Abstract
Background and purpose The systemic response after fracture is regulated by a complex mechanism involving numerous growth factors. In this study, we analyzed the kinetics of key growth factors following lower-limb long bone fracture.
Materials and methods Human serum was isolated from 15 patients suffering from lower-limb long bone fracture (tibia/femur) requiring surgical fixation. The levels of platelet-derived growth factor (PDGF-BB), vascular edothelial growth factor (VEGF), insulin growth factor-I (IGF-I), and transforming growth factor β1 (TGF-β1) were assayed by colorimetric ELISA at different time points during the first week after fracture. 10 healthy volunteers made up the control group of the study. Serum levels of the growth factors measured were compared to age, sex, and injury severity score.
Results We found that there was a decline in the levels of PDGF-BB, IGF-I and TGF-β1 during the first 3 days after fracture. However, VEGF levels remained unchanged. The levels of all the growth factors studied then increased, with the highest concentrations noted at day 7 after surgery. No correlation was found between circulating levels of growth factors and age, injury severity score (ISS), blood loss, or fluid administration.
Interpretation There are systemic mitogenic and osteogenic signals after fracture. Important growth factors are released into the peripheral circulation, but early after surgery it appears that serum levels of key growth factors fall. By 7 days postoperatively, the levels had increased considerably. Our findings should be considered in cases where autologous serum is used for ex vivo expansion of mesenchymal stem cells. There should be further evaluation of the use of these molecules as biomarkers of bone union.
Fracture healing is subject to regulation at different levels. A major part in this process depends on the ability of mesenchymal stem cells (MSCs) to proliferate and differentiate under the influence of biologically active molecules (i.e. growth factors) (Pountos and Giannoudis Citation2005, Pountos et al. Citation2006). Growth factors have been detected in fracture callus in both animals and humans, and studies have shown that if they are applied locally, they enhance fracture consolidation (Bostrom et al. Citation1995, Si et al. Citation1997, Giannoudis et al. 2009, Kanakaris et al. Citation2009).
It is currently believed that systemic signals trigger a physiological reaction—with direct influence, locally, at the fracture site (Bab et al. Citation1988, Einhorn et al. Citation1990). This belief is based on the observation that an insufficient peripheral supply of growth factors and cytokines can lead to a low number of osteoblasts available at the fracture site, while administration of TGF-β has been found to induce an anabolic effect leading to increased bone density (Manolagas and Jilka Citation1995, Gazit et al. Citation1999). In addition, the circulating level of key growth factors could be a predictive tool for early identification of potentially unsuccessful healing. This was reported by Zimmerman et al. (Citation2005), who found that early decline in the serum concentration of TGF-β is associated with delayed fracture healing.
It has been suggested that the systemic release of biologically active substances takes place in areas distant from the fracture site, as a result of the trauma sustained, and that it is not related to escape of these molecules from the fracture site (Zimmerman et al. Citation2005). This idea is further strengthened by our findings that reaming induces a substantial increase in the levels of growth factors locally, with no change in their levels in the peripheral circulation (Giannoudis et al. Citation2008).
Overall, a large number of growth factors and cytokines are known to be involved in bone-repair processes (Pountos et al. Citation2006, Citation2010a). Among them, platelet-derived growth factor-BB (PDGF-BB), insulin growth factor-I (IGF-I), vascular endothelial growth factor (VEGF), and transforming growth factor β1 (TGF-β1) exert an upregulatory effect, particularly on the osteoprogenitor cells (Pountos et al. Citation2010a). However, no studies have characterized and/or quantified the release of these molecular mediators following trauma. The aim of this study was therefore to obtain preliminary data on the kinetics of release of the above growth factors during the first week after fracture. Moreover, we wanted to determine whether patient demographics such as age, sex, and injury severity score (ISS) (Baker et al. 1974) influence the levels and the pattern of release of these molecules in the peripheral circulation.
Patients and methods
Between November 2010 and January 2011, adult patients admitted to our institution with lower-limb long bone fractures (tibia or femur) were invited to participate in this study. Approval for the study was obtained from the local ethics committee (ref. number Q1206/127). Exclusion criteria were pathological fractures, pediatric patients, use of steroids, previous radiotherapy, and patients with known metabolic bone disease or systemic inflammatory disease processes.
15 patients (9 men) were recruited in this study. Mean age was 34 (21–68) years. The most common mechanism of injury was a road traffic accident.
10 healthy volunteers (5 men) who did not suffer from a fracture or any known pathological condition and who did not take any medication on a regular basis were randomly selected, and they formed the control group. These control individuals were used to establish normal levels of the growth factors under study in the peripheral circulation, i.e. without any influence of trauma. The mean age of the controls was 34 (21–58) years.
Details such as patient demographics, mechanism of injury, ISS, associated injuries, length of hospital stay, and perioperative complications were recorded. Hemoglobin levels and blood and fluid administration during the preoperative, peroperative, and postoperative periods were also documented and analyzed. Following discharge from the hospital, all patients were followed up in the orthopedic outpatient clinic at regular intervals (6 weeks, and 3, 6, 9, and 12 months) for clinical and radiographic assessment of the injury in the affected limb.
Isolation and preparation of human serum
10 mL of peripheral venous blood was collected on admission, at induction, and on the first, third, fifth, and seventh postoperative day. All blood samples, excluding the one from admission, were collected early in the morning before drug administration and breakfast. The blood was collected into 10-mL vacutainer tubes without anticoagulant, and allowed to clot. It was processed within 2 h of collection and was kept on ice. The clotted blood was then centrifuged at 1,500 rpm for 10 min and the serum was taken. All serum was aliquoted in Eppendorf tubes and was stored in a –80°C freezer before use.
Measurement of growth factors
5 growth factors were evaluated, namely PDGF-BB, IGF-I, VEGF, and TGF-β1. Their levels were quantified using commercially available immunoassays (R&D systems, Abingdon, UK) according to the manufacturer’s instructions. All measurements were performed in duplicate.
Statistics
Assumption of normality was tested with a one-sample Kolmogorov-Smirnov test and data are expressed as mean or median accordingly. Parametric and nonparametric data were compared using the Student’s t-test, Mann-Whitney U test, or ANOVA as appropriate. Pearson’s correlation was used to define the degree of relationship between variables. The cutoff value for significance was p = 0.05.
Results
Controls
The mean levels of the growth factors studied in the 10 healthy volunteers were 4.7 (SD 2.2) ng/mL for PDGF-BB, 290 (SD 243) pg/mL for VEGF, 80 (SD 37) ng/mL for IGF-I, and 54 (SD 6.6) ng/mL for TGF-β1.
Patients
On admission, the circulating levels of PDGF-BB were 5-fold higher than in the control group (95% CI: 3.6–6.9). There was a decline in the levels of PDGF-BB, IGF-I, and TGF-β1 during the first 3 days after fracture, which was followed by a gradual increase ( and ). The highest concentrations of PDGF-BB, VEGF, IGF-I, and TGF-β1 were noted at day 7 after surgery ().
Figure 1. The kinetics of PDGF-BB and VEGF. The red line represents the mean levels obtained from the 10 normal volunteers.
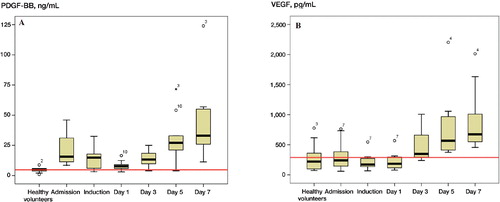
Figure 2. The kinetics of IGF-I and TGF-β1. The red line represents the mean levels obtained from the 10 normal volunteers.
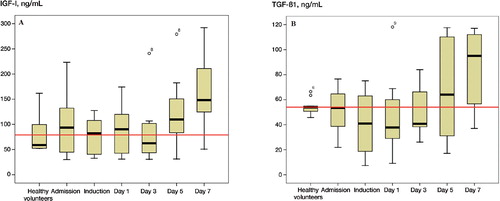
Figure 3. The kinetics of growth factors in relation to the levels found in the normal volunteer group and to levels at admission. The levels of PDGF-BB, IGF-I, and TGF-β1 followed a similar trend, being either constant or reduced in the immediate postoperative period, and they increased gradually with the highest concentrations measured at day 7. (* p < 0.05). PDGF-BB levels in fracture group were statistically significantly higher than in the normal volunteers at all time points, and by day 7 all the growth factors in the fracture patients had reached the highest levels measured.
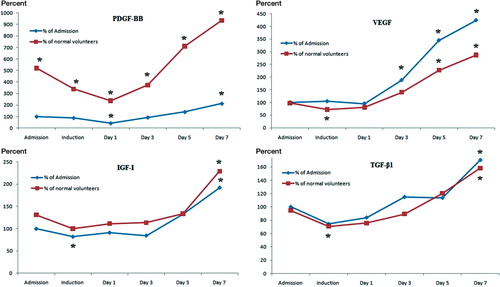
VEGF levels remained unchanged during the first postoperative day and increased after the third postoperative day, with the highest concentration measured at the seventh postoperative day. At day 7, circulating VEGF levels were 4.4 times higher than the levels measured at admission (p < 0.001, 95% CI: 2.3–5.7). Comparing the admission levels of VEGF in patients with those in the control group, no statistically significant difference was noted.
IGF-I levels decreased during induction and continued to be low until the fifth postoperative day. On day 7 postoperatively, however, the IGF-I levels were 2.3 times higher than at admission (p = 0.001, 95% CI: 1.6–3). The levels of IGF-I in patients at admission were similar to those measured in the control group.
On the first postoperative day, PDGF-BB levels decreased sharply to 0.4 times those at admission (p < 0.001, 95% CI: 0.24–0.6), with a gradual increase thereafter. There was a statistically significant difference only for the levels obtained on day 7.
TGF-β1 levels declined significantly by a factor of 0.3 on induction (p < 0.05, 95% CI: 0.5–1) and they remained unaltered until day 5. There was a statistically significant increase by day 7. Comparing levels of TGF-β1 in patients at admission to those in the control group, there was no significant difference (p > 0.05).
In all patients, the fractures had progressed uneventfully to union by a mean time of 16 (13–20) weeks. No statistically significant correlation was noted between the kinetics of levels of release of the 4 molecules studied and ISS score, age, or sex of the patients. A decrease in hemoglobin of 2.4 (0.4–4.2) g/L was noted from admission to the first postoperative day (p < 0.05). Similarly, a statistically significant increase in hemoglobin levels (mean 1.2 (–1.6 to 4.5) g/L) took place between day 1 and day 7 postoperatively (p < 0.05). Although the overall change in hemoglobin levels followed the same pattern as the growth factors, there was no statistically significant correlation between hemoglobin levels and PDGF-BB levels (r = 0.23, p = 0.4), VEGF levels (r = –0.02, p = 1.0), IGF-I levels (r = 0.14, p = 0.65), or TGF-β1 levels (r = 0.25, p = 0.37). Moreover, when we investigated the amount of blood transfusions and fluids that the patients had received for the 3 time points studied (preoperatively, peroperatively, and postoperatively), no correlation was found in the kinetics of the growth factors studied. In summary, we did not find any strong or statistically significant correlations between the levels of release of the 4 growth factors studied and patient age, ISS score, blood loss, or fluid administration.
Discussion
Growth factors play a major role in bone-repair processes (Dimitriou et al. Citation2005). Among them, PDGF-BB has mitogenic and chemotactic effects on MSCs. It increases their proliferation rate and differentiation potential, and it also promotes vasculogenesis and angiogenesis (Pierce et al. Citation1991, Bouletreau et al. Citation2002, Fieldler et al. 2002). VEGF is a key angiogenic element; it stimulates new vessel formation and increases microvascular permeability, with a direct effect on blood flow (Zelzer et al. Citation2002). In addition, it stimulates fracture repair by angiogenesis and bone turnover (Street et al. Citation2002). IGF-I has an anabolic effect on bone by upregulating the proliferation and differentiation of osteoblasts and osteocytes (Rosen and Donahue Citation1998). Thus, it increases fracture union rates (Johansson et al. Citation1992, Schmidmaier et al. Citation2003). TGF-β1 enhances the proliferation and differentiation of MSCs, promotes cartilage formation, and increases callus formation and bone strength (Bauer et al. Citation1998, Barnes et al. Citation1999, Wildemann et al. Citation2003).
The results of the present study indicate that key growth factors are released into the peripheral circulation after trauma, indicating that there is a generalized participation of the whole body in the healing of fractures. PDGF appears to be released very early after fracture, and the levels of release were found to be statistically significantly higher in the fracture patients than in the control group. Regarding the rest of the growth factors studied, their levels were statistically significantly increased at day 7 after surgery.
Our previous work showed that serum harvested at the time of surgery and on the first postoperative day had a poorer effect on the proliferation and osteogenic differentiation of MSCs than the serum obtained at admission and on the third and seventh postoperative day (Pountos et al. Citation2008). This finding is supported by the results of the present study, where an increase in the levels of the growth factors investigated was observed after day 3 and up to day 7 postoperatively. Combining the findings in our previous study with the current results, we can assume that the poor effect of human serum obtained on the first 3 days after fracture for in vitro proliferation and differentiation of MSCs can be attributed to the low content of circulating growth factors.
The decrease in the levels of the studied growth fractors noted on the first postoperative day is difficult to explain. One would expect that this finding should correlate with the blood loss and the dilution effect that the administration of blood and fluids might produce during surgery. However, no correlation was found with the drop in hemoglobin levels, the amount of fluids given, or the amount of blood transfusion that the patients received. Moreover, examination of patient demographics such as age, sex, and ISS failed to provide a robust association with the circulating levels and the pattern of release of these molecules in the peripheral circulation. The potential immunological burden and host defense mechanisms that can be induced by surgery could provide a possible explanation for this finding (Homburger and Meiler Citation2006, Nicholson and Hall Citation2011). The impact of the anesthetic drugs administered during surgery could be another reason (Homburger and Meiler Citation2006, Nicholson and Hall Citation2011). In addition, one should also consider the potential effect of the gene expression profile during fracture healing on the levels of release of the signaling molecules studied. It has been reported previously that PDGF is expressed during the first day after fracture and that expression subsides afterwards, while the expression of VEGF, IGF-I, and TGF-β1 begins at a later stage (Dimitriou et al. Citation2005). Finally, another possible explanation for the fact that we did not find any correlation between ISS and circulating levels of the growth factors studied could be the low number of patients studied (type II statistical error). Even so, the strength of this study can be considered to be the possible use of our pilot data for the design of future studies aimed at further investigation of the patterns and levels of release of growth factors, and at evaluation of their potential as potential biomarkers of fracture-healing activity.
In the clinical setting, there has been extensive interest in the use of MSCs in the treatment of musculoskeletal disorders (Pountos et al. Citation2006, Citation2007). However, the low frequency of progenitor cell populations in bone marrow aspirates has led to various approaches to obtain large numbers of MSCs. In the laboratory, the use of growth factors that can exert an effect on the proliferation of MSCs has been shown to result in an increased number of MSCs (Pountos et al. Citation2010b). Ex vivo expansion can lead to high numbers of MSCs, facilitating their re-implantation in the form of an injection or by loading them onto scaffolds for the treatment of non-unions or bone defects (Pountos et al. Citation2010b). However, ex vivo expansion requires the culture of MSCs in medium containing serum. Fetal animal sera have mainly been used in this context, after heat inactivation of the serum, which destroys viral components and microorganisms (Pittenger et al. Citation1999, Tuschong et al. Citation2002). However, MSCs cultured in fetal calf serum carry fetal calf proteins in high amounts, and this can lead to immunogenic effects (Spees et al. Citation2004). In fact, immune reactions have been reported in cells cultured in fetal calf serum (Selvaggi et al. Citation1997, Tuschong et al. Citation2002). Moreover, the risk of transmission of diseases appears to be relatively low, but it cannot be excluded (Pountos et al. Citation2006). In addition, life-threatening arrhythmias during clinical application of MSCs for cardiomyoplasty appear to be avoided when human autologous serum is used instead of FCS (Chachques et al. Citation2004). These data have forced researchers to develop serum-free medium or to use autologous serum for ex vivo expansion. Serum-free medium would seem to be the most appropriate approach, but it has not been fully explored. Based on our findings, we can propose that autologous serum harvested after the fifth day following fracture might be a better medium for expansion of MSCs due to its high content of molecular mediators (growth factors), which have a direct anabolic effect on MSCs (Dimitriou et al. Citation2005).
In conclusion, bone fracture and the associated physiological cascade of events trigger a systemic anabolic response leading to release of growth factors in the peripheral circulation. We have characterized the patterns and the levels of release of 4 molecular mediators known to contribute to bone-repair processes. A gradual increase in the levels of these molecules was observed over a 7-day period. Based on the present results, we recommend that if ex vivo expansion of MSCs is desirable, the ideal time point for isolation of autologous human serum is after the fifth day post-injury. Further studies with a larger number of patients are needed to determine whether any of these molecules could be used successfully as biomarkers for monitoring of the fracture-healing response.
IP: primary investigator, design of the study, collection of samples, assays, analysis, and writing up. TG and KH: technical support and assistance in sample collection. HB and PVG: supervisors of the study.
No competing interests declared.
- Bab I, Gazit D, Muhlrad A, Shteyer A. Regenerating bone marrow produces a potent growth-promoting activity to osteogenic cells. Endocrinology 1988;123(1): 345-52.
- Barnes GL, Kostenuik LC, Gerstenfeld LC, Einhorn TA. Growth factor regulation of fracture repair. J Bone Miner Res 1999;14(11): 1805-15.
- Bauer DC, Rosen C, Cauley J, Cummings SR. Low serum IGF-1 but not IGFBP-3 predicts hip and spine fracture: The study of osteoporotic fracture. Bone 1998;23(6): 561-2.
- Bostrom MP, Lane JM, Berberian WS, . Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. J Orthop Res 1995;13(3): 357-67.
- Bouletreau PJ, Warren SM, Spector JA, . Factors in the fracture microenvironment induce primary osteoblast angiogenic cytokine production. Plast Reconstr Surg 2002;110(1): 139-48.
- Chachques JC, Herreros J, Trainini J, . Autologous human serum for cell culture avoids the implantation of cardioverter-defibrillators in cellular cardiomyoplasty. Int J Cardiol (Suppl 1) 2004;95:S29-33
- Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury 2005;36(12): 1392-404.
- Einhorn TA, Simon G, Devlin VJ, . The osteogenic response to distant skeletal injury. Bone Joint Surg (Am) 1990;72(9): 1374-8.
- Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem 2002;87(3): 305-12.
- Gazit D, Zilberman Y, Turgeman G, . Recombinant TGF-beta1 stimulates bone marrow osteoprogenitor cell activity and bone matrix synthesis in osteopenic, old male mice. J Cell Biochem 1999;73(3): 379-89.
- Giannoudis PV, Pountos I, Morley J, . Growth factor release following femoral nailing. Bone 2008;42(4): 751-7
- Giannoudis PV, Kanakaris NK, Dimitriou R, . The synergistic effect of autograft and BMP-7 in the treatment of atrophic nonunions. Clin Orthop 2009; (467) (12): 3239-48.
- Homburger JA, Meiler SE. Anesthesia drugs, immunity, and long-term outcome. Curr Opin Anaesthesiol 2006;19(4): 423-8.
- Johansson AG, Burman P, Westermark K, Ljunghall S. The bone mineral density in acquired growth hormone deficiency correlates with circulating levels of insulin-like growth factor I. J Intern Med 1992;232(5): 447-52.
- Kanakaris NK, Mallina R, Calori GM, . Use of bone morphogenetic proteins in arthrodesis: clinical results. Injury (Suppl 3) 2009;40:S62-6
- Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med 1995;332(5): 305-11.
- Nicholson G, Hall GM. Effects of anaesthesia on the inflammatory response to injury. Curr Opin Anaesthesiol 2011;24(4): 370-4.
- Pierce GF, Mustoe TA, Altrock BW, . Role of platelet-derived growth factor in wound healing. J Cell Biochem 1991;45(4): 319-26.
- Pittenger MF, Mackay AM, Beck SC, . Multilineage potential of adult human mesenchymal stem cells. Science 1999;284(5411): 143-7.
- Pountos I, Giannoudis PV. Biology of mesenchymal stem cells. Injury (Suppl 3) 2005;36:S8-S12
- Pountos I, Jones E, Tzioupis C, . Growing bone and cartilage. The role of mesenchymal stem cells. J Bone Joint Surg (Br) 2006;88(4): 421-6.
- Pountos I, Corscadden D, Emery P, Giannoudis PV. Mesenchymal stem cell tissue engineering: techniques for isolation, expansion and application. Injury (Suppl 4) 2007;38:S23-33
- Pountos I, Georgouli T, Giannoudis PV. The effect of autologous serum obtained after fracture on the proliferation and osteogenic differentiation of mesenchymal stem cells. Cell Mol Biol (Noisy-le-grand) 2008;54(1): 33-9.
- Pountos I, Georgouli T, Henshaw K, . The effect of bone morphogenetic protein-2, bone morphogenetic protein-7, parathyroid hormone, and platelet-derived growth factor on the proliferation and osteogenic differentiation of mesenchymal stem cells derived from osteoporotic bone. J Orthop Trauma 2010a;24(9): 552-6.
- Pountos I, Georgouli T, Kontakis G, Giannoudis PV. Efficacy of minimally invasive techniques for enhancement of fracture healing: evidence today. Int Orthop 2010b;34(1): 3-12.
- Rosen CJ, Donahue LR. Insulin-like growth factors and bone: the osteoporosis connection revisited. Proc Soc Exp Biol Med 1998; 219 (1) :1-7.
- Schmidmaier G, Wildemann B, Gabelein T, and TGF-beta1 on fracture healing in rats: single versus combined application of IGF-I and TGF-beta1. Acta Orthop Scand 2003;74(5): 604-10.
- Selvaggi TA, Walker RE, Fleisher TA. Development of antibodies to fetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood 1997;89(3): 776-9.
- Si X, Jin Y, Yang L, . Expression of BMP-2 and TGF-beta 1 mRNA during healing of the rabbit mandible. Eur J Oral Sci 1997;105(4): 325-30.
- Spees JL, Gregory CA, Singh H, . Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. MolTher 2004;9(5):747-56
- Street J. Bao M, deGuzman L, Bunting S, Peale F V Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A 2002;99(15): 9656-61.
- Tuschong L, Soenen SL, Blaese RM, . Immune response to fetal calf serum by two adenosine deaminase-deficient patients after T cell gene therapy. Hum Gene Ther 2002;13(13): 1605-10.
- Wildemann B, Schmidmaier G, Ordel S, . Cell proliferation and differentiation during fracture healing are influenced by locally applied IGF-I and TGF-beta1: comparison of two proliferation markers, PCNA and BrdU. J Biomed Mater Res B Appl Biomater 2003;65(1): 150-6.
- Zelzer E, McLean W, Ng YS, . 120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development 2002;129(8): 1893-904.
- Zimmermann G, Henle P, Kusswetter M, . TGF-beta1 as a marker of delayed fracture healing. Bone 2005;36(5): 779-85.