Abstract
Background and purpose — Previous studies of soft tissue sarcoma (STS) have identified a number of possible prognostic factors; however, the majority of these include highly selected populations, with unclear validation of data and insufficient statistical methods. We identified prognostic factors in a validated, population-based 30-year series of STS treated at a single institution, using an advanced statistical approach.
Patients and methods — Between 1979 and 2008, 922 adult patients from western Denmark were treated at the Aarhus Sarcoma Center for non-metastatic STS in the extremities or trunk. The endpoints were local recurrence (LR) and disease-specific mortality (DSM). Prognostic factors were analyzed using a proportional hazard model, including continuous variables as cubic splines. Directed acyclic graphs were used to depict the causal structure.
Results — The 5-year LR was 16% and the 5-year DSM was 24%. Important prognostic factors for both LR and DSM were age, duration of symptoms, tumor size, grade, margin, and radiotherapy, while anatomical location (upper, lower extremity, trunk) was prognostic for DSM.
Interpretation — In this population-based series of adult, non-metastatic STS, we included directed acyclic graphs, cubic splines, and a competing risk model in order to minimize bias, and demonstrated that these statistical methods are feasible. Using these statistical methods on a large, validated dataset, we excluded depth as a prognostic factor and established that age, duration of symptoms, size, grade, margin, and radiotherapy were important prognostic factors for both local recurrence and disease-specific mortality.
Over the past 3 decades, numerous studies on soft tissue sarcoma (STS) have investigated prognostic factors for local recurrence and survival (Pisters et al. Citation1996, Kattan et al. Citation2002, Stojadinovic et al. Citation2002, Zagars et al. Citation2003, Weitz et al. Citation2003, Gronchi et al. Citation2005, Gutierrez et al. Citation2007, Jebsen et al. Citation2008, Biau et al. Citation2011, Citation2012). While the prognostic value of some factors, e.g. grade and surgical margin, are generally accepted, the value of other factors such as age and location is still uncertain.
To investigate this discrepancy between previous studies, we reviewed the literature and 3 core problems emerged: small sample sizes, heterogeneity of study populations, and differences in statistical methods used.
The rarity of STS, with an incidence of 1.4 per 100,000 (Maretty-Nielsen et al. Citation2013), makes a sufficient sample size challenging to obtain, and studies are often based on few patients. Thus, these studies may not have sufficient power to identify prognostic factors, or may identify them by chance.
The heterogeneity of study populations in the literature—inclusion of selected subtypes only, high-grade tumors, or patients from centers with major tertiary referral practices and a larger proportion of large, high-grade, recurrent, or otherwise complicated sarcomas—results in possible selection bias and low generalizability. Few authors (e.g. Gustafson Citation1994) have used population-based data, and when they have, the validity of data sources has not been described or is unknown.
The statistical approaches used to analyze data may introduce several problems, including a substantial overestimation of the failure risk if competing risk is not taken into account, biased estimates if the proper confounding variables are not included in the adjusted analyses, and risk of over-optimized or irreproducible estimates and residual confounding if the data are analyzed using categorical variables instead of continuous variables (Fine and Gray Citation1999, Royston et al. Citation2006, Shrier and Platt Citation2008). Previously, statistical methods for handling these problems were not easy to use; however, recent implementations in statistical software have made it feasible to address these problems properly.
The aim of this study was to identify prognostic factors in a validated, population-based series of adult STS, using an improved statistical approach with cubic spline regression in a competing risk setting.
Patients and methods
The Danish healthcare system provides tax-funded, free-of-charge healthcare for all residents. Since 1968, all the citizens of Denmark have been assigned a unique 10-digit civil personal registration number (CPR number). These numbers are used in all Danish administrative registries and clinical databases. This allows linkage of databases at the level of the individual patient and ensures complete follow-up.
Practice at the Aarhus Sarcoma Center (ASC)
Since 1979, treatment of sarcoma in western Denmark (with 2.5 million inhabitants) has been centralized at the Sarcoma Center of Aarhus University Hospital (ASC). The diagnostic program follows international guidelines and includes a clinical examination, diagnostic imaging, biopsy, and histopathological evaluation conducted by an experienced multidisciplinary team (CitationCo-operative Cancer Departments 2007). In the study period, a 3-tiered malignancy-grading system based on cellularity, necrosis, anaplasia, and number of mitoses was used (Jensen et al. Citation1991).
The primary treatment is surgery aiming at a wide margin (including radical margins), according to the principles of Enneking et al.(Citation1980). Low-grade tumors are mainly treated with marginal or wide margins (1 cm surrounding cuff of normal tissue), while deep intermediate and high-grade tumors are treated with wide margins and postoperative radiotherapy. The radiotherapy consists of fractions of 2 Gy to a total dose of 50–60 Gy.
Patients are followed for a minimum of 5 years after the last treatment, with intervals ranging from 3 to 6 months. Follow-up visits include clinical examination and chest radiography (intermediate- and high-grade tumors only), with MRI scans for patients with deep-seated tumors in recent years.
Study population
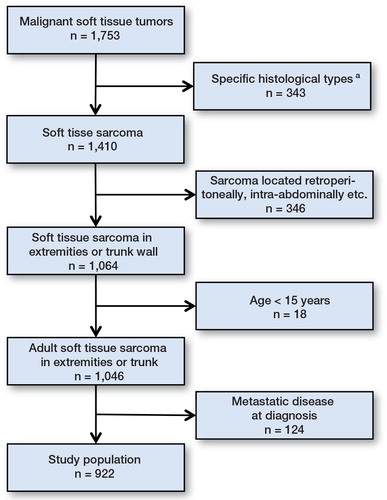
Between January 1979 and December 2008, 1,753 consecutive patients were treated for STS at the ASC and registered in the Aarhus Sarcoma Registry (ASR) (Vraa et al. Citation1998). The ASR has recently been systematically validated by reviewing the medical files (gold standard) for all patients registered in the ASR using standardized forms. The completeness of the ASR was > 85% for western Denmark in 1979–2008, when compared with the Danish Cancer Registry at the patient level (Maretty-Nielsen et al. Citation2013). The ASR collects basic patient data, specific data on tumor characteristics and treatment, and data on follow-up, local recurrence, distant metastasis, and death. The completeness of registered data is > 99%. Patients were excluded if they had certain histological types, if they had tumors not located in the extremities or trunk wall, if they were younger than 15 years, or if they had metastases at diagnosis (). The study population thus comprised 922 adult patients with non-metastatic STS of the extremity or trunk.
Prognostic factors
Based on a literature search, possible prognostic factors were selected and prioritized before the data analysis. Based on the number of events in the study (i.e. in order to have sufficient statistical power), the following factors were included: age at diagnosis, duration of symptoms, tumor size, location, depth, grade, surgical margin, radiotherapy, and calendar year of diagnosis. Directed acyclic graphs were used to depict a possible causal relationship between the prognostic factors selected, possible confounding variables, and the outcomes (Figure 2, see Supplementary data).
Tumor size was recorded as the largest diameter (in cm), based on the fixed pathological specimen. However, if patients were not treated surgically or if the pathology report was insufficient, diagnostic images were used to determine size. Tumors located in the shoulder and gluteal area were classified as trunk tumors. Margins were defined, based on the pathological specimen, as intralesional/marginal if the incision was within the tumor or the pseudocapsule, or as wide if the tumor was surrounded by a cuff of normal tissue.
Statistics
Descriptive statistics are reported as median and proportions; they were compared using the Kruskal-Wallis test and the chi-squared test. The outcomes assessed were local recurrence (LR) and disease-specific mortality (DSM). DSM was defined as death from sarcoma (including patients with locally advanced tumors) or death from metastatic disease. Patients were followed from the date of diagnosis (DSM analysis) or the date of their last primary treatment (LR analysis) to date of outcome, emigration, or end of the study period. The LR and DSM were estimated as cumulative incidence rates, with 95% confidence intervals (CIs) based on the pseudo-value approach (Fine and Gray Citation1999, Graw et al. Citation2009).
The possible prognostic factors were analyzed using a Cox proportional hazard model, estimating both crude and adjusted hazard ratios (HRs) with CI. The confounding variables, as given in and and Figure 2 (see Supplementary data), were selected based on the approach described by Shrier and Platt (Citation2008). Age, duration of symptoms, and tumor size were analyzed continuously as 4-knotted cubic splines, with the twenty-fifth percentile as reference (Smith Citation1979). Adjusted cumulative incidence curves were constructed using the Stata command stcurv (Cleves Citation2000). The proportional hazard assumption was tested for all the variables included, both crude and adjusted, using log-minus-log plots and no contradictions were found. Competing risks were death from all causes in the analyses of LR and death from causes other than sarcoma in the analyses of DSM. Only patients who were macroscopically disease-free (i.e. with no macroscopic residual tumor) after primary treatment (n = 879) were included in the analyses of LR. All tests were two-sided and any p-value ≤ 0.05 was considered significant. Analyses were performed using Stata statistical software, version 13.1.
Ethics
The study was conducted in accordance with the Helsinki Declaration. It was approved by the Danish Data Protection Agency (2007-58-0010) and the Danish Health and Medicines Authority (7-604-04-2/262/KWH).
Results
Patient and tumor characteristics
Overall, 922 adult patients in western Denmark were diagnosed with non-metastatic STS of the extremity or trunk wall from 1979 through 2008. The median age was 60 (15–95) years and 52% were males (). Over the study period, an increasing proportion of patients were referred without previous treatment and for primary diagnostics. At the same time, the proportion of patients treated with amputations decreased and the proportion of patients treated with radiotherapy increased. The proportion of wide resections decreased in the final 10-year period. The most frequent tumor types were malignant fibrous histiocytoma, liposarcoma, and leiomyosarcoma (). After primary treatment, 95% of the patients were macroscopically disease-free. The median follow-up time was 7 (0–34) years. The median follow-up time for patients who were still alive at the end of the study period was 13 (3–34) years.
Table 1. Characteristics of adult patients treated for a non-metastatic soft tissue sarcoma in the extremities or trunk wall at the Sarcoma Center of Aarhus University Hospital according to calendar year of diagnosis (n = 922). Unless otherwise stated, values are number (%)
Table 2. Distribution of non-metastatic soft tissue sarcomas in the extremities and trunk wall according to histological types in adult patients treated at the Aarhus Sarcoma Center from 1979 to 2008 (n = 922)
Local recurrence (– and )
Figure 3. Cumulative incidence rate (solid black line) of local recurrence (panel A) and disease-specific mortality (panel B) with 95% confidence intervals (dotted gray lines).
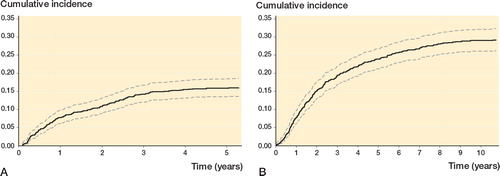
Figure 4. Adjusted hazard ratios (HR (solid line)) with 95% confidence intervals (dashed lines) for local recurrence (panels A, C, and E) and disease-specific mortality (B, D, and F) according to age, duration of symptoms, and tumor size, based on Cox proportional hazard analyses. Adjustment covariates were selected based on Figure 2 (see Supplementary data); no covariates were included in the analysis of age; duration of symptoms was adjusted for age and grade; tumor size was adjusted for duration of symptoms and grade.
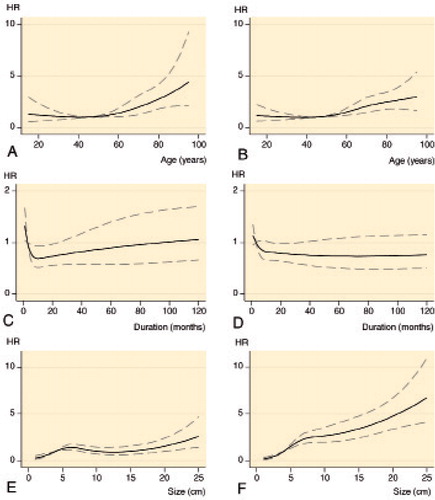
Figure 5. Adjusted cumulative incidence of local recurrence by grade (A), surgical margin (B), and radiotherapy (C) based on a Cox proportional hazard regression. Grade was adjusted for age and histological subtype; margin was adjusted for age, size, depth, location, compartmentalization, grade, and year of diagnosis; and radiotherapy was adjusted for age, depth, grade, margin, and year of diagnosis.
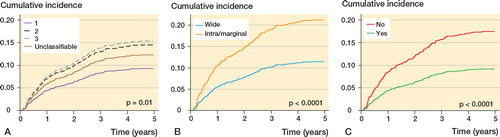
Table 3. Crude and confounder-adjusted Cox proportional hazard analyses of possible prognostic factors for local recurrence in adult patients with non-metastatic soft tissue sarcoma in the extremity or trunk wall (n = 879)
The 5-year LR rate was 16% (CI: 13–18). The correlation between age and the risk of LR was approximately linear with a constant risk from 15 to 55 years, after which the risk increased successively. The relationship between the risk of LR and symptom duration was “J”-shaped, where an increase in the duration of symptoms in the first 12 months was associated with a pronounced drop in the risk of LR, but after that the risk of LR increased steadily the longer the duration of symptoms. An increasing tumor size, for tumors ≤ 5 cm or ≥ 15 cm, was associated with an increasing risk of LR. In the confounder-adjusted analyses, age > 55 years (vs. 45 years), duration of symptoms < 3 months (vs. 3 months), tumor size 5–7 cm or > 20 cm (vs. 4 cm), grade 3, and intralesional/marginal excision were adverse prognostic factors, while duration of symptoms > 3 months and < 24 months (vs. 3 months), tumor size < 4 cm (vs. 4 cm), and treatment with radiotherapy were favorable prognostic factors.
Disease-specific mortality (, , and , and )
Figure 6. Adjusted cumulative incidence of disease-specific mortality by location (A), grade (B), surgical margin (C), and radiotherapy (D) based on Cox proportional hazard regression. Location was adjusted for histological subtype; grade was adjusted for age and histological subtype; margin was adjusted for age, size, depth, location, compartmentalization, grade, and year of diagnosis; and radiotherapy was adjusted for age, depth, grade, margin, and year of diagnosis.
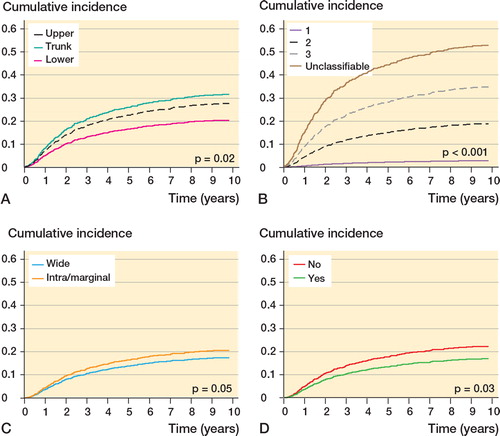
Table 4. Crude and confounder-adjusted Cox proportional hazard analyses of possible prognostic factors for disease-specific mortality in adult patients with non-metastatic soft tissue sarcoma in the extremities or trunk (n = 922)
The 5- and 10-year DSM rates were 24% (CI: 21–27) and 29% (CI: 26–32). The risk of DSM was nearly constant between 15 and 45 years, after which it increased. Increasing duration of symptoms was associated with a decreasing risk within 9 months, but after that it was approximately constant. Increasing tumor size was associated with an increasing risk, and this was most pronounced for tumors ≤ 5 cm. Adverse prognostic factors were age > 45 years (vs. 45 years), tumor size > 4 cm (vs. 4 cm), trunk and lower extremity location, grade 2–3 or unclassifiable, and intralesional/marginal excision, while duration of symptoms ≥ 15 and ≤ 30 months, tumor size < 4 cm (vs. 4 cm), and treatment with radiotherapy were favorable prognostic factors. Depth was not a prognostic factor.
Discussion
In this population-based study, using systematically validated data, we found a 5-year LR rate of 16% and a 5-year DSM of 24%. Important prognostic factors for both LR and DSM were age, duration of symptoms, tumor size, grade, margin, and radiotherapy, while anatomical location was prognostic for DSM.
Methodological considerations
The main strengths of the present study include the large sample size, the population-based design with complete follow-up, and the statistical methods used to minimize bias.
The validity of the ASR has been reported in detail in a previous paper (Maretty-Nielsen et al. Citation2013). The overall completeness of patient registration in the ASR is > 85%; however, the completeness differed according to calendar year at diagnosis, residency, and age. The completeness was lower in patients diagnosed before 1994, in patients from the southern region of Denmark, and in patients over 60 years of age. These differences may introduce a selection bias if the association between the factors investigated and the outcome is different for patients not registered in the ASR. Based on this, we would expect any selection bias to have been minor.
The possible prognostic factors we investigated were selected based on a literature search, and they were specified before the data analysis. When selecting the possible confounding factors that should be included in the adjusted analyses, many studies have used variations of forward selection, backward elimination, or simply inclusion of all factors; however, this can result in biased estimates with CIs that are too narrow and p-values that are too low. To our knowledge, this is the first study of prognostic factors in STS in which directed acyclic graphs have been used to depict causal relationships in order to select possible confounding factors and thus minimize bias.
The continuous variables were included as cubic splines (Smith Citation1979). When evaluating the results from other studies, these variables are often analyzed categorically with one cut-off point or continuous linearly (Royston et al. Citation2006). As seen in , neither of these approaches is adequate, with the categorical method resulting in residual confounding and the continuous linear method being a false assumption. Furthermore, when selecting cut-points the method is often not specified, or the “optimal” cut-point method is used, leading to over-optimized and irreproducible estimates. Likewise, different cut-points are often used, impairing the generalizability.
The WHO classification, which was used to classify STS in this study, has recently been updated, resulting in the change of some histological subtypes, e.g. malignant fibrous histiocytoma, now called undifferentiated pleomorphic sarcoma, and fibrosarcoma, which is becoming increasingly rare due to classification as other more specific types (CitationFletcher et al. 2002). The pathology of the STS patients reported in this study has not been reviewed, which may have caused misclassification when compared to the new WHO classification. However, a histopathological evaluation according to the (at that time) relevant classification was performed at the time of diagnosis by an experienced sarcoma pathologist, for all patients referred to or diagnosed/treated at the Aarhus Sarcoma Center. Furthermore, during the study period only 2–3 pathologists performed these evaluations, ensuring that the classification was consistent throughout the study period. For this reason, misclassification would be expected to be minor and of no significance to our study, where several histological types were considered together.
The comparability with other prognostic studies may have been complicated by the grading system used in the ASR (Jensen et al. Citation1991). This is primarily based on the number of mitoses, taking the cellularity, necrosis, and anaplasia into account, with some specific histopathological subtypes pre-specified, e.g. rhabdomyosarcoma and extraosseous osteosarcoma as grade 3. Currently, the most common grading systems are the National Cancer Institute (NCI) system and the Fédération Nationale des Centres de Lutte contre le Cancer (FNCLCC) system (Costa et al. Citation1984, Trojani et al. Citation1984). These grading systems are based on the same principles as in the system we used. STS patients at the ASC have been registered according to the FNCLCC system since January 2009, and based on the limited discrepancies in grading that we have found before and after January 2009, we would expect comparability issues to be minor.
We assessed the outcomes LR and DSM using a competing risk model, which was not done in most previous studies. In cases in which the competing event is frequent, not using a competing risk model can lead to overestimation of the failure outcome (Fine and Gray Citation1999). Establishment of the correct cause of death is essential in DSM, and even though we used validated data on the cause of death, this can be problematic in elderly patients, where comorbidity is frequent.
During the study period, some changes in the treatment of patients at ASC were observed. Consistent with reports from other sarcoma centers, the proportion of patients treated with amputation decreased from 24% in the period 1979–1988 to 8% in the period 1999–2008 (Gronchi et al. Citation2005, Jebsen et al. Citation2008, Biau et al. Citation2011, Citation2012). Overall, it appears that there has been a shift in treatment regime, with a tendency towards “closer” surgical removal and adjuvant treatment with radiotherapy. However, this shift did not affect the LR rate or the DSM rate, as seen in the insignificance of calendar year of diagnosis in our analyses.
Overall rates
The LR and DSM rates presented in our study were in overall agreement with those in previously published studies (Gustafson Citation1994, Pisters et al. Citation1996, Stojadinovic et al. Citation2002, Zagars et al. Citation2003, Gutierrez et al. Citation2007, Jebsen et al. Citation2008, Biau et al. Citation2011). Such studies have found 5-year LR rates between 8% and 22%, whereas there tended to be higher LR rates previously, which is possibly explained by the non-competing risk setting. The majority of previous studies found 5-year DSM rates between 20% and 45%, as compared to our results of 24%. The lower rates observed might also be explained by the fact that surgical treatment during the study period was performed by a small number of experienced oncology surgeons working in close collaboration.
Prognostic factors
Age > 45–55 years was associated with higher LR and DSM. The important effect of age on DSM, when analyzed continuously, has not been described before. Two recent studies investigated the prognostic value of age when analyzed using cubic splines: Gronchi et al. (Citation2005) reported no independent effect on either LR or DSM, while Biau et al. (Citation2011) found a statistically significantly higher LR rate with increasing age. Studies have shown increased LR and mortality with increasing age, even when reporting DSM; however, the majority of these have reported age as a binomial categorical variable (Kattan et al. Citation2002, Weitz et al. Citation2003, Zagars et al. Citation2003, Gutierrez et al. Citation2007).
Size, grade, and margin were important prognostic factors for both LR and DSM, which is supported by the previous literature (Pisters et al. Citation1996, Kattan et al. Citation2002, Stojadinovic et al. Citation2002, Zagars et al. Citation2003, Weitz et al. Citation2003, Gronchi et al. Citation2005, Jebsen et al. Citation2008, Biau et al. Citation2011, Citation2012). Unexpectedly, we found a tendency of unclassifiable tumors being associated with higher mortality than even high-grade tumors. One explanation could be that this group represents the most undifferentiated sarcomas with high aggressiveness.
Very few studies have analyzed duration of symptoms in a confounder-adjusted setting, and only as a categorical variable. Previous results have been highly contradictory; some studies found no prognostic effect of duration of symptoms (Rougraff et al. Citation2007), others found that short duration of symptoms was associated with increased mortality (Saithna et al. Citation2008, Urakawa et al. Citation2013), and finally others found that short duration of symptoms was associated with reduced mortality (Nakamura et al. Citation2011). One possible explanation of these contractions might be that the correlation between duration of symptoms and mortality is “J”-shaped, as an expression of rapid increase in size of high-grade tumors—and thus short duration of symptoms—while low-grade tumors grow slowly and in the end may de-differentiate into higher grades. This is further supported by the findings of Maguire et al. (Citation1994), namely that the effect of duration of symptoms on survival was not linear. In accordance with this, we found that both curves were almost “J”-shaped, with higher LR and DSM rates at both ends of the symptom-duration spectrum.
The literature regarding the prognostic value of depth on mortality is controversial. In contrast to the majority of published studies (Pisters et al. Citation1996, Kattan et al. Citation2002, Weitz et al. Citation2003, Gronchi et al. Citation2005), our data did not support depth as a prognostic factor for DSM, which is supported by a few studies, including a recent study comparing the sixth and the seventh version of the American joint committee’s staging system, where depth is no longer included (Rydholm and Gustafson Citation2003, Maki et al. Citation2013). In line with our study, most studies have found that anatomical location is an important prognostic factor for mortality (Pisters et al. Citation1996, Kattan et al. Citation2002, Stojadinovic et al. Citation2002, Zagars et al. Citation2003, Gutierrez et al. Citation2007). In concordance with the majority of published studies (Pisters et al. Citation1996, Weitz et al. Citation2003, Jebsen et al. Citation2008), but not all (Stojadinovic et al. Citation2002, Biau et al. Citation2011, Citation2012), we found that location and depth were not prognostic factors for LR.
Most studies (Jebsen et al. Citation2008, Biau et al. Citation2011, Citation2012), like ours, found that radiotherapy reduces the LR rate; however, the characteristics of tumors for which radiotherapy is beneficial vary. Kaytan et al. (Citation2003) reported an effect only in high-grade tumors, while Jebsen et al. (Citation2008) reported effects in low-grade tumors also. Very few studies have investigated the effect of radiotherapy on survival, and while some found a significant impact (Gronchi et al. Citation2005, Gutierrez et al. Citation2007), others found the opposite (Weitz et al. Citation2003). A retrospective study involving 6,960 patients showed that radiotherapy improved survival for high-grade tumors, while no improvement was seen for low-grade tumors.(Koshy et al. Citation2010). Based on the local practice at the Aarhus Sarcoma Center, subgroup analyses were not obtainable in our study.
Conclusion
We conducted a large study including 922 consecutive adult non-metastatic STS patients, representing the “reality” seen in everyday clinics. Since results have a direct impact on future treatment strategies as well as the prognosis given to our patients, obtaining unbiased results is essential. In order to properly address a wide range of biases we included directed acyclic graphs, cubic splines and a competing risk model, and showed that these statistical methods are feasible. Using these improved statistical methods on a large, validated dataset, we excluded depth as an independent prognostic factor, and established that duration of symptoms and radiotherapy were important prognostic factors for DSM. Moreover, the method confirmed the importance of several prognostic factors for LR and DSM previously identified in studies with major tertiary referral practices.
Supplementary data
Figure 2 is available at Acta’s website (www.actaorthop.org), identification number 6776.
Supplementary Material
Download PDF (164.6 KB)KMN: collected clinical data, performed statistical analyses, and wrote the manuscript. NAP: collected clinical data, contributed to the statistical analyses, and proofread the manuscript. AS and JK: planned the study, treated the patients included, collected clinical data, and proofread the manuscript. PHJ and BHH: operated the patients included, collected clinical data, and proofread the manuscript. SB: examined the pathological materials, collected clinical data, and proofread the manuscript. ABP: planned the study, contributed to statistical evaluation, and proofread the manuscript.
We thank the orthopedic surgeons and oncologists from the Aarhus Sarcoma Center for registering data in the Aarhus Sarcoma Registry. The study was supported by grants from “Frits, Georg og Marie Cecilie Gluds legat”, “Max og Inge Wørzners mindelegat”, the Danish Council for Independent Research, Medical Sciences, and Aarhus University.
No competing interests declared.
Notes
- Biau DJ, Ferguson PC, Turcotte RE, Chung P, Isler MH, Riad S, Griffin AM, Catton CN, O’Sullivan B, Wunder JS. Adverse effect of older age on the recurrence of soft tissue sarcoma of the extremities and trunk. J Clin Oncol 2011; 29 (30): 4029-35.
- Biau DJ, Ferguson PC, Chung P, Griffin AM, Catton CN, O’Sullivan B, Wunder JS. Local recurrence of localized soft tissue sarcoma: A new look at old predictors. Cancer 2012; 118 (23): 5867-77.
- Cleves M. stata54: Multiple curves plotted with stcurv command. Stata Technical Bull 2000; 9: 7-10.
- Co-operative Cancer Departments. Treatment of sarcoma and aggressive benign tumors. available at: http://Www.ambkir.dk/Faglige%20retningslinier/Behandling%20af%20sarkomer.pdf. 2007; 2013.
- Costa J, Wesley RA, Glatstein E, Rosenberg SA. The grading of soft tissue sarcomas. results of a clinicohistopathologic correlation in a series of 163 cases. Cancer 1984; 53 (3): 530-41.
- Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop 1980; (153): 106-20.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94 (446): 496-509.
- Fletcher C DM, Unni KK, Mertens F. Pathology and genetics. tumours of soft tissue and bone. ( WHO classification of tumours, volume 5, third edition). IARC Press, Lyon 2002.
- Graw F, Gerds TA, Schumacher M. On pseudo-values for regression analysis in competing risks models. Lifetime Data Anal 2009; 15 (2): 241-55.
- Gronchi A, Casali PG, Mariani L, Miceli R, Fiore M, Lo Vullo S, Bertulli R, Collini P, Lozza L, Olmi P, Rosai J. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: A series of patients treated at a single institution. J Clin Oncol 2005; 23 (1): 96-104.
- Gustafson P. Soft tissue sarcoma. epidemiology and prognosis in 508 patients. Acta Orthop Scand (Suppl 259) 1994: 1-31.
- Gutierrez JC, Perez EA, Franceschi D, Moffat FLJr, , Livingstone AS, Koniaris LG. Outcomes for soft-tissue sarcoma in 8249 cases from a large state cancer registry. J Surg Res 2007; 141 (1): 105-14.
- Jebsen NL, Trovik CS, Bauer HC, Rydholm A, Monge OR, Hall KS, Alvegard T, Bruland OS. Radiotherapy to improve local control regardless of surgical margin and malignancy grade in extremity and trunk wall soft tissue sarcoma: A scandinavian sarcoma group study. Int J Radiat Oncol Biol Phys 2008; 71 (4): 1196-203.
- Jensen OM, Hogh J, Ostgaard SE, Nordentoft AM, Sneppen O. Histopathological grading of soft tissue tumours. prognostic significance in a prospective study of 278 consecutive cases. J Pathol 1991; 163 (1): 19-24.
- Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol 2002; 20 (3): 791-6.
- Kaytan E, Yaman F, Cosar R, Eralp Y, Saip P, Darendeliler E. Prognostic factors in localized soft-tissue sarcomas. Am J Clin Oncol 2003; 26 (4): 411-5.
- Koshy M, Rich SE, Mohiuddin MM. Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: A SEER analysis. Int J Radiat Oncol Biol Phys 2010; 77 (1): 203-9.
- Maguire A, Porta M, Malats N, Gallen M, Pinol JL, Fernandez E. Cancer survival and the duration of symptoms. an analysis of possible forms of the risk function. ISDS II project investigators. Eur J Cancer 1994; 30A (6): 785-92.
- Maki RG, Moraco N, Antonescu CR, Hameed M, Pinkhasik A, Singer S, Brennan MF. Toward better soft tissue sarcoma staging: Building on American Joint Committee on Cancer Staging systems versions 6 and 7. Ann Surg Oncol 2013; 20 (11): 3377-83.
- Maretty-Nielsen K, Aggerholm-PedersenNKeller,J, Safwat A, Baerentzen S, Pedersen A. Population-based Aarhus Sarcoma Registry: Validity, completeness of registration, and incidence of bone and soft tissue sarcomas in western Denmark. Clin Epidemiol 2013; 5: 45-56.
- Nakamura T, Matsumine A, Matsubara T, Asanuma K, Uchida A, Sudo A. The symptom-to-diagnosis delay in soft tissue sarcoma influence the overall survival and the development of distant metastasis. J Surg Oncol 2011; 104 (7): 771-5.
- Pedersen CB. The Danish Civil Registration system. Scand J Public Health (7 Suppl) 2011; 39: 22-5.
- Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 1996; 14 (5): 1679-89.
- Rougraff BT, Davis K, Lawrence J. Does length of symptoms before diagnosis of sarcoma affect patient survival? Clin Orthop 2007; (462): 181-9.
- Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: A bad idea. Stat Med 2006; 25 (1): 127-41.
- Rydholm A, Gustafson P. Should tumor depth be included in prognostication of soft tissue sarcoma? BMC Cancer 2003; 3 (1): 17-21.
- Saithna A, Pynsent PB, Grimer RJ. Retrospective analysis of the impact of symptom duration on prognosis in soft tissue sarcoma. Int Orthop 2008; 32 (3): 381-4.
- Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol 2008; 8: 70-84.
- Smith PL. Splines as a useful and convenient statistical tool. Am Stat 1979; 33 (2): 57-62.
- Stojadinovic A, Leung DH, Hoos A, Jaques DP, Lewis JJ, Brennan MF. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg 2002; 235 (3): 424-34.
- Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, Goussot JF, David M, Bonichon F, Lagarde C. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer 1984; 33 (1): 37-42.
- Urakawa H, Tsukushi S, Arai E, Kozawa E, Futamura N, Ishiguro N, Nishida Y. Association of short duration from initial symptoms to specialist consultation with poor survival in soft-tissue sarcomas. Am J Clin Oncol 2013; [Epub ahead of print]
- Vraa S, Keller J, Nielsen OS, Sneppen O, Jurik AG, Jensen OM. Prognostic factors in soft tissue sarcomas: The aarhus experience. Eur J Cancer 1998; 34 (12): 1876-82.
- Weitz J, Antonescu CR, Brennan MF. Localized extremity soft tissue sarcoma: Improved knowledge with unchanged survival over time. J Clin Oncol 2003; 21 (14): 2719-25.
- Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS, Evans HL. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: An analysis of 1225 patients. Cancer 2003; 97 (10): 2530-43.