ABSTRACT
Strenuous exercise, such as running a marathon, is known to suppress mucosal immunity for up to 24 hr, which can increase the risk of developing an upper respiratory tract infection (URTI) and reduced performance capacity (Allgrove JE, Geneen L, Latif S, Gleeson M. Influence of a fed or fasted state on the s-IgA response to prolonged cycling in active men and women. Int J Sport Nutr Exerc Metab. 2009;19(3):209–221; Barrett B, Locken K, Maberry R, Schwamman J, Brown R, Bobula J, Stauffacher EA. The Wisconsin Upper Respiratory Symptom Survey (WURSS): a new research instrument for assessing the common cold. J Fam Pract. 2002;51(3):265; Carpenter KC, Breslin WL, Davidson T, Adams A, McFarlin BK. Baker's yeast beta glucan supplementation increases monocytes and cytokines post-exercise: implications for infection risk? Br J Nutr. 2012;1–9). While many dietary interventions have been used to combat postexercise immune suppression, most have been ineffective. The key purpose of this study was to determine if baker's yeast β-glucan (BG) could positively affect the immune system of individuals undergoing intense exercise stress using two experiments. In the first (E1; N = 182 men and women), BG was compared to placebo supplementation for the incidence of URTI symptoms for 28 days postmarathon. In the second (E2; N = 60 men and women) changes in salivary immunoglobulin A (IgA) were evaluated after 50-min of strenuous cycling when participants had been supplemented for 10 days with either BG (250 mg/day) or placebo (rice flour). For E1, subjects reported URTI symptoms using a daily health log. For E2, saliva was collected prior to, immediately, and 2-hr postexercise using a salivette. Data for E1 and E2 were analyzed using separate analyses of variance (ANOVAs) with repeated measures (p < .05). In E1, BG was associated with a 37% reduction in the number of cold/flu symptom days postmarathon compared to placebo (p = .026). In E2, BG was associated with a 32% increase in salivary IgA (p = .048) at 2 hr after exercise compared to placebo. In summary, the present study demonstrates that BG may reduce URTI symptomatic days and improve mucosal immunity (salivary IgA) postexercise.
KEYWORDS. :
INTRODUCTION
Temporary disruptions in mucosal immunity are common in the 24 hr after a strenuous exercise session, resulting in an increased risk of developing an upper respiratory tract infection (URTI) (McFarlin, Flynn, Stewart, & Timmerman, Citation2004; Woods, Davis, Smith, & Nieman, Citation1999; Walsh et al., Citation2011). Sickness can result in lost practice days, reductions in performance, and lost working days. In the case of athletes, physical laborers, police officers, firefighters, and soldiers, such illness may increase the risk of job-related injuries and fatalities due to fatigue. The first line of defense against respiratory viruses and bacteria is mucosal immunity, which is characterized by salivary immunoglobulins (Igs) and antimicrobial proteins (Walsh et al., Citation2011). Previous reports have demonstrated that salivary IgA is reduced following a strenuous exercise session and that reduced salivary IgA is associated with an increase in the number of URTI symptomatic days postexercise (Allgrove, Geneen, Latif, & Gleeson, Citation2009; Davison & Diment, Citation2010; Moreira, Arsati, de Oliveira Lima-Arsati, de Freitas, & de Araújo, Citation2011; Peters, Shaik, & Kleinveldt, Citation2010; Sari-Sarraf, Doran, Clarke, Atkinson, & Reilly, Citation2011; Usui et al., Citation2011). This established link between salivary IgA levels and URTI symptomatic days postexercise in the literature has resulted in the accepted use of salivary IgA levels as a proxy measure for mucosal immunity.
Of the many dietary interventions evaluated for potential to enhance/modulate the immune response following exercise, beta glucans (BGs) have been repeated targets (Goodridge et al., Citation2011; Harger-Domitrovich, Domitrovich, & Ruby, Citation2008; Hong et al., Citation2004; US Pharmacopiea, Citation2011; Qi et al., Citation2011; Talbott & Talbott, Citation2009). The term BG includes carbohydrates with many different linkage patterns (Qi et al., Citation2011), leading to great variation in outcomes with regard to the efficacy of BG to modulate immunity. For example, grain BGs have a linear structure 1/3, 1/4 linkage pattern while fungal (including yeast) have a branched 1,3/1,6 linkage pattern. The frequency and length of side chain branches have been shown to have important implications for biological activity; in general, the higher the degree of branching, the more biologically active the BG (US Pharmacopiea, Citation2011; Qi et al., Citation2011). Beta 1,3/1,6 glucans bind with specific immune receptors including Dectin 1 and complement receptor 3 (CR3) (Goodridge et al., Citation2011; Hong et al., Citation2004). In the body, BG is phagocytized by macrophages and broken down into smaller fragments, which are released over several days (Harger-Domitrovich et al., Citation2008; Qi et al., Citation2011; Talbott & Talbott, Citation2009). These fragments interact with and modulate the functional capacity of many innate immune cells (i.e., granulocytes and macrophages), the complement system, and antibody-mediated immunity (Harger-Domitrovich et al., Citation2008; Qi et al., Citation2011; Talbott & Talbott, Citation2009). The latter is the target of the present investigation since salivary IgA plays a role in the defense of the mucosal space.
While reducing the exercise stimulus or the stress of the work environment will improve immune outcomes; such reductions are not always possible due to the exercise or work requirements. Much research is being done to identify and describe nutritional countermeasures that may possess immune boosting potential (Allgrove et al., Citation2009; Davison & Diment, Citation2010; Sari-Sarraf et al., Citation2011); however, the results of such investigations have been mixed and limited. BG exists in various forms from different sources such as mushrooms, oats, or yeast. Previous research has documented the efficacy of using a purified, commercially available preparation of baker's yeast BG (Wellmune WGP®) to reduce URTI symptomatic days postmarathon (Qi et al., Citation2011; Talbott & Talbott, Citation2009). Similar reductions in URTI symptoms were reported in a separate study that examined forest firefighters who work in a physically stressful occupation (Harger-Domitrovich et al., Citation2008). The objective of the present twofold investigation are, first, to confirm the results of other investigators of the effect of BG supplementation on URTI symptomatic days postmarathon (Harger-Domitrovich et al., Citation2008) and, second, to evaluate the ability of BG to modulate mucosal immunity by monitoring salivary IgA levels after a strenuous bout of exercise. These objectives were achieved in two independent experiments in which participants were supplemented with either BG or placebo and monitored for either URTI symptoms for 28 days postmarathon or changes in salivary IgA after 50 min of strenuous cycling.
METHODS
Approach to the Problem
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the University of Houston (UH) Committee for the Protection of Human Subjects. Written informed consent was obtained from all subjects/patients prior to being screened for exclusion/inclusion criteria. It is important to note that both experiments utilized double-blind procedures and a placebo group to ensure the integrity of the key outcome measures (Rowe et al., Citation2009). The present study was completed using two separate experiments that employed different exercise models and are described in more detail below.
Experimental Blinding
Given that an industry partner funded the present project, great care was taken to minimize bias of the results. We followed previously published procedures regarding how to safeguard data when funding comes from an industry source (Rowe et al., Citation2009). Specifically, our partner was not involved with any aspect of the data collection, analysis, or interpretation. All supplements were provided to the project team using random code numbers. According to double-blind procedures, no member of the study staff [including the principal investigator (PI)] nor the subjects were privy to the condition codes. Once both experiments were complete and raw data reports had been submitted to Biothera, the PI and his team were decoded (unblinded) as to the study conditions in order to allow for further interpretation and preparation of the current manuscript.
Inclusion/Exclusion Criteria
Subjects who volunteered for either experiment were screened for inclusion/exclusion criteria using a brief medical history form. Inclusion criteria included low–moderate intake of alcohol (<1 drink per day), physically active for at least the past 6 months, and normal body weight [BMI (body mass index) 20.0–24.9]. Exclusion criteria included weight loss of >2 pounds in the last month, taking medications known to influence the immune system (i.e., antibiotics, corticosteroids, allergy medication, cold/flu medication, etc.), taking medications for high blood cholesterol (i.e., Lipitor, etc.), have an autoimmune disease or immune compromising disease (i.e., type I diabetes mellitus, HIV, hepatitis, etc.), taking dietary supplements purported to influence the immune system (i.e., zinc, excessive vitamin C, etc.), recently had a flu vaccine (within past 3 months), current URTI symptoms, tobacco users, asthmatics, active form of type II diabetes mellitus, active form of cardiovascular disease, and individuals who took ibuprofen, naproxen sodium, acetaminophen, and/or aspirin on a daily basis.
Baker's Yeast Beta Glucan Supplement
BG was prepared by Biothera: The Immune Health Company (Eagan, MN) and consisted of β 1,3/1,6 glucans derived from baker's yeast (Saccharomyces cerevisiae) (US Pharmacopiea, Citation2011). The Biothera source of BG is unique because it has a consistent, well-defined chemical structure with a known and reproducible branching and linkage pattern. Rice flour was used as a placebo because it was similar in color and appearance to the BG supplement. Both supplements were in powder form and were packed into VegeCap® capsules prior to packaging in individual coded bottles. In both experiments, subjects were randomly assigned to a study condition with the exception that we attempted to assign the same number of men and women to each study condition. In experiment one, subjects only participated in 1 of 3 possible supplement conditions. In experiment two, subjects were randomized to an experimental condition order because all subjects completed both conditions (random, counterbalanced design).
Experiment One: Subjects
Men and women (29–46 years) who were registered to run the 2011 Austin Live Strong Marathon (Austin, TX) volunteered to participate in our study. A total of 324 subjects were enrolled at the prerace exposition for the marathon; however, only 182 completed all study requirements (∼44% attrition). Prior to the study, we completed an a priori sample size analysis using a preliminary data set (Talbott & Talbott, Citation2009) and determined that we needed a minimum of 50 subjects in each BG group and 30 in the placebo group in order to detect BG alterations in reported URTI. Our final number of subjects who completed each condition exceeded our a priori determination, so the study was appropriately powered. Eligible subjects were provided with directions for completing and returning the forms used in the study. We also collected additional subject descriptive information that is summarized in .
TABLE 1. Experiment One: Marathon Study Subject Characteristics
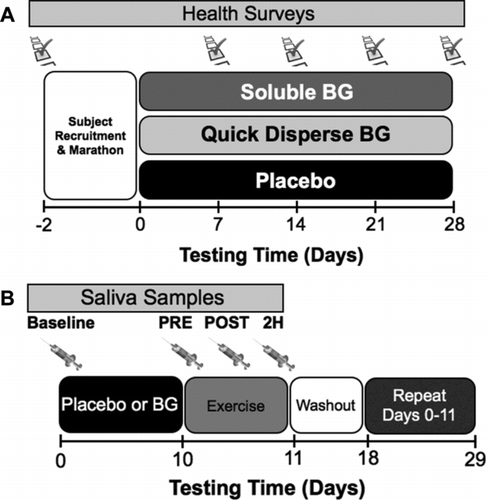
FIGURE 1. Testing Timelines for Experiment One (A) and Two (B). (A) Testing in experiment one was completed using a field study where subjects completed 28 days of supplementation with either soluble BG, insoluble BG, or placebo (rice flour) after completion of a marathon. Subjects were provided two packets of health surveys in a business reply envelope and asked to return them to the laboratory for evaluation at 14 and 28 days postmarathon. (B) In experiment two, subjects were supplemented with either insoluble BG or placebo in a randomized crossover design for 10 days prior to a cycling bout (49 ± 6 min) in a hot (45°C), humid (50% relative humidity) environment. Saliva samples were collected with a salivette at baseline (prior to supplementation), prior to exercise (PRE), immediately (POST), and 2 hr (2H) after exercise.
Experiment One: Beta Glucan Supplementation
Subjects were randomized to one of three treatment conditions by having them select a subject bag at random: soluble BG, insoluble BG, or placebo. Subjects were instructed to consume one capsule (250 mg) each morning with food for the 28 days after completing the marathon. Subjects were provided more capsules than needed in the event that they lost one or more while completing the study. Details of the testing timeline are presented in .
Experiment One: Health Questionnaires
Subjects completed daily tracking of the presence of symptoms associated with URTI. On any day when subjects indicated they were experiencing URTI symptoms, they also completed the clinically validated Wisconsin Upper Respiratory Symptom Survey (WURSS-44) (Barrett, et al., Citation2002). The University of Wisconsin granted contractual permission to the UH to use the WURSS for the present investigation. Subjects also were asked to complete a weekly profile of mood states (POMS) questionnaire to evaluate and track mental health. All surveys were created in a scannable form (ZipScan Survey, Salt Lake City, UT) that allowed for semiautomated data entry. Subjects were asked to return their completed forms at weeks 2 and 4 postmarathon in a business reply envelope. Subjects were incentivized with a $25 gift card after weeks 2 and 4 to complete and return each set of forms.
Individual surveys were scanned to a 150 dpi tiff image format using a standard Xerox office copier (Xerox, Norwalk, CT, USA). Scanned surveys were saved to a network hard drive and individually uploaded an MS Excel worksheet using the ZipScan Survey software. Once entered, data were verified to ensure that the correct data for each subject time point had been entered.
Experiment One: Statistical Analysis
Prior to formal statistical testing, data were checked for normality and constant error variance using the EXPLORE function in SPSS (v 19.0; Chicago, IL). Non-normal data were log-transformed as noted in the Results section. Each outcome variable was separately tested for significance using a 3 (Group: soluble, insoluble, or placebo) by 4 (Time: 1, 2, 3, and 4 weeks postmarathon) linear mixed model with repeated measures on the second factor (time). Significance was set at p < .05. Location of significant main effects and interactions was determined using a Tukey post-hoc test.
Experiment Two: Subjects
Subjects (18–35 years) were identified from a larger ongoing study that was designed to evaluate the effect of BG on other immune system changes as a result of intense exercise. Subject recruitment for the larger study is described in detail elsewhere (Carpenter, Breslin, Davidson, Adams, & McFarlin, Citation2012). Briefly, we screened 289 individuals and 69 met all of our inclusion/exclusion criteria (see detailed criteria mentioned earlier). Of these 69, 9 subjects either dropped out or were excused from the study for failure to follow study protocol (final N = 60). Subject demographics are presented in . The initial screening session included the measurement of height (stadiometer), weight (digital scale), body composition [whole body dual-energy X-ray absorptiometry (DXA) scan; Hologic Discovery W, Bedford, MA], and peak aerobic fitness level (VO2peak; graded exercise test on an electronically braked cycle ergometer; Velotron; Portland, OR).
TABLE 2. Experiment Two: Lab Study Subject Characteristics
Experiment Two: Beta Glucan Supplementation
Subjects were supplemented with either BG or placebo in a random, counterbalanced order. Once approved, subjects were randomly assigned to one of two orders One, placebo then BG or Two, BG then placebo. This arrangement was designed to minimize order effects associated with carryover between experimental conditions. A minimum of 7 days separated the completion of each trial condition. In a previous study, we demonstrated that the 7-day washout period was sufficient to restore the immune system to pre-BG supplementation levels (Carpenter et al., Citation2012). In both conditions, subjects consumed a daily 250-mg capsule for 10 days prior to the experimental exercise trial. Details of the testing timeline for experiment two are presented in .
Experiment Two: Exercise Trial
Exercise consisted of up to 60 min of cycling in a hot (38°C ± 2°C), humid (45% ± 2%) environment. During the exercise trial, physiological stress was monitored by the measurement of core body temperature (rectal, TC), heart rate (HR), and rating of perceived exertion (RPE) (McFarlin & Mitchell, Citation2003). Exercise sessions were stopped if the subject reached a TC > 39.2°C. In the event that the subject stopped before 60 min, then the exercise duration was matched for the next exercise trial for that individual to ensure similar amounts of exercise stress were applied between trials. This was further confirmed by monitoring the other physiological variables that we measured. Given these factors, subjects in the present study completed an average of 49 ± 6 min of exercise per experimental exercise session. Subjects were allowed water intake ad libitum during the exercise trial and after recovery from exercise; however, they refrained from drinking within 15 min of a saliva collection.
Experiment Two: Saliva Collection
Subjects arrived at the laboratory following an overnight fast (>8 hr) and abstention (>24 hr) from exercise. After at least 15 min of seated rest, subjects were instructed to place a salivette under the tongue for 2 min (Salimetrics, State College, PA). At the end of the saliva collection period, the subject placed the salivette in a salivette storage tube (without touching it with their hands), which was capped, labeled, and stored at −80°C. Saliva was collected prior to supplementation (baseline), after supplementation and prior to exercise (PRE), immediately after exercise (POST), and 2 hr after exercise (2H). This sampling schedule resulted in the collection of eight saliva samples per subject (four per arm of the trial).
Experiment Two: Salivary Immunoglobulin Analysis
After all saliva samples had been collected, collection tubes were thawed and centrifuged (30 min at 300 × g) to remove saliva from salivettes into the bottom of the storage tube. Saliva was then transferred to a freezer tube and diluted 1:750 using sterile phosphate buffered saline (PBS; Sigma-Aldrich, St. Louis, MO). The saliva dilution factor was previously determined to ensure that all raw values would fall within the linear range of the standard curve. We used the Luminex MagPix platform (Austin, TX) along with an EMD Millipore Milliplex kit (HGAMMAG-301K; Billerica, MA) optimized for the measurement of salivary IgA. In addition to study samples, a positive control (included in the kit) was a part of all assays. All of the study samples were analyzed in duplicate on the same day to minimize day-to-day assay performance. The inter- and intra-assay coefficient of variation was <11%. After Luminex analysis, calculated values were adjusted to account for the initial dilution factor.
Experiment Two: Statistical Analysis
The data presented here were collected during a larger study described previously (Carpenter et al., Citation2012). Since the larger study was ongoing, we were unable to complete an a priori sample size calculation using preliminary data; however, the larger study was powered to detect other immune system variables that had a small effect size. Based on the assumption that changes in salivary IgA were likely to have a similar or larger effect size, it is reasonable to assume that the present study was appropriately powered to detect changes in salivary IgA. Prior to formal statistical testing, data were analyzed for normality and constant error variance. Non-normal data were transformed to stabilize assumptions (noted in the Results section by variable). Exercise response variables were analyzed using a 2 (BG and placebo) by 5 (10, 20, 30, 40, and 50 min of exercise) analysis of variance (ANOVA) with repeated measures on both factors. Salivary Igs were analyzed using a separate 2 (BG and placebo) by 4 (baseline, PRE, POST, and 2H) ANOVA with repeated measures on both factors. Significance was set at p < .05 and significant p values were adjusted using the Huynh–Feldt method to account for the repeated measures design. Location of significant effects was determined using a Tukey post-hoc test. Data are reported as the mean ± SE (standard error).
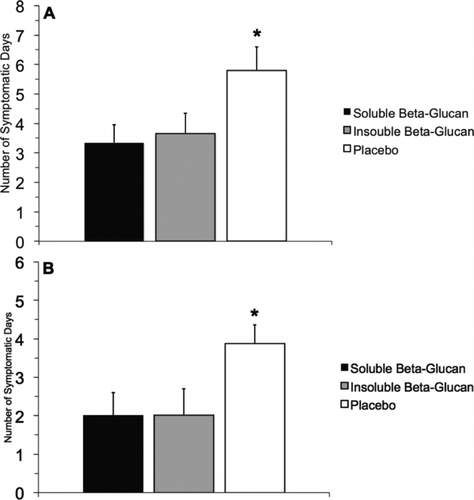
FIGURE 2. Postmarathon Sickness. (A and B) Subjects reported significant reductions in the number of URTI symptomatic days postmarathon when consuming BG compared with placebo. Values represent the average number of days a group of subjects reported health issues out of a maximum of 28 possible reporting days. BG was associated with an ∼50% reduction in URTI symptomatic days compared to placebo. The symbol “*” indicates placebo greater than both soluble and insoluble BG.
RESULTS
Experiment One: Marathon Results
Approximately 52% of the subjects in each group were women. The average marathon completion time (4:18:12 ± 0:10:11) did not differ significantly between subject conditions. Thus, we assume that given the similar characteristics of the subjects enrolled in each supplement group, the quantity of stress during the performance of the marathon was also similar. Complete race results can be found in .
Experiment One: Health Questionnaires
We found a significant main effect for time (p = .001) by global mental health (as measured by POMS), where mental health was lowest 7 days after and highest 28 days after the marathon. Data associated with post marathon health is presented in Figure 2. There were no significant differences between supplement conditions for global mental health. On the daily URTI symptom log, there was a significant condition by time interaction (p = .026) for the following question: “Did you experience any health problems today (i.e., cold, flu, etc.)?” Subjects in the placebo group reported yes to this question on 5.8 ± 0.6 days compared to 3.5 ± 0.6 (insoluble BG) and 3.5 ± 0.8 (soluble BG). There was also a trend toward significance (p = .096) for the following question: “Did you experience any cold symptoms today?” Subjects in the placebo group reported yes to this question on 3.9 ± 0.2 days compared to 2.4 ± 0.2 (insoluble BG) and 2.2 ± 0.6 (soluble BG). In general, the statistical power associated with the WURSS-44 measurements was low, so statistical significance was not achieved for this outcome.
Experiment Two: Physiologic Exercise Stress
Based on the outcome measures that we selected, there was significant main effect for time for TC (p < .001), HR (p < .001), and RPE (p < .001); however, there was no difference between conditions. TC increased by 6% over the course of exercise (37.0°C ± 0.2°C to 39.2°C ± 0.1°C), HR increased by 55% (100 ± 10 to 180 ± 11 bpm), and RPE increased by 33% (6 ± 1 to 18 ± 2). Based on these measurements, we are confident that the physiological stress associated with the experimental exercise sessions was similar between BG and placebo conditions.
Experiment Two: Salivary Immunoglobulins
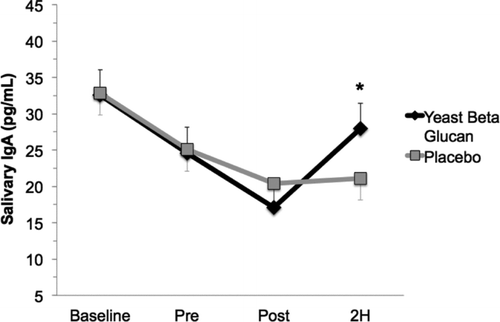
We found significant condition by time interactions for salivary IgA (p = .048; ). In the placebo condition, there was a progressive decline in salivary IgA, with the most pronounced reduction occurring at POST and 2H compared with PRE. In contrast, after 10 days of BG supplementation, salivary IgA was increased at 2H compared with placebo and increased at PRE compared with BG and placebo. This demonstrates that BG supplementation enhanced salivary IgA recovery over placebo control in an experimental model of strenuous exercise.
FIGURE 3. Salivary Immunoglobulin Response. Subjects who were participating in a larger clinical trial provided a saliva sample prior to supplementation with either placebo or BG. After 10 days of supplementation, subjects provided a second resting saliva sample. Additional saliva samples were collected immediately and 2-hr postexercise. We found a significant reduction in salivary IgA after exercise in the placebo condition at POST and 2H, which is the typical exercise response. The BG condition demonstrated a statistically significant increase in salivary IgA at 2H. The symbol “*” indicates greater than placebo (p < .05).
DISCUSSION
The key findings of the present study demonstrate that BG supplementation reduced the duration of URTI symptoms following a marathon and prevented postexercise suppression of salivary IgA following a strenuous bout of exercise. This work complements previous findings from our lab and others demonstrating that this particular source of BG with a well-characterized chemical structure improves specific immune outcomes in response to exercise, stressful working environments, and cancer (Carpenter et al., Citation2012; Harger-Domitrovich et al., Citation2008; Hong et al., Citation2004; US Pharmacopiea, Citation2011; Qi et al., Citation2011; Talbott & Talbott, Citation2009). In addition to our observations with respect to BG, it is also important to note that we observed a typical immunosuppressive response in placebo conditions, which is consistent with what others have reported after exercise (Allgrove et al., Citation2009; Davison & Diment, Citation2010; Sari-Sarraf et al., Citation2011; Usui et al., Citation2011).
Previous studies using the insoluble preparation of BG have reported reduced URTI prevalence and severity in marathon runners and forest firefighters (Harger-Domitrovich et al., Citation2008; Talbott & Talbott, Citation2009). The experiment we completed at the marathon was designed to replicate previous efforts and to compare the efficacy of different oral delivery preparations (i.e., soluble vs. insoluble) of BG on URTI symptom prevalence (Talbott & Talbott, Citation2009). These preparations of BG were selected because they are routinely used for the manufacture of beverages (soluble) or other food products (insoluble). In the present study, we found that regardless of BG preparations, similar reductions in the number of URTI symptomatic days were measured following a marathon as measured by our daily health survey. Interpretation of this finding suggests that regardless of the preparation, BG supplementation allowed subjects to recover from URTI more quickly compared with placebo. Such a recovery may have allowed the BG supplemented subjects to resume their exercise training or work routines in a timelier manner, thus reducing the health toll associated with marathon running.
In addition to measuring URTI symptoms, we also tracked the subjects’ mental health postmarathon using a POMS survey. This measure was included because it is well established that reduced mental health can cause excess stress on the immune system, increasing URTI risk (Fink et al., Citation2012). In this experiment, we did not observe any difference between groups in POMS scores during recovery from the marathon. Thus, we can rule out a change in global mental health as a confounder in our observed BG-mediated reduction in URTI symptomatic days.
While the marathon experiment provided key information regarding the effect of BG on URTI symptoms, its field study design was not well suited for tracking changes in mucosal immunity. Given this limitation, we completed a second experiment in the laboratory to evaluate the ability of BG to alter mucosal immunity. Salivary Igs along with antimicrobial proteins are major components of the mucosal immune system, whose disruption has been linked to an increased risk of URTI (Walsh et al., Citation2011). In examining the published literature, strenuous bouts of exercise tend to suppress salivary IgA concentration and/or secretion rate (Allgrove et al., Citation2009; Davison & Diment, Citation2010; Sari-Sarraf et al., Citation2011; Usui et al., Citation2011; Walsh et al., Citation2011). These previous studies used subjects and exercise conditions similar to the present laboratory experiment. The key finding from our laboratory's analysis was that BG supplementation prior to a bout of strenuous cycling increased salivary IgA concentration at 2H postexercise, compared with placebo. This increase is consistent with an improved mucosal immunity that has been summarized elsewhere (Walsh et al., Citation2011) and therefore has implications for infection risk. It is plausible that similar elevations in salivary IgA may have occurred in BG-supplemented individuals following the marathon in the first experiment. Improved mucosal immunity as a result of BG supplementation is one potential explanation for the reduced number of cold/flu symptomatic days experienced by those subjects. Since the second experiment only evaluated salivary IgA and did not track URTI symptom incidence, we are unable to determine if an improved mucosal immunity is the sole factor contributing to the reduction of URTI symptomatic days by BG. More research is needed to evaluate other immune system outcomes that may contribute to the BG supplementation effect on URTI symptom day reduction postmarathon.
Although the two experiments employed different supplementation patterns (i.e., before vs. after exercise), a statistically significant enhancement in the measured outcomes was evident in BG and placebo groups in both studies. We feel that this design difference strengthens the overall conclusions of our study by suggesting that supplementation with BG before strenuous exercise may not be necessary in order to observe a benefit to the immune system. In summary, the present study provides confirmation of the observations from other investigators that this commercially available source of baker's yeast BG decreases URTI symptom prevalence postmarathon. Here we add the novel insight that salivary IgA concentration following a strenuous exercise session is improved in subjects supplementing with BG. In future experiments, we will seek to address the limitations of the present study and further explore the mechanism of action for BG. More research is also needed to understand what type of supplementation regime maximizes the effectiveness of BG in terms of minimizing postexercise URTI.
ACKNOWLEDGMENTS
We would like to thank Tanya Halliday, Stephanie Collins, Diane Eagan, and Nicole Impero who were dietetic internship students working on this project with the authors and devoted countless hours to its success. Finally, we would like to thank all our subjects who participated in the present study, without the commitment our findings would not have been possible.
Declaration of interest: The authors were not directly paid for the completion of this project and do not report any conflict of interest. The authors alone are responsible for the content and writing of this article.
Funding for the present project was provided by Biothera: The Immune Health Company (Eagan, MN).
ABOUT THE AUTHORS
Dr. McFarlin is a faculty member and co-director of the Applied Physiology Laboratory at the University of North Texas. Dr. McFarlin received his PhD in 2003 from Purdue University. His area of research focuses on the effects of exercise and nutrition on immune system health and disease. Carpenter, Davidson, and McFarlin were all members of Dr. McFarlin's research team and participated in various aspects of data collection, analysis, and manuscript preparation.
REFERENCES
- Allgrove JE, Geneen L, Latif S, Gleeson M. Influence of a fed or fasted state on the s-IgA response to prolonged cycling in active men and women. Int J Sport Nutr Exerc Metab. 2009;19(3):209–221.
- Barrett B, Locken K, Maberry R, Schwamman J, Brown R, Bobula J, Stauffacher EA. The Wisconsin Upper Respiratory Symptom Survey (WURSS): a new research instrument for assessing the common cold. J Fam Pract. 2002;51(3):265.
- Carpenter KC, Breslin WL, Davidson T, Adams A, McFarlin BK. Baker's yeast beta-glucan supplementation increases monocytes and cytokines post-exercise: implications for infection risk? Br J Nutr. 2012;1–9.
- Davison G, Diment BC. Bovine colostrum supplementation attenuates the decrease of salivary lysozyme and enhances the recovery of neutrophil function after prolonged exercise. Br J Nutr. 2010;103(10):1425–1432.
- Fink AM, Gonzalez RC, Lisowski T, Pini M, Fantuzzi G, Levy WC, Piano MR. Fatigue, inflammation, and projected mortality in heart failure. J Card Fail. 2012;18(9):711–716.
- Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, Bose N, Chan AS, Magee AS, Danielson ME, Weiss A, Vasilakos JP, Underhill DM. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011;472(7344):471–475.
- Harger-Domitrovich SG, Domitrovich JW, Ruby BC. Effects of an immunomodulating supplement on upper respiratory tract infection symptoms in wildland firefighters. Med Sci Sports Exerc. 2008;40(5): S353.
- Hong F, Yan J, Baran JT, Allendorf DJ, Hansen RD, Ostroff GR, Xing PX, Cheung NK, Ross GD. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol. 2004;173(2):797–806.
- McFarlin BK, Flynn MG, Stewart LK, Timmerman KL. Carbohydrate intake during endurance exercise increases natural killer cell responsiveness to IL-2. J Appl Physiol. 2004;96(1):271–275.
- McFarlin BK, Mitchell JB. Exercise in hot and cold environments: differential effects on leukocyte number and NK cell activity. Aviat Space Environ Med. 2003;74(12):1231–1236.
- Moreira A, Arsati F, de Oliveira Lima-Arsati YB, de Freitas CG, de Araújo VC. Salivary immunoglobulin A responses in professional top-level futsal players. J Strength Cond Res. 2011;25(7):1932–1936.
- Peters EM, Shaik J, Kleinveldt N. Upper respiratory tract infection symptoms in ultramarathon runners not related to immunoglobulin status. Clin J Sport Med. 2010;20(1):39–46.
- US Pharmacopiea. Beta glucan from bakers yeast (Saccharomyces cerevisiae). In: Food chemicals codex seventh edition third supplement U. Pharmacopiea. US Pharmacopiea, 2011.
- Qi C, Cai Y, Gunn L, Ding C, Li B, Kloecker G, Qian K, Vasilakos J, Saijo S, Iwakura Y, Yannelli JR, Yan J. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived beta-glucans. Blood. 2011;117(25):6825–6836.
- Rowe S, Alexander N, Clydesdale FM, Applebaum RS, Atkinson S, Black RM, Dwyer JT, Hentges E, Higley NA, Lefevre M, Lupton JR, Miller SA, Tancredi DL, Weaver CM, Woteki CE, Wedral E. Funding food science and nutrition research: financial conflicts and scientific integrity. Am J Clin Nutr. 2009;89(5):1285–1291.
- Sari-Sarraf V, Doran DA, Clarke ND, Atkinson G, Reilly T. Effects of carbohydrate beverage ingestion on the salivary IgA response to intermittent exercise in the heat. Int J Sports Med. 2011;32(9):659–665.
- Talbott ST, Talbott J. Effect of BETA 1, 3/1, 6 GLUCAN on upper respiratory tract infection symptoms and mood state in marathon athletes. J Sports Sci Med. 2009;8:509–515.
- Usui T, Yoshikawa T, Orita K, Ueda SY, Katsura Y, Fujimoto S, Yoshimura M. Changes in salivary antimicrobial peptides, immunoglobulin A and cortisol after prolonged strenuous exercise. Eur J Appl Physiol. 2011;111(9):2005–2014.
- Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6–63.
- Woods JA, Davis JM, Smith JA, Nieman DC. Exercise and cellular innate immune function. Med Sci Sports Exerc. 1999;31(1):57–66.