other drugs detected. The death was determined to be due to intentional malathion poisoning.
Case 395. Acute paraquat ingestion: undoubtedly responsible.
Scenario/Substances: A 66 y/o male, upon returning to his vehicle after exercising, picked up a bottle of blue-green liquid that he thought was a sports drink and swallowed a large mouthful. He realized that this was an herbicide obtained from a friend and reported to the ED for evaluation. At that time, was not able to provide the name of the herbicide.
Past Medical History: Hypertension, hypercholesterolemia, and anxiety, no history of smoking tobacco or lung disease.
Physical Exam: Upon initial presentation to the ED, he complained of throat pain, nausea, and “feeling bad all over”. At that time, BP 186/106, HR 86, and no respiratory distress. He became diaphoretic and vomited a blue-green liquid. After vomiting, BP 129/58, RR 16, O2 sat 100% on room air, ECG normal.
Clinical Course: Within an hour of exposure, the herbicide was determined to be paraquat, concentration unknown. Although his vitals normalized, he was admitted overnight for persistent vomiting for which he received multiple doses of ondansetron. No activated charcoal was administered for fear of aspiration. Nearly 48 h after observation admission, he was discharged. He returned to the hospital that evening complaining his throat felt swollen and made it difficult to breathe. He was discharged from the ED on antibiotics and steroids. On the following day, he presented to a tertiary care center for a sore throat, swollen tongue, and persistent hiccups, treated with chlorpromazine. His mouth appeared irritated similar to a caustic injury. While in the ED, it was discovered that the patient was having renal failure with an elevated BUN 76 and Cr and 7.2. His O2 sat was 92% on room air, and he had oliguria despite administration of a large of amount of IV fluids. His O2 sat dropped into the 80s, and he was admitted to the ICU where they initiated oxygen at 3 L via CPAP. The patient developed severe, painful oral sores and swelling. The initial steroids were stopped and intense oral care started. The next morning, he was intubated and FiO2 was changed from 100% O2 to nitric oxide at 28–30% O2 and started on n-acetylcysteine, methylprednisolone, 1 g every 24 hrs, cyclophosphamide (he received only 3 doses), MES sodium salt and vitamin C. CVVH was begun. During the next several days, his oral sores continued to be severe with excessive bleeding with care, BUN peaked at 108 and Cr 12. and he was continued on nitric oxide therapy although FiO2 was frequently as high as 40% as the treatment team attempted to maintain O2 sat above 80%. Numerous CxR's showed infiltrates and atelectasis, and his lung sounds became coarse and diminished at the bases. He was sedated and started on tube feedings and electrolyte replacement while he continued on dialysis. Two weeks after the exposure, he began producing thick, creamy, blood-tinged secretions from his lungs. They were unable to wean sedation due to agitation, tachypnea, hypertension, and decreasing O2 sats. Cultures from his lungs showed several pathogens including pseudomonas. He was treated with antibiotics and antifungals. The patient continued to deteriorate, was paralyzed, nitric oxide was stopped, and FiO2 was increased to 100%. The patient expired 3 weeks after the ingestion.
Autopsy Findings: The coroner's reported that the patient's wife claimed that there were 2–3 ounces missing from the bottle. However, because the patient immediately sought care and had no evidence of suicide intent, the ingestion was ruled an accident and no further investigation or autopsy was performed.
Case 396. Acute-on-chronic carbamate insecticide ingestion: probably responsible.
Scenario/Substances: A 69 y/o male had an argument with his significant other and stated he was going to kill himself. He was later found with a can of the carbaryl, unresponsive, sweating, with signs of defecation and urination. Upon arrival, EMS noted rhonchi and rales that were audible without a stethoscope. The patient was intubated using rapid sequence with succinylcholine and transported him to the ED. A red bottle of carbaryl was found in the kitchen sink.
Past Medical History: Aortic stenosis, s/p valve repair, implanted pacemaker. Medications included atorvastatin, clobetasol, lisinopril, magnesium, and metoprolol. History of alcohol abuse, a prior suicide attempt, a daughter committed suicide “years ago.”
Physical Exam: Unresponsive, BP 112/64, HR 75 (paced rhythm), intubated.
Laboratory Data: pH 7.246-7.456, Hgb 17.7-19.1, WBC 31.1, BUN 27, Cr 3.5, Glu 129, bilirubin 2.9, AST 70, ALT 34, Na 141-149, K 3.1-4.4, CL 111-119, CO2 13-17, troponin 0.978, lactate 10.2 mmol/L, Mg 1.4, INR 1.12, serum acetaminophen, ethanol and salicylate not detected. Blood cultures showed no growth
Clinical Course: The patient was placed on ventilator, sedated with lorazepam, and had copious lung secretions needing frequent suctioning. He received 5 doses of atropine 1 mg each and 1 dose of 2 mg atropine. His secretions decreased with the atropine. BP 149/89, HR 84, RR 18, O2 sat 95%. He opened his eyes, and was placed on propofol. His BP dropped 102/65, HR 75 (paced), secretions and diarrhea increased. He became more active without muscle fasciculations but developed renal failure. Based on the prognosis, the family opted for institution of comfort measures and he expired on Day 2.
Autopsy Findings: Not available.
Case 397. Acute paraquat ingestion: undoubtedly responsible.
Scenario/Substances: A 70 y/o female who drank from an iced tea bottle later was found to contain paraquat. She was brought to the ED 30–45 min later.
Laboratory Data: Glu 130, BUN 17, Cr 1.2, AST 28, ALT 22.
Clinical Course: She presented to the ED awake, alert and vomiting. Vital signs were said to be “stable”. At Hour 24 vomiting had stopped, the patient was taking a liquid diet, but had increasing pain in the throat with swallowing or talking. On Day 2, she had increased oral discomfort, BUN 22, Cr 2.4. In subsequent days, BUN and Cr increased, throat and substernal pain continued, and extensive bilateral pulmonary infiltrates were associated with decreasing O2 sats. On Day 5, she was intubated and placed on a ventilator on. On Day 8, BUN 67, Cr 4.4. Day 9 hemodialysis was initiated, but pulmonary function continued to decline, and life support was discontinued on Day 14 and she died.
Autopsy Findings: Autopsy was not performed, but the state Department of Pesticide Regulation obtained the iced tea bottle from which the patient had ingested the liquid and confirmed the presence of a diluted paraquat solution.
Case 400. Acute mitragynine, paroxetine and lamotrigine ingestion: probably responsible.
Scenario/Substances: The 36-y/o male had a generalized tonic-clonic seizure and was found down at home by his family. EMS found the patient pulseless and apneic, intubated him, and initiated ∼30 min of CPR in the field. The patient received epinephrine and naloxone en route. He was found with empty bottles of lamotrigine, paroxetine, and an empty packet labeled “Da Pimp Bomb” with ingredients described as pure kratom.
Past Medical History: Depression, polysubstance abuse, history of suicidal ideation.
Physical Exam: After return of spontaneous circulation: unresponsive on ventilator, BP 106/63, HR 118, T 34.3°C, O2 sat 96%. Pupils dilated but sluggishly reactive, heart tachycardic, lungs with coarse breath sounds, abdomen soft and nontender, GCS 3T with 1 + reflexes bilaterally and no clonus.
Laboratory Data: Initial labs:
INR 1.42, lactate 16 mmol/L, serum acetaminophen and salicylate not detected,
Clinical Course: Upon arrival in the ED, he was found to be in asystole and received sodium bicarbonate, epinephrine, magnesium, Ca chloride, lipid emulsion, and TPA. After 40 min of CPR spontaneous circulation returned. ECG showed wide complex tachycardia with large terminal R wave in aVR that narrowed after additional sodium bicarbonate. The patient underwent a cooling protocol until Day #4 when he underwent evaluation by neurology and critical care and was declared brain dead. The body was released for organ donation the same day.
Autopsy Findings: Diagnoses included marked cerebral edema consistent with anoxic brain injury, with multifocal brainstem hemorrhage, multiple small recent pulmonary infarcts and pulmonary emboli, and recent thrombosis in prosthetic venous plexus. The autopsy revealed no other anatomic cause of death. Laboratory testing showed a qualitative positive screen for mitragynine and 7-OH mitragynine only. Cause of death was severe hypoxic encephalopathy complicating apparent mitragynine toxicity. The packet of the suspect drug was analyzed by law enforcement and found to contain only mitragynine. The manner of death is accident by the report.
Case 401. Acute cardiac glycoside ingestion: probably responsible.
Scenario/Substances: A 74 y/o male blended 7–9 oleander leaves with water in a blender and drank it as suicidal gesture. A couple of hours later his wife found him having nausea and vomiting, and brought him to the ED.
Past Medical History: Depression, GERD, chronic pain, atrial fibrillation, pacemaker, hypertension, and hyperglycemia. Patient did not have a history of taking digoxin.
Laboratory Data: Serum digoxin, 3.23 ng/mL.
Clinical Course: Awake, alert, and oriented x 3, BP 131/61, HR 60 (paced), RR: 20, O2 sat 95%. Patient was given antiemetics, activated charcoal and digoxin immune Fab and admitted overnight for observation and monitoring. On Day 2, digoxin 1.9 ng/mL, still with nausea which was treated with antiemetics. On Day 3, the patient became tachypneic (RR 37), BP 104/30 HR 60 (paced). He received IV fluid bolus. He developed hyperkalemia, WBC 30.6, and decreased renal function and started having episodes of VF. ACLS was started. The patient was defibrillated twice and given epinephrine, bicarbonate, and atropine. During the code, the family determined that he would not want to be resuscitated, opted for institution of comfort measures, and he expired.
Autopsy Findings: Not performed.
Case 404. Acute buprenorphine/naloxone (sublingual) ingestion: undoubtedly responsible.
Scenario/Substances: A 5 y/o female ingested a buprenorphine/ naloxone tablet belonging to her caregiver (her aunt). Within 1 h, the child was drowsy and nauseous. The caregiver declined repeated medical advice to bring the child to the ED. The child was later discovered unresponsive, lying on her bed and was pronounced dead at the scene
Autopsy Findings: Autopsy showed pulmonary edema. Iliac blood free buprenorphine was 2.5 ng/mL, and free norbuprenorphine was 4.3 ng/mL. Vitreous ethanol level was 19 mg/dL. Cause of death: buprenorphine intoxication. Manner of death: homicide, owing to failure of caregiver to follow medical advice.
Case 495. Chronic acetaminophen ingestion: undoubtedly responsible.
Scenario/Substances: A 27 y/o 71-kg female presented to the ED with complaints of stomach pain and was admitted. She reported received 2.6 g acetaminophen on Day 1 and 2.95 g on Day 2. On Day 3, she had an episode of loss of consciousness, hypoglycemia, and a possible seizure. It was later determined that she had been taking acetaminophen/oxycodone and acetaminophen (5 bottles) over the past several months. Her mother had passed away 5 months prior, she lost her job and had been having suicidal thoughts for which she had seeing a psychiatrist. Needles and syringes were found in her purse.
Past Medical History: Anxiety, depression, possible substance abuse, and gastric bypass surgery previous year. Medications: sucralfate, misoprostol, pantoprazole, hydromorphone, and acetaminophen/oxycodone.
Laboratory Data: ABG-pH 7.18 / pCO2 18 / pO2 256 / HCO3 6.8 on the ventilator., WBC 18.6, Hgb 10.9, Hct 32, platelets 235, Day 1: AST 27, ALT 54. Day 2: PT 12.9, INR1.1, BUN 5, Cr 0.5. Day 3: acetaminophen 123 mcg/mL, AST 2,074, ALT 1,355, bilirubin 3.4, albumin 2.6 g/dl, INR 5.9, ammonia 55, BUN 5, Cr 1, Glu 179, lactate 4.8. UDS negative for opiates. Day 4: AST 7,073, ALT 3,676, bilirubin 4.5, INR 8.9, ammonia 112, Day 5: AST 4,239, ALT 3,208, bilirubin 5.1, INR > 10, acetaminophen not detected.
Clinical Course: Vital signs (on ventilator): BP 123/65, pulse, 98, T, 37 degrees C, RR 15-16. She was moving all extremities, and pupils were 3mm, equal and reactive.
She was started on N-acetylcysteine (NAC) on Day 3 of admission and loaded with 10,500 mg and was scheduled to receive 50 mg/kg over the subsequent 4 h, the NAC dosing was then increased to 15 mg/kg/h and she was started on D10W infusion. On Day 3, she was transferred to a tertiary care hospital. She became hypotensive and received norepinephrine, vasopressin, and phenylephrine. Her transaminases continued to increase along with her INR. At this point, her family declared her a do-not-resuscitate (DNR). She was given phytonadione on Day 4, however, was having no active bleeding. On this same day, her NAC dose level was decreased despite being advised to maintain the current dose due to her critical clinical status and lack of indication for using the limited dose. Day 5 BP 105/50, HR 124, RR 11 on pressure support, T 38.1°C, O2 sat 95%. NAC was discontinued on Day 6. Based on the prognosis, the family opted for institution of comfort measures and she expired on Day 6.
Autopsy Findings: Not available.
Case 607. Acute salicylate ingestion: undoubtedly responsible.
Scenario/Substances: A 36 y/o male wrote suicide notes, ingested 500 tablets of 325 mg aspirin, and was presented to the ED ∼3 h later.
Past Medical History: Depression related to the death of his wife 2 years ago.
Clinical Course: The patient had nausea with hematemesis in the ED, and salicylate level was 84 mg/dL. He was transferred to a second hospital where his salicylate was 94 mg/dL, ABG-pH 7.45/pCO2 27/pO2 113/HCO3 19, K 4.3, and Cr 1.3. He was transferred to a tertiary care hospital for hemodialysis. His ABGs showed a mixed respiratory alkalosis with metabolic acidosis. Sodium bicarbonate was given. He was admitted to the ICU and experienced nausea, vomiting, and diarrhea for 2–3 h. He became confused, agitated, and combative. A repeat salicylate drawn an estimated 9 h after ingestion was 108 mg/dL. At 11.5 h after ingestion ABG-pH 7.22 / pCO2 38 / pO2 88 / HCO3 16. The renal team started dialysis, but the patient abruptly developed QRS widening and went into asystole. ACLS resuscitation was unsuccessful, and he died ∼12 h after ingestion.
Autopsy Findings: Not performed
Case 1057. Chronic colchicine ingestion: probably responsible.
Scenario/Substances: A 78 y/o male with multiple medical problems was discharged on colchicine for gout. He took as many as 15 tablets (0.6 mg each) over a period of 3–4 days. There was no evidence of an acute self-harm intent. He developed profuse diarrhea (7–8 stools/day) and weakness, and was brought back to the ED.
Past Medical History: Gout, end-stage renal disease on hemodialysis, hypertension, hypokalemia, leukopenia, thrombocytopenia, peptic ulcer disease, myocardial infarction, congestive heart failure, anemia, syncope, cardiogenic shock with PEA and VT arrest, methicillin-sensitive S. aureus (MSSA) sepsis. Medications included colchicine, allopurinol, aspirin, amiodarone, amlodipine, calcitriol, divalproex, pantoprazole, sevelamer, and simvastatin.
Physical Exam: He was frail-appearing but oriented, BP 94/73, HR 88, RR 27, O2 sat 93%, T 37.4°C.
Lungs clear, normal cardiac exam, and no abdominal distension.
Laboratory Data: Hgb 9.2, Hct 28.5, WBC 1.7, platelets 32,

AST 69, ALT 31, bilirubin 0.8, INR 1.7, troponin 0.4, lactate 7.4 mmol/L, CK 109. CxR showed R lung base opacity with small bilateral pleural effusion, and repeated CxR showed pulmonary edema.
Clinical Course: Patient continued to be hypotensive despite fluid resuscitation and multiple vasopressors and inotropes. He was intubated and placed on a ventilator. He had a junctional bradycardia with escape rhythm, and his ECG showed a new LBBB. He was treated with CVVH and a bicarbonate drip. He was given antibiotics for possible sepsis. He also received filgrastim for his leukopenia. His lactate level peaked at 27.9 mmol/L. He developed hepatic failure with peak AST 3,495, ALT 1,676, bilirubin 7.1, CK rose to 3,500. He died from multi-organ failure 24 h after admission.
Autopsy Findings: The ME reported colchicine 4.0 ng/mL from premortem hospital blood (1 hour after arrival in the ED).
Case 1085. Acute salicylate ingestion: undoubtedly responsible.
Scenario: An 11 m/o male was given a medicine bottle to play with by his parents and was later found with the open bottle of enteric coated 325 mg salicylic acid. The patient had orange residue on his face, 1 intact tablet was removed from his mouth by a family member, and he was brought to the ED.
Laboratory Findings: The 6-hour salicylate level was 107 mg/dL and would later peak at 123 mg/dl. Na 146, K 2.6, anion gap 29, Glu 712, BUN 13, Cr 1.2.
Clinical Course: In the ED, the patient was alert and age appropriate. HR 154, RR 30, T 37°C, O2 sat 100% on room air. Family initially reported that, at most, 7 tablets were unaccounted for. He vomited thrice with 2 aspirin tablets visible in the emesis. He was given activated charcoal, IV fluids, and sodium bicarbonate 40 meq/hr. He was admitted to the PICU where he became severely tachycardic (HR 221), tachypneic (RR 45) and hyperthermic (T 38.5°C). He experienced electrolyte abnormalities including hypokalemia, hypernatremia, and hyperglycemia. On Day 2, the patient was intubated in preparation for transfer to a HCF that could provide hemodialysis when he went into cardiac arrest and expired.
Autopsy Findings: Petechial hemorrhages of the heart, thymus, and brain. The brain had non-volumetric subdural and subarachnoid hemorrhages. The salicylate concentration of antemortem blood 7 h post ingestion was 850 mg/L (85 mg/dL). The manner and cause of death was accidental ingestion resulting in salicylate toxicity.
Case 1088. Acute methadone ingestion: undoubtedly responsible.
Scenario/Substances: Aunt of a 19 m/o female was watching the child while mom attended a recovery group meeting. When mom arrived home she noticed the child was tired, so she put her down for a nap. When mom went to wake child, she noticed her lips were blue so she took her to the ED.
Laboratory Data: UDS positive for methadone.
Clinical Course: Upon arrival to ED, child's skin was ashen and oxygen was given. UDS came back positive to methadone, naloxone was given, her color improved, and she became more alert. Continuous naloxone infusion was started at 25 mcg/kg/min. She was protecting her own airway. The next day, child developed respiratory depression, apnea, and her HR dropped to 80's. She was intubated using rapid sequence intubation with fentanyl, her HR improved, and naloxone infusion was continued. That evening, she went into acute respiratory failure and suffered a cerebral herniation. Emergency craniotomy was performed and drain inserted, but pressures in her brain remained high. Epinephrine, norepinephrine, and vasopressin were used for pressure support. She developed diabetes insipidus. Continuous EEG showed no activity. She was determined to be brain dead, and the organs were donated.
Autopsy Findings: Acute necrosis of brain tissue related to methadone toxicity. Pre-mortem: methadone 248 ng/mL, EDDP 13 ng/mL.
Case 1096. Acute sevoflurane inhalation: undoubtedly responsible.
Scenario/Substances: A 37 y/o male nurse anesthetist was found at home hooked up to an anesthesia machine with sevoflurane. Patient was found in cardiopulmonary arrest, was resuscitated, and intubated. Initial post-resuscitation rhythm was atrial flutter with rapid ventricular response. He had seizure-like activity and was given phenytoin
Past Medical History: Insomnia (reported to be using his anesthesia machine for sleep)
Laboratory Data: Initial Ca (ionized) 1.02. Toxicology screen for drugs of abuse and toxic alcohols was negative.
Clinical Course: He received Ca IV for low Ca, a calcium channel blocker IV for his atrial flutter, and was placed on 48 h post-resuscitation hypothermia protocol. BP 101/58, HR 89, O2 sat 100 % on O2, T 32°C. Head CT was consistent with anoxic brain injury. He remained paralyzed with cis-atracurium, received propofol for seizure and sedation, and was receiving norepinephrine for pressure support. After 2 EEGs, he was declared brain dead and his organs were made available for donation.
Autopsy Findings: Sevoflurane from blood drawn at admission 5.9 mcg/mL (upper reporting limit is 0.10 mcg/mL). Post mortem phenytoin 12 mcg/mL. No other injuries or pathology were found on autopsy.
Case 1100. Acute lidocaine parenteral: undoubtedly responsible.
Scenario/Substances: A 77 y/o female nursing home resident came to the ED for an unknown reason.
Past Medical History: COPD, hypertension, diabetes mellitus, seizure disorder, and s/p pacemaker placement.
Laboratory Data: K of 6.0 was reported, but ECG did not show signs of hyperkalemia.
Clinical Course: The patient was to receive 25 g dextrose and 10 U insulin for the hyperkalemia, instead she received an unknown amount (40–100 mg) of lidocaine IV.
Immediately after the bolus, she became unresponsive, possibly had a seizure, developed a wide complex bradycardia that her pacemaker did not capture, and BP 130's/80's. She received dextrose, Ca, and sodium bicarbonate to treat her hyperkalemia. She developed asystole during the next 30 min. ACLS was initiated, and the patient was given lipid emulsion, but she could not be resuscitated.
Autopsy Findings: Severe emphysema, dilated cardiomyopathy, and kidney disease. Lidocaine was 4.6 mg/L, cause of death was lidocaine toxicity, and type of death was accident (medication error).
Case 1102. Acute lidocaine ingestion: undoubtedly responsible.
Scenario/Substances: A healthy 13 m/o female was being cared for at home by her 16 y/o brother, while their parents were visiting her twin sister in the PICU at a tertiary care pediatric facility. This patient started having seizure activity, and her brother called 911. EMS arrived 9 min later to find her actively seizing, unresponsive, and cyanotic with shallow, agonal respirations. HR 150, RR 12, O2 sat 100% on room air. She was transported with bag–valve–mask ventilation.
Past Medical History: No prior medical problems or hospitalizations. The twins had unremarkable 1-year well baby checkup visits 1 week earlier. The patient's twin sister had been taken to the ED 2 days prior with seizures followed by cardiorespiratory arrest. She was resuscitated and transferred to the tertiary care pediatric hospital where she remained unresponsive and ventilator dependent. Neurologic and cardiac evaluations had not yielded the cause of her seizures and arrest.
Physical Exam: In the ED, she was dusky, foaming at the mouth, actively seizing, apneic, strong odor of stool, absent corneal reflex, abdominal distension, unresponsive, no signs of trauma, GCS 3, BP 131/99, HR 160's, apneic, O2 sat 86% on O2 via bag/mask
Laboratory Data: Glu 189, ECG rhythm strips: initial narrow-complex tachycardia, then narrow-complex bradycardia, then wide-complex agonal rhythm.
Clinical Course: In the ED, IV access was established arrival, and seizures resolved following 1 mg of lorazepam. She received 20 mg of succinylcholine for intubation. Within 3 min after these medications, she became progressively bradycardic and then pulseless. CPR was started and was intubated. She received 27 doses of epinephrine, 4 doses of atropine, 2 doses of bicarbonate, and 1 dose each of naloxone, glucagon, and calcium gluconate during the 90-min unsuccessful resuscitation. After her death, police investigated her home and found a empty bottle of viscous lidocaine 2% on the coffee table in the parlor. The medication had been prescribed to both siblings separately 3 months prior, for topical pain relief from teething. The twin sister in the PICU was found to have very high levels of lidocaine in her urine. One month later, the 16 y/o brother admitted that he had mistakenly been adding the lidocaine to the twins’ milk bottles to treat their teething pain.
Autopsy Findings: Autopsy failed to disclose an anatomic cause of death. Postmortem heart blood obtained 24 h after death: lidocaine was 6.4 mcg/mL, monoethylglycinexylidide (MEGX) was 4.1 mcg/mL. Both the concentrations are consistent with reported toxic levels. ME's final cause of death was most likely lidocaine toxicity and the manner of death accidental.
Case 1109. Chronic rivaroxaban ingestion: contributory.
Scenario/Substances: A 66 y/o male developed mild left upper quadrant pain, became pale, sweaty, weak, and had an episode of vomiting. EMS reported seizure-like activity lasting 15–20 seconds during transport to the ED.
Past Medical History: Hypertension, COPD, migraine headache, major depressive disorder, GERD, dementia, seizure disorder. S/p bilateral knee surgery, lower back surgery for degenerative disc disease, left hip fracture surgery (1 month prior). Medications: rantitidine, doxepin, memantine, lorazepam, citalopram, donepezil, levetiracetam, rivaroxaban 20mg PO daily (started 3 weeks prior), before that he was on enoxaparin)
Physical Examination: In the ED BP 58/39, HR 88, RR 20. The patient presented with pallor, diaphoresis, agitation, confusion, and altered mental status, and abdominal distention. Bowel sounds were present and stool was occult blood positive. Ecchymosis bilaterally in lower quadrant, left thigh area.
Laboratory Data: Electrolytes unremarkable, Glu 202, Hgb 10.5, Hct 33.7, WBC 10.4, PT 12.9, INR 1.2, PTT 30.
Clinical Course: Patient exhibited episodes of hypotension in the ED for which he was given IV fluids and placed on a low-dose phenylephrine drip; systolic BP increased to 90s–low100s. CT of chest and head was normal. CT of abdomen and pelvis showed hemoperitoneum with blood around the liver and spleen, without obvious liver or spleen lacerations and mild fusiform dilatation of the distal abdominal aorta without evidence of aneurysmal leakage. Initial Hgb was 10.5, and repeat was 7.8. Clinical impression was hemoperitoneum with hemorrhagic shock with coagulopathy from rivaroxaban. Four units of packed RBCs were given, and he was transferred to a tertiary care hospital via helicopter. During transport, infusions of packed RBCs and vasopressors continued. On arrival at the tertiary hospital, the patient was awake, alert, and pale with some abdominal distention. The patient remained normotensive with systolic BP 104 on phenylephrine. The trauma team ordered reversal of the rivaroxaban with 5,000 units of prothombin complex plus IV vitamin K. Patient was intubated 6 hour (tertiary care hospital) and received multiple blood products including packed RBCs, FFP, and prothombin complex concentrate. He remained hemodynamically unstable on pressors with low Hgb. EEG showed no activity. Based on the prognosis, the family opted for institution of comfort measures and he expired on Day 2.
Autopsy Findings: Not performed.
Case 1111. Acute-on-chronic enoxaparin subcutaneously: contributory.
Scenario/Substances: A 73 y/o male was inadvertently given enoxaparin Q 2 h instead of Q 12 h as prescribed s/p hip fracture complicated by deep vein thrombosis. He received a total of 320 mg subcutaneously and presented with bleeding from his gums, epistaxis, and hemoptysis.
Past Medical History: Alzheimer's dementia, alcoholic cardiomyopathy, cirrhosis, and anemia of chronic disease.
Physical Exam: Alert and oriented, BP 100/63, HR 106.
Laboratory Data: WBC 14.7, Hgb 8.5 g/dL, Hct 25.7 %, platelets 389, PT 16, INR 1.2, PTT 49.5.
Clinical Course: Patient expired from an acute GI bleed on Day 1 despite administration of FFP, IV fluids, and packed RBCs.
Autopsy Findings: Not performed.
Case 1136. Acute valproic acid ingestion: undoubtedly responsible.
Scenario/Substances: A 63 y/o female's sister called police for a welfare check when the patient did not show up for a scheduled visit. Police and EMS entered into the home, found the patient unresponsive with pin point pupils, and transported her to the ED.
Past Medical History: Bipolar disorder, anxiety, and paranoia; previous suicide attempt was with aspirin when she was 20 y/o.
Laboratory Data: Initial complete blood count, metabolic panel, and liver transaminases were unremarkable. Serum acetaminophen, salicylates, and ethanol levels were not detected; UDS negative. Ammonia 346, later 195 and finally, at 43 Hour 37. Valproic acid > 300 throughout her hospital course. ECG was unremarkable.
Clinical Course: In the ED, BP 70/40, HR 65 with a depressed level of consciousness. She was intubated and placed on mechanical ventilation. She received 3 L NS and was started on an IV norepinephrine infusion for hypotension. The patient was empirically started on levocarnitine. With maximum doses of norepinephrine, phenylephrine, and epinephrine: BP 119/87, HR 93. Hemodialysis was initiated for persistently elevated valproate, but she expired on Day 3.
Autopsy Findings: Acute bilateral pneumonia, acute hemorrhagic pancreatitis with retroperitoneal soft tissue hemorrhage, mild CAD, and moderate hepatic microvesicular steatosis. Antemortem blood valproic acid 970 mg/L. Cause of death: complications of valproic acid intoxication, manner of death: suicide.
Case 1183. Chronic lithium ingestion: undoubtedly responsible.
Scenario/Substances: A 35 y/o found at home by significant other, lethargic, and responsive with altered mental status.
Past Medical History: Bipolar disease, anxiety.
Physical Exam: Awake, agitated, shivering, maintaining her airway, pupils equal, and reactive to light, fine-hand tremor, hyperreflexia, and no seizure activity. BP 159/96, HR 80, RR 22, O2 sat 100% on room air, T 38.6°C.
Laboratory Data: ABG-pH 7.28 / pCO2 20 / pO2 103,

Serum acetaminophen, ethanol and salicylate not detected. Lithium 4.4, ECG: sinus rhythm, QRS 102, QTc 557.
Clinical Course: She received three doses of lorazepam IV for agitation. Renal failure and anion gap metabolic acidosis developed. She was intubated for airway protection. NS was given at 2 X maintenance rate. One hour after emergent hemodialysis ended, she became acutely bradycardic (HR 40s) and hypotensive (SBP 70) and required norepinephrine. Repeat electrocardiogram revealed sinus bradycardia with QRS of 110 and QTc 641. Vasopressors were continued with mild improvement in BP. On Day 2, she remained intubated and unresponsive not requiring any sedation. Repeat lithium was 1.4. On Day 4, the patient was declared brain dead. Based on the prognosis, the family opted for institution of comfort measures and she expired on Day 4.
Autopsy Findings: Not performed
Case 1200. Acute-on-chronic bupropion, diltiazem (extended release), and prednisone ingestion: undoubtedly responsible.
Scenario/Substances: A 42 y/o female was found at home with empty bottles of bupropion, diltiazem, and prednisone nearby.
Past Medical History: Current medications: zolpidem, clonazepam, and citalopram.
Laboratory Data:
Na 145, K 3.5, Cl 111, CO2 21, K 3.4, Glu 593, lactate > 7 mmol/L. ABG-pH 7.27 / pCO2 29.6 / pO2 80.3 / HCO3 13.4 on 3 L nasal cannula; serum acetaminophen and ethanol not detected, UDS positive to amphetamines and benzodiazepines.
Clinical Course: In ED, patient was initially lethargic but arousable and able to speak in complete sentences. She eventually became completely unresponsive with dilated pupils and hypotension (BP 60/40) which did not correct with fluid bolus. Patient started on norepinephrine with no response so glucagon 3 mg bolus was given and infusion of 5 mg/h started with BP responding to 83/36. She was also given ondansetron, pantoprazole, lorazepam, and sodium bicarbonate for her acidosis. Day 1 ECG: sinus tachycardia, rate 120, PR 126, QTc 522. Patient had two 10-sec tonic-clonic seizures, was intubated, and developed severe bradycardia and cardiac arrest. A temporary transcutaneous pacemaker was inserted. She became severely hypotensive with mottled skin, and no perfusion, coded again, and resuscitation was unsuccessful. Mouthful of blue and white granules/undissolved pills was discovered when endotracheal tube removed post-expiration.
Autopsy Findings: Autopsy demonstrated pill fragments in the mouth, esophagus, stomach, and small intestine along with moderate pulmonary congestion and edema. Postmortem vena cava blood bupropion > 10 mg/L, threo bupropion > 10 mg/L. Liver bupropion 14 mg/kg, threo bupropion 150 mg/kg. Antemortem blood bupropion 1.5 mg/L and threo bupropion 5.6 mg/L, diltiazem detected. Cause of death was bupropion toxicity.
Case 1268. Acute amitriptyline and diphenhydramine ingestion: undoubtedly responsible.
Scenario/Substances: A 9 m/o male was placed in his car seat to sleep the night and found unresponsive in the morning.
Past Medical History: Cystic mass in the lower lobe of his right lung, which was diagnosed in utero.
Clinical Course: Pulseless, with evidence of lividity. CPR was initiated, and an intra-osseous line was placed for fluids (25% dextrose) and 1 dose of epinephrine before resuscitation efforts were halted.
Autopsy Findings: A cystic mass involved the right lower lobe with microscopic findings suggestive of extra lobar sequestration. He had acute bronchopneumonia consistent with a period of obtunded survival and mild–moderate cerebral edema. Toxicology: amitriptyline 3.5 mg/L heart blood, 46 mg/kg liver), nortriptyline (1.7 mg/L heart blood, 28 mg/kg liver; diphenhydramine 1.9 mg/L heart blood, 8.3 mg/kg liver. These levels were felt to be inconsistent with exploratory ingestion by a 9-month old and not consistent with the initial history. The cause of death was ruled as amitriptyline and diphenhydramine toxicity with the manner of death being homicide.
Case 1272. Acute diphenhydramine ingestion: probably responsible.
Scenario/Substances: A 12 m/o 10-kg female was found with a bottle of mother's 50 mg diphenhydramine liquid gel caps. Most of the tabs were missing but exact amount unknown. The mother took the child to the closest ED.
Past Medical History: Previously healthy, no surgeries, no daily medications.
Laboratory Data: pH 7.2, Na 139, K 4.6, CO2 22, BUN 20, Cr 0.1, Glu 99, Ca 9.2, CK 261. Serum acetaminophen and salicylate were not detected.
Clinical Course: Upon arrival to the ED, the patient was awake, irritable, and tachycardic. She had a seizure and received multiple doses of midazolam. ECG showed VT at a rate of 213, QRS 160. She was given 2 boluses of 2 mEq/kg sodium bicarbonate and started on an IV infusion. The QRS narrowed to 92, ST elevation was noted in leads II, AVF, V2, V6, and QTc was 420. ECG showed sinus rhythm with PVCs. Seizure activity ceased, the patient was somnolent and intubated for airway protection after vomiting. Post intubation, she developed bradycardia, and PALS protocol was initiated; 10 ml of lipid emulsion (1 mL/kg) plus PALS medications (epinephrine, atropine) were administered. Bradycardia (HR 30–40s) persisted and lipid emulsion was repeated, while PALS was in progress. Resuscitation was unsuccessful, and she was pronounced dead 4 hours after exposure.
Autopsy Findings: Not available.
Case 1288. Acute diphenhydramine ingestion: undoubtedly responsible.
Scenario/Substances: A 43 y/o female took 325 × 25 mg diphenhydramine, spoke to her family at noon, but family was unable to contact her later in the day. EMS arrived to find her pulseless, intubated her, and started CPR.
Past Medical History: Diabetes mellitus, breast cancer, CAD, chronic renal disease, hyperlipidemia, COPD, hypertension, allergies, anemia, GERD, arthritis, hypothyroidism, and seizures. She had a long history of depression and, according to her family, was refusing medical treatment. Medications included ergocalciferol and loratadine.
Physical Exam: BP 76/42, HR 106, RR 18, left frontal abrasion, fixed and dilated pupils, absent pulses, equal breath sounds, unresponsive, GCS 3, skin warm, dry mucous membranes, absent bowel sounds, no corneal/gag reflex.
Laboratory Data: ABG-pH 6.91 / pCO2 68 / pO2 94

Hgb 12.4, WBC 13.6, platelets 282, AST 610, ALT 520, bilirubin 0.5, INR 3.4, lactate 16.8, CK 13,801, troponin 1.89, HCG negative, UDS negative, serum positive for caffeine and diphenhydramine, acetaminophen and salicylate not detected. CxR: right upper lobe atelectasis, CT C-spine: negative, CT head: diffuse cerebral edema
ECG: QRS 122, QTc 477.
Clinical Course: In the ED, she was having intermittent loss of pulses. She was given bicarbonate IV push and started on a continuous infusion. Her BP remained low despite maximum epinephrine and vasopressin was added. She was given IV lipid emulsion with transient improvement in her BP and ECG. In the ICU, she developed DIC with epistaxis and oozing blood from puncture sites; Hgb 8.3; Cr 2.2; and troponin 6.8. She was given FFP and was not felt to be a candidate for hypothermia protocol. Early on Day 2, she developed asystole and expired.
Autopsy Findings: Diffuse bronchopneumonia, autolysis of the spleen and pancreas, and cerebral edema. Heart blood diphenhydramine 28,000 ng/mL. This level is consistent with levels reported in fatalities. Cause of death: drug overdose with complications. Manner of death was suicide.
Case 1301. Acute-on-chronic amantadine ingestion: probably responsible.
Scenario/Substances: A 65 y/o female took “a lot of red pills,” was found the next morning unresponsive with shallow respirations. EMS arrived, intubated, and transported the patient. During transport, generalized seizure activity was noted. A review of the patient’s medications led to belief that the patient had overdosed on 100-mg amantadine tablets, but amount was unknown.
Past Medical History: Hypothyroidism, chronic pain, reflux, hyperlipidemia, depression. Medications: amantadine, levothyroxine, pravastatin, citalopram, carvedilol, gabapentin, oxybutynin, omeprazole, sertraline, tramadol, vitamin D, and acetaminophen/hydrocodone.
Laboratory Data: AST 23, ALT 11, bilirubin 0.2, CK 120,
Serum acetaminophen and salicylates were not detected, UDS positive for cocaine.
Clinical Course: In the ED, the patient was placed on ventilator, BP 122/72, HR 68, RR 12 (ventilator). Generalized seizure activity was treated with lorazepam. Initial ECG QRS 128, QTc 540. Her K level was repleted, she was transferred to the ICU, had another seizure, and sodium bicarbonate was given IV and an infusion started at 100 mL/hour. Without sedation, she would grimace in response to sternal rub and gag on endotracheal tube when stimulated. BP 175/71, HR 61, T 36°C. ECG Day 2 normal QRS QTc of 585. Na 132, K 3.0, Cl 94, CO2 28, BUN 14, Cr 1.1, Glu 88. She demonstrated intermittent bursts of VT. Sedation with fentanyl and midazolam infusions was started and then with antibiotics (vancomycin and piperacilln–tazobactam). On Day 2, the patient developed recurrent seizures and was started on with levetiracetam and valproic acid. She also had a period of hypotension that improved with saline bolus and phenylephrine. She had increasing oxygen requirement on the ventilator with FiO2 of 80%. Her urine output decreased and urine became dark in color. K 6.0 treated with calcium gluconate, Kayexalate, furosemide and insulin. She developed a widened QRS with bradycardia. Hemodialysis was started on Day 4 for worsening hyperkalemia. On Day 5, she became tachypneic (RR 24) and pH 7.24. Sedation was changed to propofol and reduced on Day 7, but she remained without purposeful movements. Diltiazem was initiated for cardiac ectopy. EEG on Day 10 demonstrated anoxic encephalopathy. Based on the prognosis, the family opted for institution of comfort measures and she expired on Day 11.
Autopsy Findings: The ME reviewed the case, but no autopsy or body viewing was performed. Cause of death was undetermined with cocaine abuse as a contributing factor and some consideration to “drug overdose”.
Case 1307. Acute-on-chronic methotrexate ingestion: probably responsible.
Scenario/Substances: A 82 y/o female received 2.5 mg of methotrexate per day instead of 2.5 mg thrice per week of methotrexate for 1 month at her extended care facility. She was admitted to the hospital with renal failure, mucositis, neutropenia, and infection. The error was discovered, and methotrexate dosing was stopped.
Past Medical History: Arthritis, diabetes, hypertension, colon cancer, and chronic kidney infections.
Laboratory Data: WBC 0.5, Hgb 9.8, Hct 28.8, RBC 3.22, Platelets 3,000, BUN 86, Cr 4.2 (1 year prior BUN 52, Cr 1.8).
Clinical Course: She was initially awake alert, but drowsy and slightly confused after receiving analgesics. HR 111, BP 127/69, RR 19, O2 sat 98% on 2L O2, T 37°C. She received several units of platelets daily and leucovorin 100 mg/6 h IV. Her urine output was low (60 cc per 8 hrs) on IV furosemide. Based on the prognosis, the family opted for institution of comfort measures on Day 36. By Day 37 she had developed sores all over her body, on her arms, legs and in the area of her perineum which opened up. Her WBC 4.6, platelets 22, BUN 114, Cr 4.2, K + 4.2. On Day 38, she was transferred to hospice and expired.
Autopsy Findings: Not done.
Case 1318. Chronic nitroprusside parenteral: contributory.
Scenario/Substances: A 23 y/o female was admitted to the ICU with acute decompensated heart failure and started on with nitroprusside infusion x 3 days with mild improvement in her condition. On Day 3, she suffered a PEA arrest with return of spontaneous circulation after 15 min of ACLS resuscitation, after which she required multiple vasopressors.
Past Medical History: Congestive heart failure, severe dilated cardiomyopathy of unknown cause, methamphetamine abuse.
Physical Exam: Lethargic, but arousable to voice, jugular venous pulses elevated to angle of jaw, bibasilar crackles and diminished breath sounds, S3 present, 2/6 systolic murmur at apex, abdominal ascites with distension and positive hepatojugular reflex, diffuse lower extremity edema and anasarca, poor capillary refill with cool fingers/toes. BP 109/69, HR 147, RR 30, O2 sat 98% on 3L O2, T 38.7°C.
Laboratory Data: ABG-pH 7.32 / pCO2 67 / pO2 36, lactate 13.7 mmol/L. UDS negative for amphetamine and methamphetamine. Blood: nitroprusside: 7,170 ng/L, cyanide (pre-treatment) 6.289 mg/L, cyanide (post-treatment) 0.128 mg/L.
Clinical Course: Cyanide toxicity considered, cyanide levels sent, and patient treated with 300 mg sodium nitrite and 12.5 g sodium thiosulfate, followed by second dose of 150 mg sodium nitrite and 6.25 g sodium thiosulfate. The patient did not fully recover from the PEA arrest, developed acute renal failure treated with hemodialysis, and required increasing vasopressors to maintain perfusion. Based on the prognosis, the family opted for institution of comfort measures, and she expired on Day 4.
Autopsy Findings: Not performed.
Case 1381. Unknown, amlodipine/benazepril ingestion: undoubtedly responsible.
Scenario/Substances: A 51-year-old female ingested unknown quantities of amlodipine/benazepril 10/20 and presented to the ED complaining of blurred vision.
Physical Exam: BP 122/100, HR 84, RR 14, O2 sat 95% on room air.
Laboratory Data: Ca 9.4, Mg 2.0, AST 13, ALT 18, PT 14.1,

INR 1.13, serum acetaminophen, ethanol, and salicylate not detected.
Clinical Course: Shortly after ED arrival, the patient became hypotensive to BP 51/38, HR 84. Calcium gluconate, glucagon, norepinephrine, bicarbonate, and atropine were given. The patient remained awake and oriented at 6 h. At Hour 7, HR 73, BP 93/59, RR 23, O2 sat 96% on 2L O2. A high-dose insulin infusion was initiated at 60 U/h, with supplemental glucose. Dobutamine and then vasopressin were administered. Attempts to wean insulin were followed by sudden hypotension. Insulin was increased to 2U/kg/h with BP 93 systolic. At Hour 52 the patient suffered a cardiac arrest, was resuscitated and had multiple episodes of bradycardia and repeated cardiac arrests. She expired on Day 3.
Autopsy Findings: The cause of death was polysubstance overdose. The manner was suicide.
Case 1407. Acute verapamil ingestion: undoubtedly responsible.
Scenario/Substances: A 59 y/o male was brought to the ED after his family noticed an altered level of consciousness. EMS found him hypotensive and bradycardic. They applied an external pacemaker and transported him to the ED.
Past Medical History: Hypertension, hyperlipidemia, migraines, and benign prostatic hypertrophy. Medications included verapamil, sumatriptan, lisinopril, topiramate, tamsulosin, terazosin, methocarbamol, pravastatin, and aspirin.
Physical Exam: On arrival to the ED, he was intubated for impending respiratory failure. BP 40's, HR 30's. He had a brief period of cardiac arrest which responded to CPR and epinephrine. Dopamine infusion was started, and he was admitted to the ICU for a suspected verapamil overdose.
Laboratory Data: ABG-pH 7.12 / pCO2 48 / pO2 113,

Hgb 10.9, WBC 16.8, platelets 214, lactate 6.8 mmol/dL,
UDS positive for methamphetamines, MDMA, amphetamines, and phencyclidine. Initial ECG showed complete heart block with intraventricular escape rhythm.
Clinical Course: In the ICU, a transvenous pacemaker was placed. Several doses of IV Ca were given, and broad-spectrum antibiotics were started. Dopamine, epinephrine, vasopressin, and sodium bicarbonate infusions were given. The poison control center recommended continued use of IV calcium, and starting high-dose insulin plus dextrose infusions. Despite normalization of his BP and HR, he remained unresponsive. Head CT showed cerebral edema most likely secondary to anoxic encephalopathy, and scan showed no brain blood flow. Neurology was consulted, and he was declared brain dead on Day 4
Autopsy Findings: Hospital blood was positive for caffeine, lidocaine, midazolam, topiramate, verapamil, phenylpropanolamine, amphetamine, and methamphetamine. Verapamil 1,500 ng/mL, topiramate 3.4 mcg/m, midazolam 15 ng/mL, phenylpropanolamine 22 ng/mL, amphetamine 250 ng/mL, methamphetamine 850 ng/mL. Cause of death: medication overdose along with use of controlled substance. From the laboratory results and the clinical course, it is most likely that he died as a result of acute verapamil overdose.
Case 1411. Acute-on-chronic diltiazem ingestion: undoubtedly responsible.
Scenario/Substances: A 60 y/o male ingested ∼90 diltiazem 180 mg extended release tablets, had a seizure at home (∼1 min) witnessed by family. He denied co-ingestants. An empty bottle found at the scene had been filled (90 tablets) earlier that day.
Past Medical History: Depression, anxiety, previous suicide attempts, diabetes, hypertension, liver cancer, COPD, diabetic neuropathy, degenerative joint disease, hyperlipidemia, multiple falls, myofascial pain dysfunction syndrome, radiculopathy to bilateral lower extremities after laminectomy, shoulder pain, transient ischemic attack, GERD, lumbago with chronic back pain, BPH, urinary retention.
Physical Exam: He was postictal for 10–15 min, waking minimally able to verbalize complaint of back pain, was tremulous and hypotensive. BP 70/40, HR 67, RR 18, O2 sat 94% on 4 L of oxygen.
Laboratory Data: WBC 11.5. AST 8, ALT 26. calcium 8.3,

Mg 2.1, troponin 0.01, lactic acid 7.0 mmol/l, albumin 3.3, Serum acetaminophen and salicylate not detected, UDS positive for benzodiazepines.
Clinical Course: Pupils were 3 mm, and he had jerky movements. He was treated with calcium gluconate, and glucagon with no response and was given lorazepam for seizures. IV fluids were started with no improvement in hypotension; norepinephrine was added with little response. ECG showed AV dissociation with accelerated junctional rhythm, QRS 96, QTc 442. He had a tonic-clonic seizure that resolved with a dose of lorazepam. After the seizure, he was alert with slurred speech. BP 95/37 (rapidly falling to 62/43), HR 64, RR 14, and O2 sat 93% on 4 L of oxygen. Activated charcoal was given Hour 3. He complained only of generalized weakness. Head CT was negative for acute pathology. He was treated with D50W with high-dose insulin, but it was initiated at the time that a bradysystolic cardiac arrest occurred. Hour 5.5. CPR with epinephrine, atropine was unsuccessful.
Autopsy Findings: Urine positive for caffeine, benzodiazepines, gabapentin, lidocaine, and nicotine. Urine oxycodone 0.17 mg/L, oxymorphone 0.022 mg/L. Urine oxycodone and oxymorphone concentrations consistent with normal use. Antemortem blood diltiazem 8.5 mg/L. ME listed the probable cause of death as diltiazem toxicity with contribution from hypertension, TIA/Stroke, COPD and diabetes, with the manner being suicide.
Case 1501. Acute sodium bicarbonate ingestion: undoubtedly responsible.
Scenario/Substances: A 33 y/o male ingested large quantities of sodium bicarbonate to cleanse his system prior to drug testing. Found unconscious in bed by his significant other and transported to the ED.
Laboratory Data: Initial labs: ABG-pH 7.56 / pCO2 51.8 / pO2 83 / HCO3 44.9 / BE 20.1 on FiO2 70%. Ca 11.6, Hgb 19.3, WBC 19.3,

anion gap 53, Mg 4.3, ammonia 39, serum acetaminophen, ethanol and salicylate not detected, UDS negative. Hour 6: Na 167, CK 1,602. Hour 12: Na 166, K 3.0, Glu 194, Cr 2.36, Cl, 119, CO2 37, Ca 7.9. Hour 24: Na 164, K 3.4, Cl, 131, Glu 138, Cr 2.31, Ca 8.0, CK 2,979.
Clinical Course: In the ED seizures developed, given levetiracetam and phenytoin and intubated. Initial ECG QT/QTC 300/463. BP 80/43, HR 104, RR 44, O2 sat 94%. He received etomidate, lidocaine, lorazepam, propofol, vecuronium, and D50W. CT showed diffuse extensive subarachnoid hemorrhage, mild, dilation of temporal horns of the lateral ventricles. Hemorrhage was thought to be owing to massive osmotic shift as a result of the sodium load. Patient was transferred to a tertiary care hospital where he received pentobarbital but was still having seizures per EEG. Received piperacillin and tazobactam for suspected aspiration. HR 130, BP 117/67, T (bladder) 39.3°C. Based on the prognosis, the family opted for institution of comfort measures and he expired on Day 3.
Autopsy Findings: Not available.
Case 1546. Unknown, carisoprodol and meloxicam ingestion: undoubtedly responsible.
Scenario/Substances: A 41 y/o female took unknown amounts of meloxicam and carisoprodol, was found unresponsive 1.5 h after talking to a friend. She was found to be in cardiac arrest, and transported to the ED.
Past Medical History: Non-insulin dependent diabetes mellitus, systemic lupus erythematosus, hypertension, bipolar disorder, fibromyalgia, and depression.
Laboratory Data: K 2.9, Mg 1.5, UDS positive for tricyclic antidepressants, serum acetaminophen was not detected.
Clinical Course: In the ED, she was intubated, and naloxone was given with no response. She was also given flumazenil, epinephrine, and dopamine. ECG showed sinus tachycardia with depression in the lateral leads. Systolic BP 160, HR 120, and she had a metabolic acidosis. She was transferred to a tertiary care hospital and admitted to the ICU. She was on norepinephrine and phenylephrine, and her urine output was characterized as “good”. She received potassium replacement for hypokalemia, was placed on post cardiac arrest cooling protocol and started on propofol with BP 97/72, HR 70, T 32.8°C. On Day 2, she was rewarmed and sedation was stopped HR 102, BP 110/65. She had no neurological activity, consistent with anoxic brain injury. Based on the prognosis, the family opted for institution of comfort measures and she expired on Day 3
Autopsy Findings: Ischemic brain injury, bronchocentric pneumonia and pulmonary edema. Urine from hospital admission positive for amitriptyline, diphenhydramine, nicotine, nortriptyline. Antemortem (Day 1) serum: 7-aminoclonazepam, < 0.020 mg/L; carisoprodol, < 16 mg/L; and meprobamate, 46 mg/L. Antemortem (Day 1, sample 1) blood: carisoprodol 19 mg/L, diphenhydramine < 0.25 mg/L, and meprobamate 35 mg/L. Antemortem (Day 1, sample 2) blood carisoprodol 6.7 mg/L, lamotrigine < 4.0 mg/L, nortriptyline < 0.25 mg/L, and meprobamate 43 mg/L, amitriptyline not detected. The ME-determined cause of death was hypoxic ischemic brain injury owing to meprobamate toxicity, more likely the death was due to carisoprodol toxicity with contribution from its metabolite, meprobamate since the parent compound carisoprodol was found on multiple samples as well as being initially suspect from the history.
Case 1643. Acute pentobarbital/phenytoin, embutramide/ mebezonium/tetracaine parenteral: undoubtedly responsible.
Scenario/Substances: A 59 y/o female veterinarian was found unresponsive in her veterinary clinic. She appeared to have injected herself with either pentobarbital/phenytoin or embutramide/mebezonium iodide/tetracaine solution as a suicide attempt. She was intubated in the field for respiratory arrest. She received naloxone prior to arrival at the ED with no response.
Past Medical History: Depression, dementia.
Physical Exam: Unresponsive on ventilator, BP 90/60, HR 90.
Laboratory Data: ABG-pH 7.3 / pCO2 42 / pO2 200, Na 134, K 2.9, Cl, 102, CO2 24, BUN 20, Cr 1; phenytoin 6 mcg/mL, phenobarbital 4 mcg/mL, valproate 33.6 mcg/mL; serum acetaminophen and salicylate not detected.
Clinical Course: In the ED, she received flumazenil without response. She remained hypotensive and bradycardic, and received IV fluids and multiple vasopressors without measurable effect. She had a cardiac arrest and died on Day 3.
Autopsy Findings: Postmortem toxicological tests included hospital blood levels of lorazepam 18.9 ng/mL, valproic acid 19.5 mcg/mL, mirtazapine 9.7 ng/mL, caffeine-positive, pentobarbital 74.3 mcg/mL, venlafaxine 149 ng/mL, norvenlafaxine 503 ng/mL, and urine pentobarbital > 10, 000 ng/mL. Cause of death was reported as pentobarbital toxicity, and manner of death is reported as suicide.
Case 1671. Acute hallucinogenic amphetamine ingestion: undoubtedly responsible.
Scenario: A 17 y/o male snorting “bath salts” at a party experienced seizure activity, and was found unconscious. EMS-administered benzodiazepines, paralyzed, endotracheally intubated, and transported the patient to the ED. “Bath salts” were found at the party by law enforcement
Clinical Course: The patient had a cardiac arrest upon arrival to the ED. His pupils remained dilated and non-reactive. Upon return of spontaneous circulation, his BP was 70/40 on vasopressors, HR 75, T 37°C, RR 24 (ventilator), O2 sat 89% on 100% FIO2. ABG-pH 7.12 / pCO2 48 / pO2 53 / HCO3 15.3, K 8.1, Hgb 5.3, Hct 15.8, CK 689, troponin I 2.3, serum acetaminophen, ethanol, and salicylate not detected. The UDS was negative for amphetamines. The patient was admitted to the ICU, place on a sodium bicarbonate drip, epinephrine, norepinephrine, phenylephrine and amiodarone. He developed DIC, and was given FFP and cryoprecipitate. Lipid emulsion therapy was given for persistent hemodynamic instability. He expired on Day 1.
Autopsy Findings: Evidence of DIC, diffuse organ failure, massive pulmonary edema, bilateral pleural effusions, small subdural hematoma, and anoxic brain abnormalities. Cause of death: 2, 5-dimethoxy-N-(2-methoxybenzl) phenethylamine derivative (NBOMe) toxicity. Analysis of powder residue on the patient confirmed the substance. The drug was not detected in the patient’s Hour 12 blood. amphetamines found in his blood and urine on medical examiner screens were thought to be NBOMe metabolites. Manner of death was accidental.
Case 1687. Acute methylenedioxy-methamphetamine (MDMA) ingestion: undoubtedly responsible.
Scenario/Substances: A 19 y/o female collapsed at the nightclub after ingesting “Molly”. EMS on scene noted apnea and a weak pulse, rescue breathing was performed, and she was transported to the ED.
Physical Exam: The patient was seizing and unresponsive.
Laboratory Data: Na 155, K 6.5, Cl 110, anion gap 21, BUN 14, Cr 1.99, Ca 8.0, Mg 2.5, Hgb 12, WBC 17.0, platelets 296, PT 18, INR 1.45, PTT 31.7, CK 523, CKMB 6.0, troponin 0.3. Acetaminophen and salicylate were not detected, UDS was negative. INR 10 and amylase > 1000 U/L 12 h later.
Clinical Course: In the ED, the patient was diaphoretic, T 39.7°C (rectal), pupils dilated, minimally responsive; was intubated, sedated with midazolam, ventilated. Phenytoin was given for “seizure-like activity”. BP 70/30. IV fluids with bicarbonate were administered with little improvement in BP so vasopressor support was initiated and she was admitted to the ICU. After 12 h, despite the use of maximal vasopressor support, her BP was 80/27, HR 100s, and urine output was minimal. Blood products and N-acetylcysteine were administered. The patient suffered a cardiac arrest and expired 17 h after admission to the hospital.
Autopsy Findings: Cause of death was 3, 4-methylenedoxymethamphetamine (MDMA) intoxication.
Case 1691. Acute hallucinogenic amphetamine (methylone) ingestion: undoubtedly responsible.
Scenario/Substances: A 20 y/o male and was found down while attending a concert. When seen previously by his friends, he had exhibited an increased RR.
Past Medical History: Good general health.
Physical Exam: Obtunded, GCS 3, HR 140, T normal on presentation.
Laboratory Data: Na 139, later 146, K 7.1, later 2.7, Cr 2.5, pH 7.02, CO2 15, platelets 36, PT > 120 s, PTT > 180 s, AST 494, ALT 164, Alk phos 90, bilirubin 2.0, CK initially normal, later 8,900. lactate 4 (decreased from earlier peak)
Clinical Course: He was intubated for airway protection, T 41.7°C, later 38.9°C within 1 hour of treatment. The patient received N-acetylcysteine IV for fulminant hepatic failure. The patient developed bradycardic/PEA arrest 2–3 times during the initial phases of treatment which responded to ACLS. Despite aggressive supportive care, the patient experienced an uncontrollable hemorrhage on Day 1 secondary to DIC and subsequently experienced a cardiac arrest from which he could not be resuscitated.
Autopsy Findings: Cause of death was hyperthermia due to methylone intoxication and environmental exposure. Heart blood contained 0.71 mg/L methylone and cannabinoids were detected.
Case 1724. Hallucinogenic amphetamine ingestion: undoubtedly responsible.
Scenario/Substances: A 23 y/o male found obtunded after a reported ingestion of “Molly” while at an electronic music concert was brought to the ED by EMS.
Physical Exam: Obese, diaphoretic, GCS 3, pupils dilated and reactive, lower extremity rigidity. Initial BP 88/58, HR 160, T 42.7°C, respirations agonal.
Laboratory Data: ABG-pH 7.15 / pCO2 48 / pO2 141 / HCO3 16, O2 sat 98%, Hgb 15, Hct 44, WBC 16, lactate 10,

anion gap 21, troponin 0.27, UDS negative for cocaine, barbiturates, benzodiazepines, opioids, phencyclidine, and cannabinoids.
Clinical Course: In the ED, the patient was intubated via rapid sequence intubation with etomidate, rocuronium, and midazolam. T was reduced from 42.7–39.4°C over ∼30 min with a cooling blanket and 6L cold NS. He remained hypotensive despite fluid resuscitation, was transferred to the ICU where he was found to pulseless. Resuscitative efforts lasting 50 min were unsuccessful.
Autopsy Findings: Final diagnoses: Acute intoxication with methylenedioxy-methamphetamine and methylone with hyperthermia, cardiac hypertrophy with left ventricular hypertrophy, aortic atherosclerosis, slight, obesity. Cause of death: Acute intoxication by the combined effects of methylenedioxy-methamphetamine and methylone with hyperthermia. MDMA 2.6 mg/L, methyleneoxyamphetamine 0.12 mg/L, methylone 0.22 mg/L. Manner of death: Accident (substance abuse).
Case 1783. Cocaine and levamisole exposure: probably responsible.
Scenario/Substances: A 28 y/o white female was found unresponsive, lying in her feces and urine at home, EMS was summoned by her significant other. When EMS arrived, they found the patient not breathing and pulseless. They started bagging her with a bag valve mask, performed cardio pulmonary resuscitation and within a minute were able to feel a pulse. Because of emaciation and necrotic limbs, the emergency medicine team did not attempt to place IV access or an intraosseous needle before transport.
Past Medical History: Chronic IV drug use including heroin, cocaine, and methamphetamine. She presented several weeks prior to another hospital with skin lesions strongly suggestive of vasculitis.
Laboratory Data:

anion gap measured 24, Ca 7.9, Ca (ionized) 1.22, Phos 14, Mg 3.2, Tprot 5, albumin 1.2, bilirubin 0.6, bili (direct) 0.5, Alk phos 122, AST 60, ALT 13, WBC 14.3, Hgb 4.4, platelets 51, urine pregnancy test negative, serum ethanol was not detected, UDS positive for cocaine and opiates and negative for amphetamines, benzodiazepines, and methadone.
Clinical Course: In the ED BP 46/24, HR 46, RR 22, GCS 3. Emaciated and poorly nourished, multiple gangrenous wounds and a decubitus ulcer. General surgery and intensive care teams were consulted to determine if the patient was treatable. The decision was made to let the patient expire. The patient died in the ICU ∼4 h after arriving at the ED.
Autopsy Findings: The antemortem and postmortem blood levamisole 0.28 mcg/mL. Postmortem blood benzoylecgonine 3,300 ng/mL. Death was due to complications of sepsis, complicating gangrenous wounds, most likely related to levamisole associated vasculitis. The manner of death was ruled natural, as it was the sequelae of chronic drug use.
Case 1836. Acute methamphetamine ingestion: undoubtedly responsible.
Scenario/Substances: A 32 y/o male in police custody developed a sympathomimetic toxidrome and became unresponsive after presumably body stuffing methamphetamine.
Past Medical History: Polysubstance abuse: amphetamines, alcohol, and marijuana.
Physical Exam: BP 109/87, HR 143, RR 29, T 38.7°C.
Obtunded, diaphoretic, pale, no signs of trauma; pupils 8 mm, symmetric, and reactive; lungs clear; abdomen soft, non-distended, bowel sounds present; GCS 4, not responsive to painful stimuli;
Laboratory Data: ABG-pH 7.24 / pCO2 189 / pO2 43.6 / HCO3 18.5, WBC 11.9, K 4.9, CO2 15, BUN 29, Cr 2.2, lactate 4.0 mmol/L, CK 1029. Serum acetaminophen and salicylate were not detected. UDS positive for amphetamine, methamphetamine and THC metabolite. UA showed myoglobinuria.
Clinical Course: In the ED, he was intubated with a neuromuscular blocker, received benzodiazepines for sedation, and activated charcoal. T increased to T 43.3°C requiring placement of IV cooling device: norepinephrine was started for hypotension, sodium bicarbonate was given for acidosis, and calcium was replaced. He was admitted to the ICU with hypotension unresponsive to IV fluids and multiple vasopressors. He developed anuric renal failure, DIC and bleeding per rectum. He was given steroids, cryoprecipitate and FFP. Due to hemodynamic instability, he was considered to not be a candidate for hemodialysis or CVVH. Whole bowel irrigation was initiated: He developed shock liver with abdominal distension with increasing lactate, thought to be due to bowel infarction. Based on the prognosis, comfort measures were instituted and he expired on Day 1.
Autopsy Findings: Bilateral pulmonary edema; upper GI bleeding with 700 cc of coffee ground material in the stomach; hemorrhages of the pericardium, omentum, and stomach erosions; endocardial hemorrhage. Cause of death: acute methamphetamine toxicity.
Case 2062. Chronic dimethylamylamine ingestion: contributory.
Scenario/Substances: A 59 y/o female was found on the floor by her family. Family found an empty bottle of dietary supplement at her home.
Past Medical History: Obesity, depression, COPD and chronic pain. Medications: Benzodiazepines, acetaminophen/hydrocodone, and dietary supplement containing 1,3-dimethylamylamine (DMAA). She was evaluated by her primary care physician over the last 2 weeks for jaundice and elevated liver function tests with negative viral hepatitis panel and undetectable acetaminophen level.
Physical Exam: Obese, jaundiced, nonverbal but able to opens eyes to verbal stimulation. No abdominal distention. BP 157/75, HR 88, RR 10, O2 sat 96% room air.
Laboratory Data: Bilirubin 39.7, AST 933, ALT 959, Alk phos 186, INR 2.8, PTT 48, serum acetaminophen 15 mcg/mL, serum salicylate not detected, hepatitis A negative, hepatitis C negative, previous hepatitis B infection, anti-mitochondrial antibody and ANA negative, HIV negative, abdomen CT negative for mass lesions.
Clinical Course: Patient was found to have fulminant hepatic failure upon arrival to the ED. N-Acetylcysteine was started and continued for the duration of hospitalization. Further evaluation for cause of hepatic failure was unrevealing. Her mental status deteriorated, and she required endotracheal intubation. She was transferred to a tertiary care hospital. Despite aggressive management, there was no improvement of her hepatic synthetic function. She was unresponsive without sedation and did not qualify for liver transplantation. Based on the prognosis, comfort measures were instituted and she expired.
Autopsy Findings: Extensive liver necrosis (> 95%) and cholestasis. Cause of death was fulminant hepatic failure as a result of of dietary supplement containing 1,3-dimethylamylamine (DMAA).
Case 2080. Acute cocaine ingestion: undoubtedly responsible.
Scenario/Substances: A male in his 20’s was arrested by police for a possible drug deal. The patient swallowed 2 baggies cocaine during the arrest and shortly thereafter suffered a cardiac arrest in the field. The patient was reported to have had a seizure in his vehicle prior to EMS arrival. EMS found the patient in cardiac arrest, intubated, began CPR, and transported him to the ED
Past Medical History: Unknown
Laboratory Data: ABG-pH 6.46 / pCO2 81 / pO2 198,

lactate18 mmol/L, Ca 7.0, Hgb 8.4, platelets 98, INR = 2.51, UDS positive cocaine, serum acetaminophen, ethanol and salicylate not detected, ECG showed a LBBB.
Clinical Course: In the ED, normocephalic, atraumatic, pupils fixed and dilated, no pulse, no BP. Pulses returned transiently for a brief time on arrival. He received naloxone, epinephrine, and sodium bicarbonate, and was placed on a ventilator. The patient developed a PEA arrest that progressed to asystole. Despite aggressive supportive care, he was unable to be resuscitated and was declared dead.
Autopsy Findings: Autopsy report confirmed cause of death as massive cocaine toxicity. Patient also had THC in his system but was not attributed to cause of death. Serum cocaine at time of death was 2,900 ng/mL and benzoylecgonine 2,700 ng/mL.
Abbreviations and Normal ranges for Abstracts
Disclaimer—all laboratories are different and provide their own normal ranges. Units and normal ranges are provided here for general guidance only. These values were taken from Harrison's (11), Goldfrank (12), or Dart (13)
Serum electrolyte summary table
serum electrolytes have units of mmol/L = mEq/L

∼ = approximately
ABG-pH/pCO2/pO2/HCO3/BE
ABG = arterial blood gases
ABG-pCO2 = partial pressure of carbon dioxide [38–42]
ABG-pH = hydrogen ion concentration [7.38–7.42]
ABG-pO2 = partial pressure of oxygen [90–100]
Base Excess = [− 2 to + 2 mmol/L]
ACLS = advanced cardiac life support, protocol for the provision of cardiac resuscitation
ADHD = attention deficit hyperactivity disorder
AF = atrial fibrillation
AICD = automatic implanted cardio defibrillator
Alk phos = alkaline phosphatase [13–100] U/L
ALT = Alanine aminotransferase [7–41] U/L = (SGPT)
AMA = against medical advice
Ammonia = [25–80] mcg/dL = [15–47] mcmol/L
amp = ampoule
APLS = advanced pediatric life support, protocol for the provision of cardiac resuscitation
ARDS = acute respiratory distress syndrome
AST = Aspartate aminotransferase [12–38] U/L = (SGOT)
AVblock = atrioventricular block
BAL —British anti-Lewisite
BE = base excess, mmol/L
Bicarbonate = [22–26] mmol/L
bili (direct) = direct bilirubin [0.1, 0.4] mg/dL
bili (indirect) = indirect bilirubin [0.2, 0.9] mg/dL
Bilirubin = total [0.3–1.3] mg/dL, direct [0.1, 0.4] mg/dL, indirect [0.2, 0.9] mg/dL
BLQ = below the limit of quantitation
BMI = body mass index
BP = Blood Pressure, systolic/diastolic, (Torr)
BPH = benign prostatic hypertrophy
BUN = see Urea nitrogen
C = degrees Centigrade
Ca (ionized) = ionized calcium, [4.5–5.6] mg/dL
Ca = calcium, [8.7–10.2] mg/dL
CABG = coronary artery bypass graft
CAD = coronary artery disease
Carbon Dioxide = CO2 [22–26] mmol/L
CIWA = Clinical Institute Withdrawal Assessment for Alcohol
CK = creatinine kinase (CPK), total: [39–238] U/L females, [51–294] U/L males
CKMB = MB fraction of CK [0.0–5.5 mcg/L = 0.0–5.5 ng/mL] Fraction of total CK activity [0–0.04 = 0–4.0%]
Cl = chloride [102–109] mmol/L
CNS = central nervous system
CO2 = carbon dioxide serum or plasma [22–26]mmol/L
COHb = carboxyhemoglobin
COPD = chronic obstructive pulmonary disease
CPR = cardiopulmonary resuscitation
Cr = creatinine [0.5–0.9] mg/dL females, [0.6–1.2] males,
CRRT = continuous renal replacement therapy
CSF = cerebrospinal fluid
CT = computed tomography (CAT scan)
CVA = cerebrovascular accident
CVVHD = continuous venovenous hemodiafiltration
CxR = chest radiograph, chest X-ray
D10W = 10% dextrose in water
D50W = 50% dextrose in water
D5NS = 5% dextrose in normal saline
D5W = 5% dextrose in water
Day = when capitalized, Day = hospital day, that is, days since admission
DIC = disseminated intravascular coagulation
Dx = diagnosis
ECG = electrocardiogram (EKG), leads = I, II, III, aVR, aVL, aVF, V1, V2, V3, V4, V5, V6
ECMO = extracorporeal membrane oxygenation
ED = emergency department, in these abstracts refers to the initial health care facility
EDDP = principal methadone metabolite, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine
EEG = electroencephalogram
EF = ejection fraction
ELISA = enzyme-linked immunosorbent assay
EMS = emergency medical services, paramedics, the first responders
ER = extended release (sustained release)
FFP = fresh frozen plasma
FiO2 = fraction of inspired oxygen
g = grams
g/dL = grams per deciliter
GCS = Glasgow Coma Score, ranges from 3 to 15
GERD = gastroesophageal reflux disease
GI = gastrointestinal
Glu = glucose, fasting [75–110] mg/dL
h = hours
HCF = health care facility
HCG = human chorionic gonadotropin test for pregnancy
HCO3 = bicarbonate
HCP = health care provider
Hct = hematocrit [35.4–44.4] females, [38.8–46.4]% males
Hgb = hemoglobin [12.0–15.8] g/dL females, [13.3–16.2] g/dL males
HIV = human immunodeficiency virus
Hour = when capitalized, Hour = hours since admission
HR = HR, beats per min
ICP = intracranial pressure
ICU = intensive care unit
IgE = immunoglobulin E
IM = intramuscular
INR = international normalized ratio (PT to control) [0.8–1-2]
IU/L = international units per Liter
IV = intravenous
K = potassium, [3.5–5] mmol/L
kg = kilogram
L = Liter
Lactate = lactic acid [4.5–14.4] mg/dL arterial, [4.5–19.8] mg/dL venous
LBBB = left bundle branch block on ECG
Leukocyte count = white blood count [3.54–9.06] 103/mm3
m/o = months old
MAP = mean arterial pressure
mcg/dL = micrograms per deciliter
mcg/L = micrograms per Liter
mcg/min = micrograms per minute
mcg/mL = micrograms per milliliter
mcmol/L = micromoles per liter
MDA = 3,4-methylenedioxyamphetamine
MDMA = methylenedioxymethamphetamine (ecstasy, molly)
ME = medical examiner
mEq = milliequivalents
mEq/L = milliequivalents per liter
Mg = magnesium [1.5–2.3] mg/dL
mg = milligrams
mg/dL = milligrams per deciliter
mg/kg = milligrams per kilogram
mg/L = milligrams per liter
min = minutes
ml = milliliter
mmol/L = millimoles per liter
mosm/kg = milliosmoles per kilogram
mosm/L = milliosmoles per liter
MRI = magnetic resonance imaging
ms = milliseconds
Narrative Headers:
Scenario/substances: concise narrative of EMS and pre-HCF events
Past medical history: available relevant past medical history
Physical examination: initial physical examination if available
Laboratory data: initial results, give units except for units given in abbreviations
Clinical course: concise narrative of HCF & beyond with outcome
Autopsy findings: = medical examiner and/or autopsy results
NG = nasogastric
ng/mL = nanograms per milliliter
not detected = analyte below the level of quantitation, negative
NPO = nil per os, nothing by mouth
NS = normal saline
O2 sat = oxygen percent saturation [94–100]% at sea level
OR = operating room
Osm = osmole
PALS = pediatric advanced life support
PC = poison center (= PCC, or poison control center)
PCC = prothrombin complex concentrate
PCP —primary care provider
PEA = pulseless electrical activity
PEEP = positive end expiratory pressure
PICU = pediatric intensive care unit
Platelets = platelet count [150–400] x109/L
PO = per os (“by mouth” in Latin)
Potassium = [3.5–5] mmol/L
ppm = parts per million
PR = P-R interval [120–200] msec on the ECG
prn = as needed
PT = prothrombin time, INR is preferred, but PT may be used if INR is not available
PTA = Prior to admission
PTT = partial thromboplastin time [26.3–39.4] sec
PVC = prematureventricular contraction
QRS = ECG QRS complex duration [60–100] msec
QT = Q to T interval on the ECG waveform, varies with HR
QTc = QT interval corrected for HR, usually QTcB = QT/RR½ (Bazett correction) 1–15 y-o [< 440] msec, adult male [< 430] msec, adult female [< 450] msec
RBBB = right bundle branch block on ECG
RBC = red blood cell(s)
RR = respiratory rate, breaths per minute
s/p = status post
sec = seconds
SL = sublingual
SVT = supraventricular tachycardia
Synthetic stimulant = one or more of the products (6-APB, bath salts, plant food, Bliss, Ivory Wave, Purple Wave, Vanilla Sky, et al.) or chemicals (3,4 methylenedioxypyrovalerone [MDPV], 6-(2-aminopropyl)benzofuran [6-APB], butylone, desoxypipradrol [2-DPMP], ethylone, flephedrone, naphyrone, mephedrone, methylenedioxypyrovalerone, methylone, methcathinone, et al)
T (oral) = temperature (oral) [36.4, 37.2]°C
T (rectal) = temperature (rectal) [36.4, 37.2]°C
T (tympanic) = temperature (tympanic) [36.4, 37.2]°C
t-bili = total bilirubin
THC = tetrahydrocannabinol
THC homolog = one or more of the products (Blaze, Dawn, herbal incense, K2, Red X, spice, et al) or chemicals (cannabicyclohexanol, CP-47,497, JWH-018, JWH-073, JWH-200, et al)
TPN = total parenteral nutrition
Tprot = total protein
Troponin I = normal range [0–0.08] ng/mL, cut-off for MI > 0.04 ng/mL
U = units
U/dL = units per deciliter
U/L = units per liter
U/mL = units per milliliter
UA = urinalysis
UDS = urine drug screen
Urea nitrogen (BUN) = [6–17] mg/dL
VBG = venous blood gases
VF = ventricular fibrillation
VT = ventricular tachycardia
WBC = white blood count, see leukocyte count
WNL —within normal limits
y/o = years old