Abstract
Background and purpose — Current techniques for epiphysiodesis involve opening of cortical windows; use of staples, screws, and tension devices; and fusion with curettes or drills. Complications may have serious consequences. There is a need for a more reliable, precise, and less traumatic procedure that overcomes the known complications from existing techniques. We analyzed a new epiphysiodesis technique using radio-frequency ablation (RFA) in a porcine model.
Methods — Six 35-kg and two 25-kg immature pigs were used. 1 hind leg of each animal was randomly selected and the proximal tibia growth plate was ablated laterally and medially. The contralateral leg was used as a control. MR images were obtained immediately after the ablation and 12 weeks later for 6 animals, and 24 weeks later for the other 2 animals. CT was done for the 2 animals that were followed for 24 weeks for proof of bone bridges.
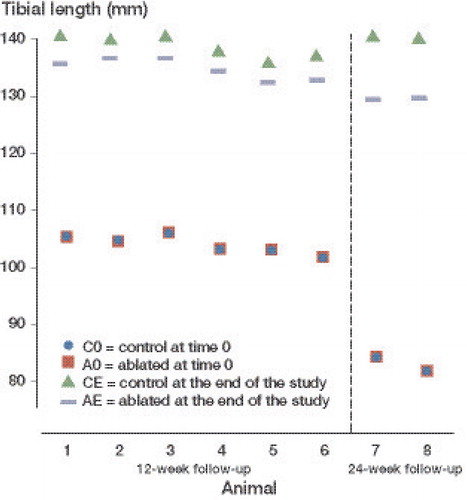
Results — Both tibias were equal in length initially. At the 12-week follow-up, there was an average leg length discrepancy of 3.9 mm (95% CI: 3.0–4.8), and at 24 weeks the difference was 8.4 mm and 7.5 mm. No damage to the adjacent tissue was found. Bone bridges and physeal closure were found after 24 weeks. The pigs showed no discomfort after the intervention.
Interpretation — We found RFA to be feasible for epiphysiodesis in a pig model. The method is minimally invasive and recovery may be quick compared to conventional methods. We recommend that the method should be tested in larger-scale safety studies before clinical application.
Epiphysiodesis was first described in 1933 by Phemister, who introduced an open surgical technique for growth inhibition (Edmonds et al. Citation2007). Alternative techniques such as the percutaneous technique described by Bowen have largely replaced the open technique during the past 20 years (Rosenthal et al. Citation2003, Edmonds et al. Citation2007). Percutaneous techniques produce bony bridges and disorganize growth plate structure, which results in growth inhibition (Dodge et al. Citation2007, Gunderson et al. Citation2013). Percutaneous methods reduce length of hospital stay, operating time, and postoperative pain, and they result in minor surgical scars compared to open surgery. Based on experimental rabbit models (Ghanem et al. Citation2009, Widmann et al. Citation2010, Moon et al. Citation2012), epiphysiodesis using radio-frequency ablation (RFA) is a successful minimally invasive method. However, this species behaves differently to humans when studying bone. The pig is considered to be one of the best animal models to study the musculoskeletal system (Aersens et al. 1998).
We assessed the feasibility of epiphysiodesis using RFA in a pig model.
Material and methods
Animals
Healthy, skeletally immature female pigs from the Yorkshire-Landrace-Duroc race were used in all procedures. The animals were fed standard Danish diet taking care of the energy concentration compound, which was 30% less than normal to obtain constant weight gain. Study A included six 15-week-old pigs weighing 35 (32–40) kg on average. Two 10-week-old animals weighing 29 kg and 28 kg were used in study B.
Design
Study A, 12 weeks of follow-up (n = 6). A paired randomized design was used with the animal as its own control. 1 tibia was randomly selected in each animal using a random number generator. The selected tibia was RF ablated at the proximal tibial physis. The other tibia was not treated. Both tibiae were MR-scanned under general anesthesia immediately after the ablation. After 12 weeks, the animals were MR-scanned under general anesthesia for a second time and then killed.
Study B, 24 weeks of follow-up (n = 2). Both animals were treated similar to the animals in study A and then followed for 24 weeks. MRI was performed immediately after surgery and after 24 weeks.
Sample size (study A)
Growth inhibition was expected in all ablated legs producing leg length discrepancy (100% success). The desired power was 90% and α = 0.05. According to these parameters, 6 animals were included in the study.
For study B, no sample size was calculated; this additional group was only added to investigate the tendency of the procedure after a longer follow-up.
Methods
Reference parameters. The MRI result obtained from the non-ablated tibiae was considered normal material and was used as a reference.
MRI. Images were obtained immediately after the procedure and at the end of the follow-up, before killing. The orientation of the MR slices was corrected once the animal was lying on the scanner table to obtain true coronal, sagittal, and axial projections. All the MR sequences were run using the same orientation. 27 images were analyzed for each specimen. The MR procedures were done under general anesthesia. A full body scanner (Siemens Magnetom Avanto 1.5 Tesla; Siemens, Erlangen, Germany) was used to acquire 4 image sets from each specimen. Tibiae from the 2 animals that were followed for 24 weeks were analyzed using CT. No other analysis was done on these animals.
Bone bridges and closure of the gap between the bones at the epiphysis (physis) were investigated.
Anesthesia and medication. All the procedures were performed under general anesthesia. For premedication, the animals received an intramuscular injection of ketaminol (5 mg/kg) and midazolam (0.5 mg/kg). Anesthesia was maintained with an intravenous infusion of propofol (5 mg/kg/h) and fentanyl (0.025 mg/kg/h). Before the ablation, the animals received 1 prophylactic intramuscular dose of penicillin (1 mL/10 kg, procaine penicillin G 150,000 IU/mL), and then on a daily basis for 3 days. The animals were administrated intramuscular Flunixin (2.2 mg/kg) for 3 days after the procedure.
Epiphysiodesis. All the procedures were done using a 7-mm, 18G radio-frequency probe without cooling (Cool-tip RF ablation system; Covidien, Mansfield, MA). Ablation power (in W) was corrected constantly during the procedure to maintain the temperature between 92°C and 98°C. Impedance fluctuated between 90 Ω and 700 Ω during the procedure.
Operation technique. Under fluoroscopic guidance, the proximal tibia growth plate was identified and a 14G penetration cannula (Bonopty Bone Biopsy System; AprioMed AB, Uppsala, Sweden) was inserted under sterile surgical technique 90 degrees from the vertical plane towards the growth plate. A stylet was drilled approximately 1 mm into the growth plate. The radio-frequency probe was inserted 1 cm into the growth plate and the ablation was done for 8 min, while maintaining the temperature of the probe between 92°C and 98°C. The procedure was done through lateral and medial approaches to the selected tibial physis. In all procedures, the tip of the radio-frequency probe was placed in the first attempt.
Follow-up. Animals had free access to food and water. Any signs of discomfort or dysfunction were assessed and based on changes in gait, activity, posture, and attitude (Swindle et al. Citation1992, Citation1998).
Killing. After the final MR scan, the animals received a lethal intravenous injection of pentobarbital (0.5 mL/kg).
Analysis
All the MR images were analyzed using “syngo fastView” software (Siemens). The proximal tibial physis was identified and then analyzed for discontinuity. The distance between the proximal and distal physes () was measured in 3 standardized positions: medial, central, and lateral on MR image 12, 13, and 14. Based on these measurements, a mean medial, central, and lateral value was calculated and a mean overall value was used as tibial length. 9 independent observations were done for each tibia. Also, lateral, central, and medial regions on the physis were delimited () and their T1-map values were calculated using the T1-map analysis software Siswin version 0.9. The presence of bone bridges was analyzed on the CT images.
Figure 1. High-resolution T1 MR images 12 weeks after the procedure. The distance between the 2 tibial physes (proximal and distal) was measured laterally, centrally, and medially (yellow lines) to compare tibial length in the control leg (left) and in the treated leg (right). Both lateral and medial sites were ablated in the treated tibia, but it is difficult to visualize both in the same MR slice.
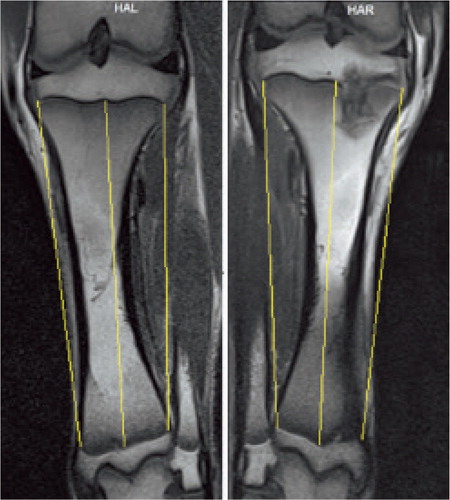
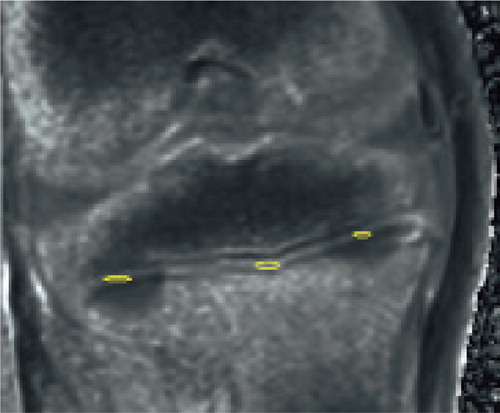
Figure 2. Water content. This image corresponds to an MR done immediately after the procedure; physis disruption is not evident. Water content was calculated in the ablation sites (yellow circles) and in a non-ablated region (in the middle) to compare the effect of the ablation.
Reproducibility. Measurements were done twice by the same observer. A coefficient of variation (CV) was calculated using the formula CV% = (δ /x¯;) 100, where δ is the sample standard deviation andx¯ is the sample mean. Also known as “relative variability”, CV describes the variation of a test, providing information about the performance of a method when being implemented several times.
Reliability. Intraclass correlation coefficients were calculated for length measurement and T1-map value calculation.
Statistics
Wilcoxon rank sum test using SOCR software (University of California Statistical Resources). We assumed randomly sampled data from a population that was distributed according to a Gaussian distribution.
Ethics
The study was conducted in compliance with the Danish Animal Research Guidelines, and it was accepted and authorized by the Danish Animal Experiment Committee (no. 2008/561-329).
Results
The mean length of the control tibiae at the beginning of the study was 102 mm (SD 6.5), and that of the ablated ones was 104 mm (SD 6.0). There was a mean difference of 0.26 mm (95% CI: 0.06–0.46).
No discomfort or dysfunction was observed immediately after the anesthesia.
Study A. At the end of the study, the mean length of the control tibiae was 132 mm (SD 10) and the mean length of the treated ones was 128 (SD 10). There was a mean difference of 3.9 mm (95% CI: 3.0–4.8) ().
Study B. A difference of 8.6 mm was seen in 1 pig and 7.5 mm in the other.
MRI. No damage to the articular cartilage or the knee was observed in the high-resolution T1-weighted and T2-weighted images at any time. On the final scanning images, the growth plate was found to be disrupted in both T1-weighted and T2-weighted images. An extended lesion into the metaphysis was observed in all cases, below the ablation site.
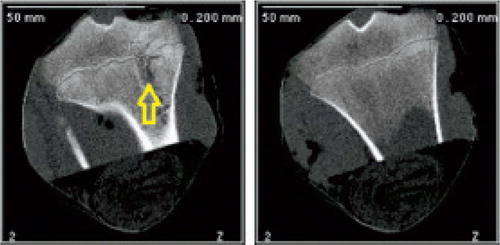
CT. A bone bridge was observed at the ablation site (), preceded and followed by physeal closure.
Figure 4. CT images taken 24 weeks after the procedure. Left panel: treated tibia. A bone bridge can be seen at the ablation site (arrow). Right panel: control, contralateral tibia.
Calculation of water content. In study A, at week 0, a mean T1-value of 1,338 (SD 134) was calculated for the control physes and 903 (SD 194) was calculated for the intervention physes. There was a mean difference of 436 (95% CI: 267–605). At 12 weeks, the mean T1-value of the control physes was 1,169 (SD 171) and that for the ablated physes was 866 (SD 110), with a mean difference of 433 (95% CI: 245–620). The difference between the values obtained at the beginning and at the end of the study was not statistically significant (p = 0.2).
Reliability. The intraclass correlation coefficient for length measurement using MR was 0.2 and that for T1-map value calculation was 0.1.
Precision. All measurements were performed twice by the same observer. The CV was 1.7% for the length measurements and 8.3% for the T1 intensity value measurements.
Discussion
In a study on rabbits using RFA, growth inhibition was achieved by radio-frequency coagulation of the physis (Ghanem et al. Citation2009). Positive results in rabbit experiments have also been reported in 2 later studies (Moon et al. Citation2012, Widmann et al. Citation2010). In these studies, RFA was done in the growing physis and the authors concluded that RFA may be useful for epiphysiodesis. However, better knowledge is needed about the ideal temperature for achievement of spontaneous irreversible growth inhibition (Campens et al. Citation2010) and experiments in large animal models are warranted before clinical introduction.
In our study on pigs, leg length discrepancy was achieved by RFA of the proximal tibial physis. This is in accordance with previous publications (Ghanem et al. Citation2009, Widmann et al. Citation2010, Moon et al. Citation2012), although these results were obtained in a rabbit model. The postoperative well-being of the animals studied indicates the clinical potential of the technique, with fast recovery after the procedure. A pig model is more clinically relevant than rodent models, because of the volume of the growth plate and the similarities in bone quality between pigs and humans (Aerssens et al. Citation1998). The limitations are that pig growth differs from that in humans because of a relatively poor longitudinal growth, but they are particularly fast in gaining weight (up to 0.7 kg a day) (CitationBriggs 1983). We ablated the proximal physis of the tibia, which contributes approximately 57% of the tibial growth estimated in humans (Pritchett Citation1992). The intact distal tibial physis kept growing normally during the follow-up period; therefore, only a small difference was found between the treated leg and the untreated leg in study A. Consequently, we added a second group (study B) to prove that the same tendency to have a shorter leg on the treated side was more evident after a longer follow-up. We did not find any previous literature regarding longitudinal growth in pigs. However, our data from study B suggest that the longitudinal growth in the proximal tibia might even exceed the growth in a human. We found a leg length difference after 24 weeks of 8 mm, which exceeds the growth expected in humans, which is approximately 6 mm. This pig model may therefore be valuable in growth-plate research.
Physeal injuries may lead to bone bridge formation, which can slow or arrest growth. These calcified structures are radio-opaque and can be visualized using X-ray-based image techniques. After percutaneous physiodesis, bone bridges can be observed using CT. These bone bridges inhibit growth and affect the normal function of the physis (Gunderson et al. Citation2013). Our results indicate that RFA gives permanent growth inhibition, as bony bridges were documented by CT—which suggests that RFA could replace current methods for epiphysiodesis. RFA involves the application of energy, resulting in a reproducible local thermal coagulate necrosis. Several studies have shown the usefulness of RFA as a minimally invasive image-guided technique. It has been increasingly used in the treatment of musculoskeletal tumors (Rosenthal et al. Citation2003). This technique has advantages over conventional surgical treatment. The method is feasible in an outpatient setting. It has a low complication rate and short recovery time (Ghanem et al. Citation2009). This technique has been reported to be a clinical success (Cairns Citation2003 Rosenthal et al. Citation2003, Cioni et al. Citation2004, Moon et al. Citation2012 ).
Complications of using RFA clinically for tumor treatment are rare (Sailhan et al. Citation2004, Papathanassiou et al. Citation2011, Schmidt et al. Citation2011). A previous study in dogs showed promising results in thermal treatment of the growth plate. The animals only showed slight discomfort and were able to walk with their full weight immediately after the treatment (Corby et al. Citation2008). RFA can be safely performed close to the joint surface without damaging the cartilage (Martel et al. Citation2008). RFA induces an energized volume that can be predicted as long as it occurs in solid tissue with high water content (Sang et al. Citation2012). The bone above the physis is able to contain the energy and heat, preventing it from proceeding and damaging the surrounding structures. Below the physis, the metaphysis is rich in blood vessels that contain a large amount of water. This conducts heat and causes coagulation, as observed below the ablation sites on the MR images from the end of the study. MRI can be used to evaluate and map physeal closure, which can be caused by lack of physeal activity or bone bridges (Sydner et al. Citation2001, Friend and Widmann Citation2008). The physis is well seen on most MRI sequences and has signal characteristics that are similar to those of articular cartilage (Moon et al. Citation2012). Based on T1-value calculations, we found reduction in the water content of the physis immediately after the procedure. This decrement persisted after 12 and 24 weeks. The first and the second MR-based water content measurements on the treated physis were similar, which can be interpreted as the effect of epiphysiodesis: after causing necrosis, the area cannot have the same amount of water as it had before, when healthy. The amount of water should be less, which is what we tended to find. Water content adds important information about the reaction of the physis and metaphysis to RFA. The metaphyseal lesion that we found could be related to coagulation. It is possible that the metaphyseal lesion caused contributed to growth inhibition. Vascularization and oxygenation of the plate itself as a local microenvironment have been described as the main regulators of physis function because chondrocytes react to oxidative stress as a stimulus for cell death (Shapiro et al. Citation2005).
In conclusion, epiphysiodesis using RFA can disrupt the growth plate, it causes bone bridge formation, and it leads to growth inhibition in a large animal model.
JSM: experimental setup and interventions, imaging studies, data analysis, and writing of the manuscript. OR: assessment of the experimental setup and manuscript correction. AHA: assisted with experimental interventions. HSJ: assessor of imaging studies. BMM: main supervisor of the study.
We thank Søren Juul Hansen for his pilot study and Covidien for facilitating the RF generator and providing technical assistance.
No competing interests declared.
- Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: Potential implications for in vivo bone research. Endo 1998; 139 (2): 663-70.
- Briggs HM. Danish Landrace. International Pig Breed Encyclopedia, 1983. Elanco Aimal Health. Oklahoma State University.
- Cairns R. Magnetic resonance imaging of the growth plate: pictorial essay. JACR 2003; 54 (4): 234-42.
- Campens C, Mousny M, Docquier PL. Comparison of three surgical epiphysiodesis techniques for the treatment of lower limb length discrepancy. Acta Orthop Belg 2010; 76: 226-33.
- Cioni R, Armillotta N, Bargellini I, Zampa V, Cappelli C, Vagli P, et al. CT-guided radiofrequency ablation of osteoid osteoma: long term results. Eur Radiol 2004; 14: 1203-8.
- Corby RR, Stacy GS, Peabody TD, Dixon LB. Radiofrequency ablation of solitary eosinophilic granuloma of bone. AJR 2008; 190: 1492-4.
- Dodge GR, Bowen JR, Oh CW, Tokmakova K, Simon BJ, Aroojis A, Potter K. Electrical stimulation of the growth plate: A potential approach to an epiphysiodesis. Bioelectromagnetics 2007; 28: 463-70.
- Edmonds EW, Stasikelis PJ. Percutaneous epiphysiodesis of the lower extremity. A comparison of single- versus double-portal tTechniques. J Pediatr Orthop 2007; 27 (6): 618-22.
- Friend L, Widmann RF. Advances in management of limb length discrepancy and lower limb deformity. Curr Opin Pediatr 2008; 20: 46-51.
- Ghanem I, El Hage S, Diag M, Saliba E, Khazzaka A, Aftimos G, et al. Radiofrequency application to the growth plate in the rabbit: A new potential approach to epiphysiodesis. J Pediatr Orthop 2009; 29 (6): 629-35.
- Gunderson RB, Horn J, Kibsgård T, Kristiansen LP, Pripp AH, Steen H. Negative correlation between extent of physeal ablation after percutaneous permanent physiodesis and postoperative growth. Acta Orthop 2013; 84 (4): 426-30.
- Martel J, Bueno A, Domínguez MP, Llorens P, Quirós J, Delgado C. Percutaneous radiofrequency ablation: relationship between different probe types and procedure time on length and extent of osteonecrosis in dog long bones. Skeletal Radiol 2008; 37: 147-15.
- Moon SG, Kang HS, Hong SH, Kim NR, Lee JW, Lim SD. Chronologic change in the growth plate after radiofrequency-induced thermal injury: MRI-histologic correlation. AJR 2012; 198: 163-72.
- Papathanassiou ZG, Petsas T, Papachristou D, Megas P. Radiofrequency ablation of osteoid osteomas: Five years experience. Acta Orthop Belg 2011; 77: 827-33.
- Pritchett JW. Longitudinal growth and growth-plate activity in the lower extremity. Clin Orthop 1992; (275): 274-9.
- Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: Percutaneous treatment with radiofrequency energy. Radiology 2003; 229: 171-5.
- . Sailhan F, Chotel F, Guibal A-L, Gollogly S, Adam P, Bérard J, et alThree-dimensional MR imaging in the assessment of physeal growth arrest. Eur Radiol 2004; 14: 1600-8.
- Sang Z, Aarya I, Gueorguieva M, Liu D, Luo H, Mandfredi L, et al. Image-based 3D modeling and validation of radiofrequency interstitial tumor ablation using a tissue-mimicking breast phantom. Int J CARS 2012; 7: 941-8.
- Schmidt D, Clasen S, Schaefer JF, Rempp H, Duda S, Trübenbach J, et al. CT-guided radiofrequency (RF) ablation of osteoid osteoma: Clinical long-term results. Fortsch Röntgenstr 2011; 183: 381-7.
- Shapiro IM, Adams CS, Freeman T, Srinivas V. Fate of the hyperthrophic chondrocyte. Microenvironmental perspectives on apoptosis and survival in the epiphyseal growth plate. Birth Defects Research (Part C) 2005; 75: 330-9.
- Swindle MM. Swine as models in biomedical research, Ames: Iowa State University Press; 1992.
- Swindle MM. Surgery, anesthesia and experimental techniques in swine.Ames: Iowa State University Press; 1998.
- Sydner M, Harcke HT, Conard K, Bowen JR. Experimental epiphysiodesis: magnetic resonance imaging evaluation with histopathologic correlation. Int Orthop 2001; 25: 337-42.
- Widmann RF, Amaral TD, Yildiz C, Yang X, Bostrom M. Percutaneous radiofrequency epiphysiodesis in a rabbit model. A pilot study. Clin Orthop 2010; (468): 1943-8.