Abstract
Introduction. The United Nations Globally Harmonized System of Classification and Labelling of Chemicals (UN-GHS) is developed to harmonize the criteria for hazard communication worldwide. The European Regulation on classification, labeling, and packaging of substances and mixtures [CLP Regulation (European Commission, EC) No 1272/2008] will align the existing European Union (EU) legislation to the UN‐GHS. This CLP Regulation entered into force on January 20, 2009, and will, after a transitional period, replace the current rules on classification, labeling, and packaging for supply and use in Europe. Both old and new classifications will exist simultaneously until 2010 for substances and until 2015 for mixtures. Hazard classification. The new hazard classification will introduce new health hazard classes and categories, with associated new hazard pictograms, signal words, Hazard (H)-statements, and Precautionary (P)-statements as labeling elements. Furthermore, the CLP Regulation will affect the notification of product information on hazardous products to poisons information centers (PICs). Product notification procedures. At this moment product notification widely varies in procedures and requirements across EU Member States. Article 45 of the CLP Regulation contains a provision stating that the EC will (by January 20, 2012) review the possibility of harmonizing product notification. The European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) is recognized as an important stakeholder. For cosmetic products, the new Cosmetics Regulation will directly implement a new procedure for electronic cosmetic product notification in all EU Member States. Conclusion. Both the CLP Regulation and the Cosmetics Regulation will develop their own product notification procedure within different time frames. Harmonization of notification procedures for both product groups, especially a common electronic format, would be most effective from a cost-benefit viewpoint and would be welcomed by PICs.
Introduction
Two European regulations, one recently introduced and one forthcoming, will have impact on poisons information centers (PICs) practice. First, the new regulation on classification, labeling, and packaging of substances and mixtures [CLP Regulation (European Commission, EC) No 1272/2008]Citation1 will introduce a new hazard classification. The CLP Regulation will also affect the notification of product information on hazardous products to PICs by the intention to harmonize the notification procedures in European Union (EU) Member States. Second, the forthcoming Cosmetics Regulation (replacing Directive 76/768/EEC) will legally implement a new procedure for cosmetic product notification in EU Member States.Citation2
This article will first present an overview of the new classification for health hazards according to the CLP Regulation and compare it with the old classification according to the Dangerous Substances Directive (67/548/EEC) and the Dangerous Preparations Directive (1999/45/EC). Furthermore, the separate developments in the product notification of hazardous mixtures and cosmetic products will be discussed as well as the possibility of a single notification procedure, especially a common format, for both product groups.
Hazard classification and labeling
The CLP Regulation will align the EU legislation on classification and labeling of substances and mixtures for supply and use with the United Nations Globally Harmonized System of Classification and Labelling of Chemicals (UN-GHS). The international mandate for the development of a globally harmonized system for hazard classification and hazard communication was adopted in the 1992 United Nations Conference on Environment and Development (UNCED). The UN-GHS itself is not legally binding, it will come into effect only once implemented in regional/national legislation.
The CLP Regulation has entered into force on January 20, 2009, in the EU and will, after a transitional period, replace the current European rules on classification, labeling, and packaging of substances (Directive 67/548/EEC) and mixtures (Directive 1999/45/EC). Both old and new classifications will exist simultaneously until 2010 for substances and until 2015 for mixtures. PICs will be confronted with the new hazard classification. The new classification will consist of new health hazard classes and categories and will use new hazard pictograms, signal words, hazard (H)-statements, and precautionary (P)-statements as labeling elements. In the CLP Regulation, the old terms “dangerous” and “preparations” will be replaced by “hazardous” and “mixtures.”
Health hazard classes and categories
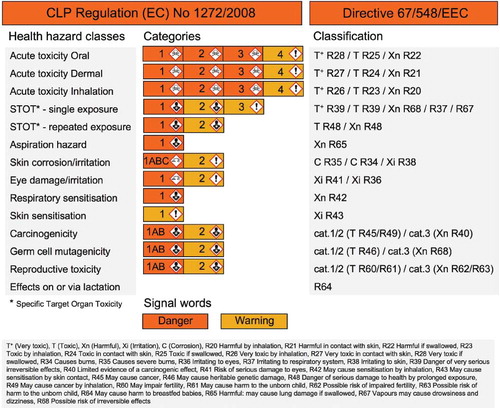
shows a complete overview of the new classification for health hazards according to the CLP Regulation and also the corresponding old classification according to Directive 67/548/EEC. The CLP Regulation introduces new health hazard classes that are subdivided in hazard categories. For example, the hazard class “acute toxicity oral” is subdivided in the categories 1, 2, 3, and 4 of decreasing toxicity. The corresponding old classifications that fall in this hazard class are T+ with R28 (Very toxic if swallowed), T with R25 (Toxic if swallowed), and Xn with R22 (Harmful if swallowed). To give another example, preparations with R37 (Irritating to respiratory system) will now fall in the new hazard class “specific target organ toxicity” (STOT) for single exposure.
Fig. 1. Health hazard classification of substances and mixtures according to the CLP Regulation (EC) No 1272/2008 (entered into force from January 20, 2009) compared with the classification according to Directive 67/548/EEC.Citation1,Citation7
For each hazard category specific hazard communication elements are introduced consisting of pictograms, signal words, hazard statements, and precautionary statements.
Health hazard pictograms and signal words
In the CLP Regulation, four pictograms are used to indicate the health hazards and these are shown in . In the classification according to the CLP Regulation, each category of a hazard class will have one of these four symbols (see ). Already existing are the “skull and crossbones” pictogram that will be used for acute toxicity category 1/2/3 and the “corrosion” pictogram that will be used for skin corrosion category 1A/1B/1C and eye damage category 1. New is the “health hazard” pictogram that will be used for serious health hazards that will not result in serious acute effects, for example, for STOT single/repeated exposure category 1/2 and for all categories of the hazard classes aspiration hazard, respiratory sensitization, carcinogenicity, mutagenicity, and reproductive toxicity. An “exclamation mark” pictogram will be used for all remaining categories of lower toxicity, which are acute toxicity category 4, STOT single exposure category 3, skin irritation category 2, eye irritation category 2, and skin sensitization. The “St. Andrews cross” pictogram will no longer be used in the classification system as it is not included in the UN-GHS. The CLP Regulation further introduces signal words that are associated with the hazard categories: “danger” for categories of higher hazard and “warning” for lower hazard categories (differently colored in ).
Fig. 2. Comparison between the new CLP Regulation (EC) No 1272/2008 (entered into force from January 20, 2009) pictograms and the old Directive 67/548/EEC pictograms for health hazards. Risk (R)-phrases and safety (S)-phrases are no longer used. The new classification system uses hazard (H)-statements and precautionary (P)-statements. A few new “poison center” P-statements are shown.Citation1

H-statements and P-statements
In the CLP Regulation risk (R)-phrases and safety (S)-phrases are no longer used. The new classification system uses H-statements and P-statements (). Some statements will be exactly the same, for example, “Toxic if swallowed” is R25 in the old classification and H301 in the new classification. Others will be slightly different, “Very toxic if swallowed” (R28) will change into “Fatal if swallowed” (H300). There will also be entirely new statements. Especially relevant for PICs are the new P-statements that advise to call a PIC, like P311: “Call a POISON CENTER or doctor/physician.” There are two other “poison center” P-statements advising to call either “immediately” (P310) or “if you feel unwell” (P312) (see ) and five combinations with other P-statements. One example of such a combination statement is P342 + P311: “If experiencing respiratory symptoms: Call a POISON CENTER or a doctor/physician.” In countries where the PIC is only open to inquiries from the health service and not from the general public (like in the United Kingdom and in the Netherlands),Citation3 such statements on a product label could increase the inquiries from the general public and so have consequences for the poisons information supply in these countries.
The CLP Regulation gives for each hazard classification advices for P-statements and states that in general the number of P-statements on a label should be limited to six. However, for some individual hazards like oral acute toxicity category 1, already seven P-statements are advised. For the selection among the relevant P-statements, limited guidance exists at the moment. Whether the advice to contact a PIC is included on the final label will depend on choices of the individual company responsible for placing a product on the market.
New cutoff values
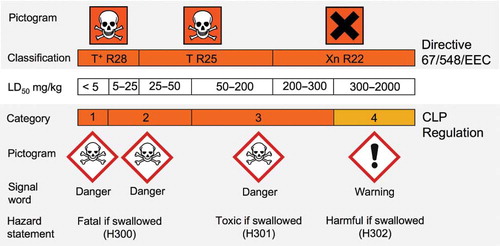
Although the scope covered by classification in the CLP Regulation is equal to the scope covered in the Dangerous Substances Directive (67/548/EEC), the cutoff values to separate the individual hazard categories of several health hazard classes will change. For example, shows in more detail the transition from the old to the new classification for the CLP hazard class “acute toxicity oral.” Both old and new classifications are based on the LD50 value in mg/kg. The effect of new cutoff values for hazard categories in the CLP Regulation is apparent. For example, substances/mixtures with an LD50 value between 200 and 300 mg/kg were formerly classified as Xn with R22 (Harmful if swallowed) and will now fall under category 3 with corresponding “skull and crossbones” pictogram, signal word “danger”, and the hazard statement “Toxic if swallowed.” In other words, some substances/mixtures will be classified as more hazardous when the CLP Regulation is applied.
Fig. 3. From the old classification according to Directive 67/548/EEC to the new classification according to the CLP Regulation (EC) No 1272/2008 (entered into force from January 20, 2009) for the CLP hazard class “acute toxicity oral” (see legend of . for risk (R)‐phrase explanation).Citation1,Citation7
Notification of product information on hazardous products
The main task of PICs is to inform medical personnel and/or the public about the symptoms and treatment of acute intoxications. To perform this task adequately, PICs at least need detailed information on the composition of products to which consumers can be exposed.
Article 17 of Directive 1999/45/EC states that each EU Member State must appoint an authority where the information regarding dangerous preparations must be notified. The appointed authority is responsible for supplying information exclusively for medical purposes. Article 17 does not define which information should be notified and how.
In 2007, we conducted a survey to make an overview of the notification procedures in EU Member States.Citation4 The general conclusion was that specific guidelines in Article 17 were missing, resulting in a considerable diversity in (electronic) notification procedures, used formats, and country-specific requirements on product composition and ingredient concentrations. It was pointed out that the forthcoming CLP Regulation (referred to as “EU-GHS” at that time), replacing Directive 1999/45/EC and also Article 17, would give an opportunity for harmonization of product notification within the EU Member States. It was suggested that product notification requirements should be incorporated in the new legislation that was at that moment under development. Two years later, by joint efforts of industry, Member State representatives, and the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT), Article 45 in the final version of the CLP Regulation (the replacement of Article 17 of Directive 1999/45/EC) was extended with a provision that the EC shall review the possibility to harmonize product notification to the appointed bodies.Citation1 From entry-into-force (on January 20, 2009), the EC has 3 years to review this possibility, which also includes establishing a format and possibly incorporating the result in an Annex to the CLP Regulation. Furthermore, in Article 45, the EAPCCT is recognized as an important stakeholder to be consulted to achieve this harmonization.
How to move forward
In our report on product notification,Citation4 three steps were defined to reach harmonization of product notification in EU Member States. The first step is to reach consensus among the EU PICs on the required product information and especially the necessary detail of the product composition. Already in 1996, the EAPCCT presented “guidelines for completion of a product information form.”Citation5 The following requirements were proposed for the product composition and ingredient concentrations. All constituents of a product, whatever their toxicity, must be mentioned. Actual concentrations on very toxic (T+), toxic (T), and corrosive (C) constituents should be mentioned and specified concentration ranges for the others (0–1%, 1–5%, 5–10%, 10–20%, 20–30%, 30–50%, 50–75%, >75%). When comparing the requirements in different EU Member States, the EAPCCT proposal still seems a reasonable compromise and can be a starting point for the discussions.
The next step toward harmonization is the development of a uniform electronic format used by all EU Member States. Very promising is the XML format, as this has become a general electronic standard for data exchange between existing systems. Databases of competent authorities should be adapted to process the agreed format. Finally, the requirements on product notification and the electronic format should be taken up in the CLP Regulation, so the new harmonized notification procedures will be legally implemented in EU Member States. The EC intends to do so in an Annex to the CLP Regulation.
Translating the EAPCCT requirements
If the requirements on product composition and ingredient concentrations as presented in the EAPCCT guidelinesCitation5 will be a starting point for the discussions, it is necessary to translate the requirements in terms of the new hazard classification. In other words, it should be defined for which hazard classes and categories of the CLP Regulation, an exact concentration of the ingredient is required. For all the other ingredients defined concentration ranges can be used.
A first approach could be a direct translation of the EAPCCT proposal into the new classification. This means that an exact concentration should be required for ingredients in the CLP hazard categories that correspond with the old classification T+, T, and C of Directive 67/548/EEC (). Because the CLP Regulation uses different cutoff values, an exact translation is not possible. Acute toxicity category 3 corresponds partly with the old classification T (with R25) and partly with Xn (with R22) (see also ). The entire category should be included because defining only a part of category 3 is not practical. A second approach could be a division based on the CLP hazard pictograms “skull and crossbones,” “health hazard,” and “corrosion.” These pictograms are used for the more hazardous health categories. An exact concentration could be required for ingredients in the hazard categories associated with these pictograms. shows that more categories are included in the first option. The reason is that the pictogram “health hazard” is assigned not only to the hazard categories that correspond to the old classification of T+ and T but also to Xn (harmful). A third approach for a suitable division is a compromise between approaches 1 and 2 and is based on signal words. Requiring an exact concentration for ingredients in hazard categories with the signal word “danger” will be closest to the exact translation of the EAPCCT proposal in 1996 and still presents a clear division in terms of the classification according to the CLP Regulation.
Table 1. Translation of the EAPCCT requirements on product composition in terms of the classification of the CLP Regulation (EC) No 1272/2008Citation1,Citation7
Notification of product information on cosmetics
In the second half of 2009, a new Cosmetics Regulation will replace the Cosmetics Directive 76/768/EEC.Citation2 Important to know for PICs is that the new Cosmetics Regulation will directly implement a new notification procedure and the use of frame formulations for the notification of cosmetic product information in all EU Member States.
Frame formulations will be used as developed by Colipa (the European Cosmetics Association) in collaboration with the EAPCCT.Citation6 These have been in use since the year 2000. Essentially, these frame formulations only provide the type of ingredients and their maximum concentration. However, there are situations where more detailed information is required. First of all, there is always the obligation to provide additional information on specific more hazardous ingredients, for example, ethanol, and hydrogen peroxide. Furthermore, if a product does not comply with a frame formulation or if a frame formulation is not available, the formulation details, both qualitative and quantitative, need to be provided. Finally, a detailed formulation is always necessary for certain products, for example, nail varnish removers.
The EC will collect electronically (from companies) as well as make available to PICs and governmental authorities (GAs) all relevant cosmetic product information. The EC intends to set up a notification portal in one of the EU countries (personal communication). The companies will submit the cosmetic product information through a secured website. After registration, the information should be made available to the PICs in two ways. Either the notification portal can be used as a database to access and download (selected) notifications or the notifications can be sent electronically to the PICs either in PDF format (for archiving) or in XML format (for upload in a database). To be able to process the XML format, PICs probably will have to adapt their databases.
One notification procedure for both hazardous products and cosmetic products
The CLP Regulation and the new Cosmetics Regulation might result in different notification procedures for both product groups if these notification procedures are not harmonized. A complication for harmonization is the different time frame of the development of the individual notification procedures. For cosmetic products the EC already started a working group to establish a notification procedure, and for hazardous mixtures the EC will only assess the different possibilities for a harmonized notification procedure by January 20, 2012. The consequence will be that PICs/governmental authorities (GAs) might need to develop different processing procedures for each product group to make the product information available for poisons information supply to physicians and/or to the public. From a cost–benefit perspective this is undesirable. Thus, one notification procedure for dangerous products and cosmetic products would be the most appropriate approach.
This can be realized if one uniform XML format could be developed to process both cosmetic products and hazardous products. The format should be made suitable to contain the specific product information required for both product groups. For hazardous products it is important that such a format can contain detailed information on product composition and ingredient concentration. In a similar manner, this detailed information is also necessary for cosmetic products that do not or only partially fit the frame formulations or for which a detailed formulation is obligatory.
Conclusion
PICs should be aware that the new hazard classification and its new labeling elements for hazardous products introduced by the CLP Regulation (EC) No 1272/2008 will affect their daily practice. The old classification will coexist with the new classification until 2010 for substances and until 2015 for mixtures. The CLP Regulation also contains a provision that states that the EC will (by January 20, 2012) review the possibility of harmonizing product notification at the EU level. The EAPCCT is recognized as an important stakeholder to propose possibilities to harmonize product notification. Important for EU PICs is to reach consensus on this matter. The new Cosmetics Regulation will directly implement a new notification procedure in EU Member States. It will be most effective from a cost-benefit viewpoint to have only one notification procedure, especially a common electronic format, for both hazardous mixtures and cosmetic products.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. http://eur-lex.europa.eu/Notice.do?val=486098:cs&lang=en&list=490877:cs,486098:cs,&pos=2&page=1&nbl=2&pgs=10&hwords. Accessed 1 December 2009.
- European Parliament legislative resolution of 24 March 2009 on the proposal for a regulation of the European Parliament and of the Council on cosmetic products (recast). http://www.europarl.europa.eu/sides/getDoc.do?type=TA&language=EN&reference=P6-TA-2009-0158. Accessed 1 December 2009.
- Andrew E. Comparison between eight Poisons Information Centers in Europe. Clin Toxicol 2006; 44:345–350.
- de Groot R, Brekelmans PJAM, Meulenbelt J. Article 17 of the Preparations Directive 1999/45/EC is differently implemented in EU member states. RIVM report 233900001/2007. http://www.rivm.nl/bibliotheek/rapporten/233900001.html. Accessed 1 December 2009.
- EAPCCT Newsletter of April 1996: 5–14.
- Cosmetic Frame Formulations, Colipa guidelines realised in collaboration with The European Association of Poison Centres and Clinical Toxicologists (EAPCCT), January 2000. http://www.colipa.eu/publications.html?Itemid=71&catid=2&task=viewprod&id=22. Accessed 1 December 2009.
- DRAFT Comparison between EU and GHS criteria Human Health and Environment, December 2007. http://ec.europa.eu/enterprise/sectors/chemicals/files/ghs/ghs_comparison_classifications_dec07_en.pdf. Accessed 1 December 2009.