Abstract
Late stent thrombosis represents a life-threatening event, usually triggered by inadequate antiplatelet therapy and promoted by multiple risk factors, such as stenting of a chronic total occlusion, overlapping stenting, an abnormal vascular response to the eluted drug, stent malapposition and stent fracture. A 57-year-old man with aspirin hypersensitivity underwent successful percutaneous revascularization of a chronic total occlusion of the left anterior descending artery (LAD). He received two sirolimus-eluting stents overlapping for 2 mm and was discharged on clopidogrel and picotamide. Two years later, 15 days after clopidogrel discontinuation, he experienced an anterior ST-segment elevation myocardial infarction and underwent rescue percutaneous LAD thrombectomy after unsuccessful fibrinolysis. Coronary angiography showed fracture of the distal stent, with a 5 mm gap between the two portions, as well as severe late stent malapposition, confirmed by optical coherence tomography. Despite treatment with clopidogrel and picotamide, in the following days the patient experienced two new episodes of stent thrombosis, treated with thrombectomy and deployment of bioengineered stents. The patient underwent successful oral aspirin desensitization, with a complete in vitro inhibition of platelet function, and was discharged on aspirin, clopidogrel and warfarin, without experiencing other events at 6-month follow-up.
Keywords::
Very late drug eluting stent (DES) thrombosis represents a life-threatening event, usually triggered by antiplatelet therapy discontinuation. Its management can be very difficult, in particular in patients with multiple prothrombotic risk factors, such as the presence of multiple or long stents, positive vascular remodelling, dissections, stent malapposition and stent fracture. Stent strut thickness, nonerodable polymers and cytotoxic drugs loaded on DES also play an important prothrombotic role (Citation1). Recent advances in intravascular imaging allow an accurate in vivo detection of these prothrombotic vascular conditions.
A 57-year-old man with cutaneous hypersensitivity to aspirin underwent successful percutaneous revascularization of a chronic total occlusion of the left anterior descending artery (LAD). He received two sirolimus-eluting stents (Cypher, Cordis, Johnson and Johnson Company) overlapping for 2 mm and was discharged on clopidogrel 75 mg/day and picotamide 300 mg/day.
Two years later, 15 days after clopidogrel discontinuation, he experienced an anterior ST-elevation myocardial infarction and underwent successful rescue percutaneous LAD thrombectomy after unsuccessful fibrinolysis. Coronary angiography showed fracture of the distal stent with a 5 mm gap between the 2 portions (, Online Video 1), as well as late stent malapposition despite a preserved stent expansion (, Online Video 2). Optical coherence tomography (OCT, ImageWire, LightLab imaging) confirmed stent malapposition (, Online Video 3) and showed microdissection of the distal LAD ().
Figure 1. A. Angiographic anteroposterior straight view of the LAD, showing stent fracture (arrow). B. Angiographic left-anterior-oblique cranial view of the LAD, showing micro-aneurisms due to stent malapposition (arrow) after successful percutaneous thrombectomy.
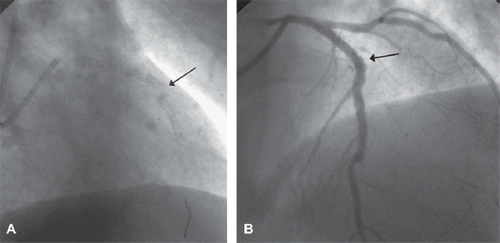
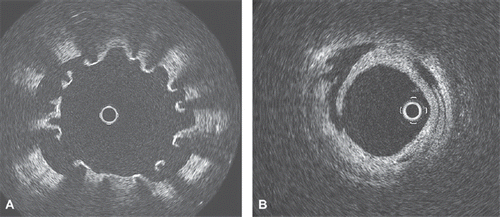
Figure 2. A. OCT image of the distal stent deployed in mid LAD after successful percutaneous thrombectomy, showing a separation of stent struts from the arterial wall, due to stent malapposition. B. OCT image of the distal LAD after successful percutaneous thrombectomy, showing residual stratified thrombus and dissection of the vascular wall.
Despite treatment with clopidogrel and picotamide, a second episode of stent thrombosis occurred a week later, treated with percutaneous thrombectomy. Coronary angiography and OCT confirmed a marked vascular remodelling, treated with deployment of bioengineered stents coated with antibodies to attract endothelial progenitor cells and favour rapid endothelialisation: one stent (Genous 2.75/15, OrbusNeich) was placed between the stent fracture, two overlapping stents (Genous 2.75/18 and Genous 2.75/15) downstream in the mid LAD and other two overlapping stents (Genous 2.75/18 and MiniVision 2.25/15) in the distal LAD. Complete stent expansion was obtained with balloon post-dilation and confirmed with OCT imaging. Moreover, clopidogrel and picotamide were double-dosed.
One day before hospital discharge, the patient experienced a third episode of ST-segment elevation. Coronary angiography confirmed stent thrombosis, treated with percutaneous thrombectomy. After this third episode, the patient underwent successful oral desensitization to aspirin (Citation2) and introduction of oral anticoagulant therapy. Laboratory analysis showed a complete inhibition of platelet function, and ruled out the presence of antiphospholipid auto-antibodies or abnormal levels of coagulation factors. He was discharged on aspirin 100 mg/day, clopidogrel 150 mg/day and warfarin, remaining asymptomatic at six-month follow-up.
In the present case, discontinuation of clopidogrel probably triggered the first episode of very late DES thrombosis, in a markedly remodelled vessel, with severe late stent malapposition and stent fracture. This suggests the ineffectiveness of picotamide monotherapy in high risk patients and the importance of a lifelong clopidogrel therapy. The subsequent two episodes of stent thrombosis occurred despite double antiplatelet therapy with clopidogrel and picotamide, suggesting the ineffectiveness of this combination in an acute setting of platelet hyper-reactivity, and were successfully managed with aspirin desensitization and warfarin introduction. In particular, early aspirin desensitization in patients with known hypersensitivity, besides a thieno pyridinic antiplatelet agent, should always be considered prior to coronary interventions. In high risk patients, new antiplatelet agents such as cilostazol or prasugrel could be considered, in particular prasugrel is a third-generation thienopyridine with a faster and more potent antiplatelet action than clopidogrel (Citation3).
Stent thrombosis might have been favoured also by stent malapposition, i.e. the separation of stent struts from the arterial wall, serving as a local nidus for thrombus formation. Late acquired malapposition has been observed more frequently with DES versus bare-metal stent (7–21% versus 2–5%, respectively), particularly with overlapping stents and stenting of chronic total occlusions (Citation4). Several mechanisms of malapposition have been proposed: positive arterial remodelling; dissolution of plaque debris; stent under expansion (i.e. persistent malapposition already present at the time of stent deployment); chronic stent recoil without any change in arterial dimension (Citation4). Alternatively, malapposition may be a marker for other thrombogenic mechanisms, such as delayed re-endothelialization, impaired vasomotion, and chronic inflammation.
Stent fracture is another possible prothrombotic risk factor, frequently associated with aneurysms and malapposition, occurring in particular with longer stents, overlapping stents, hinge movements, angulated vessels (Citation5,Citation6). Nearly all stent fractures reported occurred in sirolimus-eluting stents, with an incidence of 0.8% to 7.7% (Citation5).
In this patient, intravascular OCT contributed to explain the underlying vascular substrate of the recurrent stent thrombosis triggered by inadequate antiplatelet therapy, and to suggest a tailored therapy with high-dose antiplatelet drugs, oral anticoagulants and deployment of bioengineered stents. In particular, OCT allows imaging of vascular wall remodelling similarly to intravascular ultrasound, but with a 10 times higher resolution and fewer artefacts. Had OCT imaging been performed after initial stent deployment, it could have identified whether stent malapposition was present from the very first procedure, allowing an immediate treatment with balloon post-dilation, or it developed later.
Supplementary Material
Download PDF (163.8 KB)Online Videos 1–3.
Download Microsoft Video (AVI) (2.5 MB)Online Videos 1–3.
Download Microsoft Video (AVI) (2.3 MB)Online Videos 1–3.
Download Microsoft Video (AVI) (6.3 MB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lüscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, . Drug-eluting stent and coronary thrombosis: Biological mechanisms and clinical implications. Circulation 2007;115:1051–8.
- Rossini R, Angiolillo DJ, Musumeci G, Scuri P, Invernizzi P, Bass TA, . Aspirin desensitization in patients undergoing percutaneous coronary interventions with stent implantation. Am J Cardiol. 2008;101:786–9.
- Coccheri S. Antiplatelet drugs—do we need new options? With a reappraisal of direct thromboxane inhibitors. Drugs 2010;70: 887–908.
- Cook S, Wenaweser P, Togni M, Billinger M, Morger C, Seiler C, . Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation 2007;115:2426–34.
- Doi H, Maehara A, Mintz GS, Tsujita K, Kubo T, Castellanos C, . Classification and potential mechanisms of intravascular ultrasound patterns of stent fracture. Am J Cardiol. 2009;103:818–23.
- Kandzari DE, Rao SV, Moses JW, Dzavik V, Strauss BH, Kutryk MJ, . Clinical and angiographic outcomes with sirolimus-eluting stents in total coronary occlusions. J Am Coll Cardiol Interv. 2009;2:97–106.