Abstract
This study examined the effects of cryopreservation on DNA integrity of spermatozoa from 34 fertile subjects and 166 infertile subjects comprised of 80 teratospermic, 32 normospermic, 30 astheno-teratospermic, and 24 oligo-astheno-teratospermic individuals. Semen samples were prepared by swim-up and the Percoll density gradient centrifugation method (Pdgc) prior to freezing in liquid nitrogen. Neat and prepared samples were supplemented with cryoprotectant (SpermFreez) in cryoampoules and were frozen using the static phase vapor cooling procedure. Sperm DNA integrity of all thawed samples was determined using the alkaline comet assay. It was noticed that the sperm DNA integrity of frozen samples of fertile subjects was considerably higher than that of infertile subjects with greater catch-up integrity similar to the fresh samples. Freezing caused less chromatin damage to sperm of Pdgc samples from both fertile and infertile subjects as was compared to the neat and swim-up samples. It is concluded that the increase in comet frequency of frozen-thawed samples from infertile subjects was more prominent (8.25–22.78%; P<0.01) than in the fresh samples. Frozen-thawed samples from Ts (Teratospermic individuals) and ATs (Astheno-teratozoosspermic) showed higher level of OTM (Olive tail moment) indicating a higher level of chromatin fragmentation than fertile, Ns (Normospermics), and OATs (Oligo-astheno-teratozoospermics).
| Abbreviations | ||
| Pdgc: | = | Percoll density gradient centrifugation |
| Ts: | = | terato-spermic individuals |
| ATs: | = | astheno-teratozoosspermic |
| OTM: | = | olive tail moment |
| Ns: | = | normospermics |
| OATs: | = | oligo-astheno-teratozoospermics |
| ART: | = | assisted reproductive technology |
| FCM: | = | flow cytometric |
| CL: | = | comet length |
| CTL: | = | comet tail length |
| CHDP: | = | comet head DNA percentage |
| CTDP: | = | tail DNA percentage |
| TM: | = | tail moment |
| SCSA: | = | Sperm Chromatin Structure Assay |
| MEM: | = | minimum essential medium |
| ROS: | = | Reactive Oxygen Series |
INTRODUCTION
Freezing spermatozoa is a technique used in the assisted conception unit prior to ART (Assisted Reproductive Technology) [Bauman et al. Citation2007], cancer therapy [Stahl et al. Citation2008], or vasectomy [Spano et al. Citation1999; Donnelly et al. Citation2001a,Citationb; Allamaneni et al. Citation2005]. Temperature variations (e.g. during cooling, freezing, and thawing) can cause detrimental changes in sperm structure at the levels of the membrane and mitochondria, impairing sperm function. The detrimental effect of cryopreservation on spermatozoa, reduction in motility [Donnelly et al. Citation2001b; Gandini et al. Citation2006], and damage to the nuclear chromatin has been documented in humans [Spano et al. Citation1999; Gandini et al. Citation2006; Thomson et al. Citation2009], rhinoceros [Portas et al. Citation2009] rhesus macaques [Li et al. Citation2007], and rainbow trout and gilthead sea bream [Cabrita et al. Citation2005]. To optimize post-freezing motility, several different cryopreservation methods have been tested to determine optimal freezing rates [Morris et al. Citation1999] using various cryopreservatives [Gilmore et al. Citation2000; Hallak et al. Citation2000; Li et al. Citation2008]. Semen quality has been observed to be better preserved in fresh and cryo-preserved semen processed through a PureSperm density gradient than swim-up. Higher recovery rates of mature motile sperm obtained after Pure-Sperm sperm preparation may be beneficial for successful ART [Allamaneni et al. Citation2005], having an improved nuclear maturity as assessed by microscopy [Le Lannou and Blanchard Citation1988; Colleu et al. Citation1996] or flow cytometric (FCM) techniques [Molina et al. Citation1995; Golan et al. Citation1997; Spano et al. Citation1999]. Cryopreservation of human semen in liquid nitrogen at −196°C is considered superior to mechanical freezing at −70°C. The latter is associated with a greater decrease in motility after 7 days of storage. This difference is accentuated following 3 months of storage; although no differences in sperm morphology is observed [Trummer et al. Citation1998]. Freezing can be detrimental to the cell because of osmotic imbalances and ice crystal formation formed at different rates during the cooling process. If this occurs at a rate that is either too fast or too slow it can disrupt cellular membranes and organelles [Morris et al. Citation1999; Glimore et al. Citation2000]. Slow freezing and gradual dehydration may promote cell survival whereas rapid freezing and thawing is considered more lethal for cells [Donnelly et al. Citation2001a]. Interestingly, no difference in DNA integrity was observed when human semen samples from men of 21–50 years of age were flash frozen with or without cryopreservatives [Duty et al. Citation2002]. Anderson et al. [Citation1997] projected the same pattern of sperm DNA integrity, by using the alkaline comet assay, in frozen samples of fertile and infertile subjects. Another study by Donnelly et al. [Citation2001a] showed that DNA of semen and spermatozoa prepared from fertile samples was unaffected by cryopreservation with cryoprotectant, in marked contrast to spermatozoa from infertile samples that was significantly damaged by freezing-thawing. However, semen morphology in both fertile and infertile samples was significantly affected by cryopreservation. The various conclusions of these studies aroused our interest to examine the influence of sperm pre-preparation methods and cryopreservation on chromatin integrity in fertile and teratospermic, normospermic, atheno-teratospermic, and oligo-astheno-teratospermic infertile subjects.
RESULTS
As shown in , each sample was divided into four aliquots. Aliqout 1 was subjected to semen analysis and aliquot 2 was used for the neat sample procedure. In comparison, aliquots 3 and 4 were subjected to swim up or precoll density gradient centrifugation, respectively, prior to analysis as described below.
FIGURE 1 Division of semen samples used for different sperm preparation methods, cryopreservation and comet assay.
Comparison between Comet Variables of Fresh and Cryopreserved Samples from Fertile and Infertile Subjects' Semen
Among fertile subjects, olive tail moment of neat, swim-up, and Percoll density gradient centrifugation (Pdgc) frozen-thawed sperms were considerably (P< 0.001) higher than that observed in fresh sperm. All other comet assay parameters were similar when fresh sperm samples were compared with their corresponding frozen-thawed samples, as shown in . However, a small increase in chromatin fragmentation of 6.66, 7.59, and 8.49%, respectively, was observed when frozen-thawed samples from neat, swim-up, and Pdgc aliquots, were compared. In contrast, an increase in the number of DNA damaged sperm from frozen-thawed neat (8.25%; P= 0.9603), swim-up (20.61%, P<0.001), and Pdgc (22.78%; P<0.001) samples was observed in infertile subjects (). In contrast the other comet parameters including, comet length (CL), comet tail length (CTL), comet head DNA percentage (CHDP), tail DNA percentage (CTDP), tail moment (TM), and olive tail moment (OTM) in frozen-thawed samples with neat, swim-up, and Pdgc preparation from infertile subjects showed no significant difference (P>0.05) when compared to fresh samples ().
FIGURE 2 Comparison of fresh and frozen samples as a function of fertility and preparation. Top panel, no-significant increase in the comet frequency of frozen-thawed samples with neat swim-up and Pdgc prepared samples from fertile subjects when compared with fresh samples. Bottom panel, the infertile subjects' comet frequency was significantly (P<0.001***) higher in frozen-thawed samples with swim-up and Pdgc compared to neat samples.
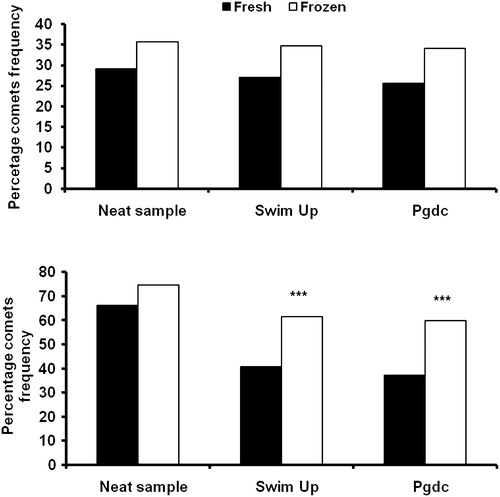
TABLE 1 Fertile and Infertile Sperm Comet Analysis Profile. Comet Length (μm) (CL), Comet Tail Length (μm) (CTL), Comet Head DNA Percentage (CHDP), Comet Tail DNA Percentage (CTDP), Tail Moment (TM), and Olive Tail Moment (OTM) in Fresh and Cryopreserved with Each Method of Sperm Preparation, Neat, Swim-up, Pdgc Preparation.
Comparison of Sperm Chromatin Damage Induced by the Freezing and Thawing Procedure of Fertile Samples With Ts, IDs, ATs, and OATs Infertile Samples
Mean comet number and intact chromatin sperm number obtained from frozen-thawed samples from neat, swim-up, and Pdgc fertile sperm were compared with four categories of infertile subjects (Ts (teratospermic individuals), Ns (normospermics), ATs (astheno-teratozoosspermic), and OATs (oligo-astheno-teratozoospermics) ().
TABLE 2 Fertile and Infertile Sperm Comet Analysis Profile. Comet Number (CN), Intact DNA Number (IDN), Comet Length (lm)(CL), Comet Tail Length (lm)(CTL), Comet Head DNA Percentage (CHDP), Comet Tail DNA Percentage (CTDP), Tail Moment (TM) and Olive Tail Moment (OTM) of Fertile Subjects, Teratospermics (Ts), Normospermic (Ns), Astheno-teratospermic (ATs) and Oligo-astheno-teratospermics (OATs) in Cryopreserved Neat, Swim-up, and Pdgc Prepared Samples.
The fertile subjects showed a significantly (P<0.001) highest frequency of intact DNA and the lowest frequency of comets when compared with all categories of infertile subjects. Sperm from Pdgc samples had a considerably (P<0.01) higher frequency of intact DNA, with a lower number of comets formed than neat samples. This reflects a protective effect on DNA integrity even after the frozen-thaw procedure. Identical results were also seen in infertile subjects, where a partial improvement in DNA integrity was observed in swim-up samples.
Among infertile subjects, Ts sperm were disrupted to the greatest extent (P<0.001) as shown by the frequency of fragmented chromatin, when compared with other categories of infertility. ATs and OATs had greater fragmented sperm chromatin from neat and Pdgc samples than Ns infertile subjects. OATs neat samples possessed a greater level of damaged DNA than ATs.
Among the infertile subjects, the highest comet length and tail length were recorded in Ts. This was independent of isolation procedure. In comparison, the ATs were similar when compared to Ns (P<0.001). The neat sample comet parameters from ATs were generally higher (P<0.01) than those observed in OATs. CTDP and TM from ATs prepared by swim-up or Pdgc were significantly higher than Ns (P<0.001), though, ATs prepared with neat and swim-up sperm showed the same increase in these variables when compared to OATs (P<0.01). OTM, considered the most sensitive comet variable, was higher in ATs independent of the isolation strategy when compared to Ns (P<0.01), whereas ATs prepared by neat or swim-up was higher than OATs (P<0.01). This variable, again, indicates that Pdgc is a better suited method to select the sperm with intact DNA than others.
In contrast to the neat samples, the best chromatin integrity (comet number) was obtained when samples were prepared from both fertile subjects as well as infertile subjects by Pdgc. The neat samples from Ts, Ns, and ATs all showed a considerable increase in DNA damage variables (CN, CL, CTL, TM, and OTM), when compared to the sperm prepared by swim-up or Pdgc (P<0.01). In comparison to neat samples, all comet variables increased (P<0.001) when OATs sperm was isolated by Pdgc or swim up (P>0.05).
DISCUSSION
The present study was aimed to address the detrimental effects of cryoperservation as reflected by sperm DNA damage or sperm DNA integrity. Samples were prepared from neat, swim-up, and Pdgc isolated sperm from fertile and infertile subjects (teratospermics, normospermics, astheno-tertospermics, and oligo-astheno-teratospermics). Comet variables in the three fertile preparations and in the different categories of infertile subjects (Tables 1 and 2) indicate a greater level of DNA damage when sperm were isolated by swim up or neat compared to Percoll density gradient.
Sperm cryopreservation is considered a routine practice prior to ART [Bauman et al. Citation2007], cancer therapy, or vasectomy. It is associated with a decrease in motility and pregnancy rate compared to fresh semen [Subak et al. Citation1992; Sharma and Agarwal Citation1996] but has not been associated with adverse results compared with fresh semen [Duty et al. Citation2002; Bauman et al. Citation2007]. In the present study the data suggest that fresh samples from fertile subjects isolated as neat, or prepared by the swim-up and Pdgc preparation methods (70.93%, 72.94%, and 74.37%, respectively) were of similar DNA integrity as compared to the frozen samples (64.25%, 65.35%, and 65.90% respectively).
Hammadeh et al. [Citation1999] observed significant damage to sperm chromatin, morphology, and membrane integrity in fertile and infertile human sperm due to freeze-thawing. DNA damage from cryopreserved semen of infertile men was subsequently confirmed [Donnelly et al. Citation2001b] and spermatozoa from infertile men were more susceptible to freezing damage than those from fertile men. Similarly, in the present study sperm DNA damage as revealed by percent comet frequency increased 8.25%, 20.61%, and 22.78%, respectively, in neat, swim-up, and Pdgc samples from cryopreserved samples when compared to fresh samples of infertile subjects. These results are consistent with those of others [Gandini et al. Citation2006] showing that cryopreservation caused a significant decrease of 24% (neat) and 40% (Percoll prepared) and swim-up preparation in DNA integrity of semen from infertile men. These results suggest that semen and sperm preparations from fertile men are not as susceptible to DNA damage as infertile men with freeze-thaw technique. Together this suggests that cryopreservation has some negative effects on sperm DNA integrity both in fertile and infertile subjects.
Steel et al. [Citation2000] found no appreciable difference in the percentage of comet head DNA of fresh (81.7 ± 1.2%) and frozen-thawed (81.9 ± 2.8%) testicular sperm in contrast to recent studies that showed an increased percentage of DNA fragmentation in cryopreserved sperm samples from human [Donnelly et al. Citation2001a, Citation2001b; Labbe et al. Citation2001; Thomson et al. Citation2009]. Furthermore, results from equine [Linfor and Meyers Citation2002] and rhesus macaques [Li et al. Citation2007] have revealed significantly higher sperm DNA damage in the absence of cryoprotectants prior to freezing. In contrast, Duty et al. [Citation2002], reported the most appropriate method for human sperm cryopreservation was flash-freezing without the use of a cryopreservative.
Recently, Ricci et al. [Citation2009] have reported that sperm prepared by both swim-up and density gradient centrifugation yield high quality sperm, in agreement with Sharma and Agarwal [Citation1996] and Yogev et al. [Citation1999]. As shown in this communication infertile subjects had significantly higher levels of DNA damage in cryopreserved neat samples and a gradual decline was observed in samples prepared by swim up and Percoll density gradient centrifugation. Samples prepared from fertile individuals by Percoll density gradient centrifugation had significantly less sperm DNA damage compared to neat samples. There was no appreciable difference with respect to DNA damage when sperm was prepared by either swim up or Percoll density gradient centrifugation. However, Donnelly et al. [Citation2001a,Citationb] observed a significant decrease of 40% in DNA integrity even after sperms were prepared by Percoll density centrifugation or by the direct swim up technique before freezing.
Other techniques including the Sperm Chromatin Structure Assay (SCSA) [Ollero et al. Citation2001] or in situ nick translation [Tomlinson et al. Citation2001] have been used to assess the efficacy of the Percoll method of sperm preparation. Kooij et al. [Citation2004] studied normospermic donors' fresh samples that were prepared by swim up and Percoll density centrifugation. In comparison with neat sperm exhibiting 15–25% double strand DNA breaks as revealed by the neutral comet assay, the frequency of sperm with double strand DNA breaks was reduced to about one third by swim up and high density Percoll fraction.
The results of the present study for the first time indicate that fertile and infertile Ts, Ns, ATs, and OATs subjects have a higher comet frequency including comet length, comet tail length, comet tail DNA percentage, tail moment, and olive tail moment in neat samples as compared to samples prepared by swim-up and Pdgc. Patients with teratozoospermia are at greater risk of developing pathogenic levels of ROS, apopto-sis, and sperm DNA damage [Agarwal and Said Citation2005], due to a high frequency of sperms with morphologically abnormal heads [Ahmad et al. Citation2007]. As shown in , the significantly higher level DNA damage was observed in teratospermics compared to normo-spermics which can be reflected in the sperm RNA population [Platts et al. Citation2007; Goodrich et al. Citation2007]. Kalthur et al. [Citation2008] also observed a three-fold increase in DNA damage in teratospermics as compared to normospermics. They suggested that compared to normal sperms, abnormal sperms exhibit increased susceptibility to DNA damage due to freeze-thaw.
According to Rogers et al. [Citation1983] and Mortimer [Citation2000] there are certain factors in the seminal plasma which obstruct the fertilizing ability of spermatozoa and due to these factors the induction of the capacitation reaction in spermatozoa is reduced. For the isolation of functionally normal spermatozoa, sperm migration techniques and gradient centrifugation remain the most popular methods [Mortimer Citation2000; Ahmad et al. Citation2007]. In a previous report on fresh semen, Donnelly et al. [Citation2000] studied men whose semen profiles were either teratospermics [Ahmad et al. Citation2007], normozoospermic, or asthenozoospermic. In comparison to neat samples, DNA integrity was significantly improved when spermatozoa were purified. In the present study, comparison of sperm DNA damage parameters including comet frequency, comet length, tail length, tail DNA percentage, tail moment, and olive tail moment of Ts from neat and sperm prepared by all methods from frozen samples improved significantly. Similar results with fresh semen have been described by others [Tomlinson et al. Citation2001; Irvine et al. Citation2000; Ahmad et al. Citation2007].
Grizard et al. [Citation1999] and Oehninger et al. [Citation2000] reported a 50% loss of normal sperms in response to cryopreservation induced lethal and sublethal damage including loss of sperm membrane integrity [Bell et al. Citation1993], loss of acrosome function [McLaughlin et al. Citation1993], DNA fragmentation [Medeiros et al. Citation2002], loss of motility [Grizard et al. Citation1999; Oehninger et al. Citation2000; Li et al. Citation2008], and poor fertilization [Oehninger et al. Citation2000]. The cell membrane can be damaged by freezing that usually resolves as rupture by intra-cellular [Muldrew and McGann Citation1990; Watson Citation1995] or extracellular ice crystal formation, osmotic effects [Mohammad et al. Citation1997]. Cryoprotectants such as glycerol or propendiol can be added to the cells to reduce freezing damage by lowering the salt concentrations and increasing the fraction of water that remains in a liquid state, thereby reducing osmotic stress and ultimately fracture [Watson Citation1995; Li et al. Citation2008]. Damage to the sperm can also occur during thawing [Wolley and Richardson Citation1978; McLaughlin et al. Citation1993; Mohammad et al. Citation1997; Calamera et al. Citation2008]. The severity of damage during the freeze-thaw cycle varies considerably between different individuals and different samples from the same individual. Oligozoospermic and asthenotertospermic [Erenpreiss et al. Citation2008] samples are more susceptible to damage than normozoospermic samples [Lin et al. Citation1998; Hughes et al. Citation1999; Grizard et al. Citation1999], as seen in the current study with frozen ATs and OATs samples. Independent of the sperm preparation method the frozen-thawed AT samples had higher mean comet values compared to fertile subjects and IDs, whereas, no appreciable difference of comet length, tail length, tail DNA percentage, tail moment, and olive tail moment of ID and OATs was observed.
In conclusion, sperm DNA damage was higher in infertile subjects than fertile subjects both in fresh and cryopreserved samples. The level of DNA integrity was improved in frozen-Pdgc prepared samples as compared to samples prepared by swim-up. Normospermic infertile subjects exhibited elevated levels of chromatin fragmentation in frozen sperms compared to fertile subjects. Frozen-thawed Ts and ATs sperm were considered more susceptible to chromatin damage than others. In summary, in comparison with fresh samples cryopreserved samples exhibit higher levels of sperm DNA damage.
MATERIALS AND METHODS
Fertile (n = 34) and infertile (n = 187) males, 87 teratospermics (Ts), 36 normospermics (Ns), 39 terato-asthenospermics (ATs), and 25 oligo-astheno-teratospermics (OATs), were recruited from midwifery, hospitals, and andrology laboratories in Rawalpindi, Pakistan and surroundings. Normospermic subjects were identified on the basis of their medical history and through semen analysis carried out following WHO [Citation1999] standards. Due to procedural unforeseen problems, 21 semen samples from infertile subjects were not included in the study. Hence, 166 semen samples from infertile subjects (Ts = 80, Ns = 32, ATs = 30 and OATs = 24) were further processed for cryopreservation. Healthy male subjects without any fertility problem histories were recruited as fertile subjects; their partners had previously proven pregnancy potential or were pregnant, after one year unprotected intercourse, during this study. Informed consent for participation was obtained from each patient/subject prior to sample production. Participants provided semen samples, collected in sterile polypropylene containers, by masturbation at home or at the andrology laboratory after abstaining from sexual relations for a period of 3–5 days.
Semen Analysis
A routine semen analysis, after one hour (liquefaction time), was performed under the light microscope to determine sperm concentration, motility percentage, motility gradation, morphology (Kruger Strict Criteria, 14% considered morphologically normal), morphology gradation, and sperm abnormality indices as presented in the World Health Organization [WHO Citation1999] manual.
Division of Sample
As summarized in , each sample was divided into four aliquots. Aliquot 1: (150–200 μl) was used for semen analysis, aliquot 2: (200–250 μl) was subjected to the neat sample procedure while aliquot 3: (0.5 ml–1.0 ml) was purified by swim up and aliquot 4: (remaining semen) was purified by the Percoll density gradient centrifugation procedure (Pdgc). Upon preparation, samples 2–4 were adjusted to contain 20–30 × 106 spermatozoa/ml with Horwel chamber then further divided into two 250 μl aliquots a and b. The first aliquot was immediately used for fresh comet assay and the second cryopreserved prior to assessment by the comet assay.
Preparation of Samples
Semen sample of aliquot 2 was diluted 1:1 with MEM (Minimum Essential Medium; Eagle (Sigma) HEPES modification, supplemented with 10% pasteurized plasma albumen) as described by Kooij et al. [Citation2004]. Samples were mixed then divided into equal volume Ra and Rb aliquots. The sperm swim up preparation method was adapted from several published protocols [Gandini et al.Citation2000; Younglai et al. Citation2001; Donnelly et al.Citation2001a]. Briefly, aliquot 3 was diluted with 1:2 in MEM then centrifuged for 10 min at 300 × g. Following centrifugation, the supernatant was discarded and 0.5 ml of MEM was layered onto the pellet. The spermatozoa were allowed to migrate for 30 min at 30°C, in 5% CO2. The supernatant was then gently aspirated into Sa and Sb aliquots. Aliquot 4 was subjected to a modified two-step 45%–90% discontinuous Percoll gradient [Donnelly et al. Citation2001a]. The liquefied semen was gently layered on the top of the Percoll gradient then centrifuged at 450 × g for 12 min. The resulting sperm pellet was diluted with 1 ml of MEM, mixed then centrifuged at 200 × g for 6 min. The sperm pellet was again resuspended with 1 ml MEM medium then centrifuged at 200 × g for 6 min then the final sperm preparation suspended in 500 μl of MEM, adjusted to 20–30 × 106/ml and Pa and Pb aliquots prepared.
Cryopreservation of Samples
The cryopreservation of sperm was achieved by the static-phase vapor cooling method [Donnelly et al. Citation2001a; Duty et al. Citation2002]. In brief, aliquots Rb, Sb, and Pb were pipetted into cryovials and mixed 1:1 with room temperature SpermFreeze® (FertiPro NV, Sint-Martens-Latem, Belgium). The mixture remained at room temperature for 10 min prior to suspention in liquid nitrogen vapor (10 cm above the level of liquid nitrogen; −80°C) for 30 min. The samples were then plunged into liquid nitrogen (−196°C) and stored until required.
Sample Thawing
Samples were removed from the liquid nitrogen and the caps of the cryovials loosened before being placed at (25–30°C) for 20–30 min to thaw. Once thawed, an equal volume of MEM was added to each cryovial and the cells centrifuged at 200 × g for 6 min. The Spermfreeze® supernatant was removed and the pellets were resuspended in 250 μl of MEM.
Comet Assay
The procedure was carried out in low indirect incandescent light (60 watt) to minimize light induced sperm chromatin damage [Ahmad et al. Citation2007] Briefly, labeled 25.4 × 76.2 mm microscopic glass slides (Sail) were dipped to the half way mark in 1% agarose in Ca2+ and Mg2+ free phosphate buffer saline. Agarose attached to the underside was removed and laid down on the ice packs to solidify then dried at room temperature. Neat samples containing 1 × 105 spermatozoa were mixed with 70 μl of 0.5% low melting point agarose and spread to form the second layer. A large 24 × 50 mm Marienfeld cover slip was placed to flatten the molten cell suspension. After 3–5 min, the cover slip was gently removed and the third layer of agarose (70 μl) of the same concentration was added to fill any residual holes in the second layer and to increase the distance between cells and gel surface.
The slides were then immersed for 1 h at 4°C in freshly prepared cold lysing solution (2.5 M NaCl, 100 mM EDTA disodium salt, 10 mM Tris, pH 10, buffer, containing 1% Triton X-100) to maintain the agarose gel stability. Slides were then treated with overnight incubation at 37°C with 0.2% proteinase K to eliminate the protamines that otherwise impede DNA migration. Following overnight incubation, slides were placed in a horizontal gel electrophoresis chamber filled with fresh alkaline electrophoresis solution comprised of pH 12.5 300 mM NaOH plus 1 mM EDTA buffer at 12–15°C. The slides were placed side by side, sliding them as close together as possible. The end containing the agarose faced the anode. The electrophoresis buffer was set at a level of 0.25 cm above the slide surface. The slides were left in this alkaline electrophoresis buffer for 30 min to allow DNA in the cells to unwind. The DNA fragments were separated by electrophoresis for 10 min at 25 V (0.714 V = cm). To remove alkali and detergents, the slides were flooded with three changes of neutralization buffer [0.4 M = L Tris. pH 7.5] each for 5 min after electrophoresis. This enhances acridine orange staining. The slides were dehydrated by immersion in absolute ethanol for 5 min, and instantly dried and stored at room temperature [Klaude et al. Citation1996]. Slides were overlaid with 300–500 μl of 20 μg/ml acridine orange and covered with a fresh cover slip (24 × 50 mm). Any excess liquid was bottled away from the back and edges of the slides. After scoring, cover slips were removed and were rinsed in 100% alcohol to remove the stain, dried, and stored. Coded slides were viewed using a Nikon (AFX-II) Optiphot epi-fluorescence microscope equipped with filters (blue DM 510 and green DM 430) for Acridine orange visualization. A green filter was used for Acridine orange staining that yielded varying red colored comets. The comet color was a milder dark red when Ns were compared to fertile and Ts. For each sample, 200 randomly selected nuclei were evaluated. Imagess were captured by using a Nikon FX 35 fluorescence camera.
The comets were analyzed using TRITEK software. The parameters for the comet assay were: tail fluorescence, mean tail DNA percentage; relative tail fluorescence (tail DNA percentage); and olive tail moment (the product of the tail length and percentage of DNA in the tail expressed as the mean). Tail fluorescence and olive tail moment were used as a measure of primary DNA damage. Tail length is defined as the distance from the center position of the maximum fluorescence intensity over the nucleus, to the end of the tail. The product of DNA and mean distance of DNA migration in the tail were combined and defined as the tail moment [Olive et al. Citation1990].
Statistical Analysis
Comet assay parameters from fertile and infertile subjects were expressed as Mean ± SEM. The t-test was performed using statistical analysis package, Graph pad prism version 5 to compare the different comet parameters of cryopreserved neat sample, swim up, and Pdgc samples from fertile and all categories of infertile subjects.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Agarwal, A. and Said, T. M. (2005) Oxidative stress, DNA damage and apoptosis in male infertility - a clinical approach. BJU International 95:503–507.
- Ahmad, L., Jalali, S., Shami, S. A. and Akram, Z. (2007) Sperm Preparation: DNA Damage by Comet Assay in Normo- and Teratozoospermics. Sys Bio Reprod Med 53(6):325–338.
- Allamaneni, S. S., Agarwal, A., Rama, S., Ranganathan, P. and Sharma, R. K. (2005) Comparative study on density gradients and swim-up preparation techniques utilizing neat and cryopreserved spermatozoa. Asian J Androl 7(1): 86–92.
- Anderson, D., Dobrzyska, M. M., Yu, T. W., Gandini, L., Cordelli, E. and Spano, M. (1997) DNA integrity in human sperm. Terat Carcinog Mutagen 17:97–102.
- Baumann, K. H., Weidner, A., Kalff-Suske, M. and Bock, K. (2007) Assisted Reproduction Using Cryopreserved Sperm - a Mini Review. Reproduktionsmed Endokrinol 4(2):97–100.
- Bell, M., Wang, R., Hellstrom, W. J. G. and Sikka, S. C. (1993) Effect of cryoprotective additives and cryopreservation protocol on sperm membrane lipid peroxidation and recovery of motile human sperm. J Androl 14:472–178.
- Cabrita, E., Robles, V., Rebordinos, L., Sarasquete, C. and Herráez, M. P. (2005) Evaluation of DNA damage in rainbow trout (Oncorhynchus mykiss) and gilthead sea bream (Sparus aurata) cryopreserved sperm. Cryobiol 50:144–153.
- Calamera, J. C., Buffone, M. G., Doncel, G. F., Brugo-Olmedo, S., de Vincentiis, S., Calamera, M. M., Storey, B. T., Alvarez, J. G. (2008) Effect of thawing temperature on the motility recovery of cryopreserved human spermatozoa. Fertil Stril 10: .[Epub ahead of print]
- Colleu, D., Lescoat, D. and Gouranton, J. (1996) Nuclear maturity of human spermatozoa selected by swim-up or by Percoll gradient centrifugation procedures. Fertil Steril 65:160–164.
- Donnelly, E. T., McClure, N. and Lewis, S. E. (2001a) Cryopreservation of human semen and prepared sperm: effects on motility parameters and DNA integrity. Fertil Steril 76:892–900.
- Donnelly, E. T., O'Connell, M., McClure, N. and Lewis, S. E. M. (2000) Differences in nuclear DNA fragmentation and mitochondrial integrity of semen and prepared human spermatozoa. Hum Reprod 15:1552–1561.
- Donnelly, E. T., Steel, E. K., McClure, N. and Lewis, S. E. (2001b) Assessment of DNA integrity and morphology of ejaculated sperm from fertile and infertile men before and after cryopreservation. Hum Reprod 16:1191–1199.
- Duty, S. M., Singh, N. P., Ryan, L., Chen, Z., Lewis, C., Hunag, T. and Hauser, R. (2002) Reliability of the comet assay in cryopreserved human sperm. Hum Reprod 17:1274–1280.
- Erenpreiss, J., Elzanaty, S. and Giwercman, A. (2008) Sperm DNA damage in men from infertile couples. Asian J Androl 10:786–790.
- Gandini, L., Lombardo, F., Lenzi, A., SpanŒ, M. and Dondero, F. (2006) Cryopreservation and Sperm DNA Integrity. Cell Tissue Banking 7(2):91–98.
- Gandini, L., Lombardo, F., Paoli, D., Caponecchia, L., Familiari, G., Verlengia, C., Dondero, F. and Lenzi, A. (2000) Study of apoptotic DNA fragmentation in human spermatozoa. Hum Reprod 15(4): 830–839.
- Gilmore, J. A., Liu, J., Woods, E. J., Peter, A. T. and Critser, J. K. (2000) Cryoprotective agent and temperature effects on human sperm membrane permeabilities: convergence of theoretical and empirical approaches for optimal cryopreservation methods. Hum Reprod 15:335–343.
- Golan, R., Shochat, L., Weissenberg, R., Soffer, Y., Marcus, Z., Oschry, Y. and MLeuin, L. M. (1997) Evaluation of chromatin decondensation in human spermatozoa: a flow cytometric assay using acridine orange staining. Mol Hum Reprod 3:47–54.
- Goodrich, R., Johnson, G. and Krawetz, S. A. (2007) The preparation of human spermatozoal RNA for clinical analysis. Arch Androl: J of Repro Sys 53:161–167.
- Grizard, G., Chevalier, V., Griveau, J. F., Le Lannou, D. and Boucher, D. (1999) Influence of seminal plasma on cryopreservation of human spermatozoa in a biological material-free medium: Study of normal and low quality semen. Int J Androl 22:190–196.
- Hallak, J., Sharma, R. K., Wellstead, C. and Agarwal, A. (2000) Cryopreservation of human spermatozoa: comparison of TEST-yolk buffer and glycerol. Int J Fertil Womens Med 45:38–42.
- Hammadeh, M., Al-Hasani, S., Doerr, S., Stieber, M., Rosenbaum, P., Schmidt, W. and Diedrich, K. (1999) Comparison between chromatin condensation and morphology from testis biopsy extracted and ejaculated spermatozoa and their relationship to ICSI outcome. Hum Reprod 14:363–367.
- Hughes, C. M., McKelvey-Martin, V. J. and Lewis, S. E. (1999) Human sperm DNA integrity assessed by the Comet and ELISA assay. Mutag 14(1):71–75.
- Irvine, D. S., Twig, J. P., Gordon, E. L., Fulton, N., Milne, P. A. and Aitken, R. J. (2000) DNA integrity in human spermatozoa: relationship with semen quality. J Androl 21(1):33–44.
- Kalthur, G., Adiga, S. K., Upadhya, D., Rao, S. and Kumar, P. (2008) Effect of cryopreservation on sperm DNA integrity in patients with teratospermia. Fertil Steril 89(6):1723–1727.
- Klaude, M., Eriksson, S., Nygren, J. and Ahnstrom, G. (1996) The comet assay: mechanism and technical considerations. Mutat Res 363:89–96.
- Kooij, R. J., de Boer, P., De Vreeden-Elberts, J. M., Ganga, N. A. and Singh, N. (2004) The neutral comet assay detects double strand DNA damage in selected and unselected human spermatozoa of normospermic donors. Int J Androl 27(3): 140–146.
- Labbe, C., Martoriati, A., Devaux, A. and Maisse, G. (2001) Effects of sperm cryopreservation on sperm DNA stability and progeny development in rainbow trout. Mol Reprod Dev 60:397–404.
- Le Lannou, D. and Blanchard, Y (1988) Nuclear maturity and morphology of human spermatozoa selected by Percoll density gradient centrifugation or swim-up procedure. J Reprod Fertil 84:551–556.
- Li, M., Meyers, S., Tollner, T. L. and Overstreet, J. W. (2007) Damage to Chromosomes and DNA of Rhesus Monkey Sperm Following Cryopreservation. J Androl 28:493–501.
- Li, P., Wei, Q. and Liu, L. (2008) DNA integrity of Polyodon spathula cryopreserved sperm. J Appl Ichthyol 24:121–125.
- Lin, M. H., Morshedi, M., Srisombut, C., Nassar, A. and Oehninger, S. (1998) Plasma membrane integrity of cryopreserved human sperm: An investigation of the results of the hypoosmotic swelling test, the water test, and eosin—Y staining. Fertil Steril 70:1148–1155.
- Linfor, J. J. and Meyers, S. (2002) Detection of DNA damage in response to cooling injury in equine spermatozoa using single cell electrophoresis. J Androl 23:107–113.
- McLaughlin, E. A., Ford, W. C. L. and Hull, M. G. R. (1993) Effects of cryopreservation on the human sperm acrosome and its response to A23187. J Reprod Fertil 99:71–76.
- Medeiros, C. M. O., Forell, F., Oliveira, A. T. D. and Rodrigues, J. L. (2002) Current status of sperm cryopreservation: Why isn't it better? Therology 57:327–344.
- Mohammad, S. N., Barratt, C. L., Cooke, and Moore, H. D. (1997) Continuous assessment of human spermatozoa viability during cryopreservation. J Androl 18:43–50.
- Molina, J., Castilla, J. A., Gil, T., Hortas, M. L., Vergara, F. and Herruzo, A. (1995) Influence of incubation on the chromatin condensation and nuclear stability in human spermatozoa by flow cytometry. Hum Reprod 10:1280–1286.
- Morris, G. J., Acton, E. and Avery, S. (1999) A novel approach to sperm cryopreservation. Hum Reprod 14:1013–1021.
- Mortimer, D. (2000) Sperm preparation methods. J Androl 21:357–366.
- Muldrew, K. and McGann, L. E. (1990) Mechanism of intracellular ice formation. Bio J 57:525–533.
- Oehninger, S., Duru, N. K., Srisombut, C. and Morshedi, M. (2000) Assessment of sperm cryodamage and strategies to improve outcome. Mol Cell Endocrinol 169:3–10.
- Olive, P. L., Banath, J. P. and Durand, R. E. (1990) Heterogeneity in radiation induced DNA damage and repair in tumor and normal cells using the comet assay. Radiat Res 122:86–94.
- Ollero, M., Gil-Guzman, E., Lopez, M., Sharma, R., Agarwal, A., Larson, K., Evenson, D., Thomas, A. J., Jr. and Alvarez, J. G. (2001) Characterization of subsets of human spermatozoa at different stages of maturation: implications in the diagnosis of male infertility. Hum Reprod 16:1912–1921.
- Platts, A. E., Dix, D. J., Chemes, H. E., Thompson, K. E., Goodrich, R., Rockett, J. C., Rawe, V. Y., Quintana, S., Diamond, M. P., Strader, L. F. and Krawetz, S. A. (2007) Success and failure in human spermato-genesis as revealed by teratozoospermic RNAs. Human Molecular Genetics 16:763–773.
- Portas, T., Johnston, S. D., Hermes, R., Arroyo, F., Lopez-Fernadez, C., Bryant, B., Hildebrandt, T. B., Goritz, F. and Gosalvez, J. (2009) Frozen-thawed rhinoceros sperm exhibit DNA damage shortly after thawing when assessed by the sperm chromatin dispersion assay. Theriogenology 72:711–720.
- Ricci, G., Perticarari, S., Boscolo, R., Montico, M., Guaschino, S. and Presani, G. (2009) Semen preparation methods and sperm apoptosis: swim-up versus gradient-density centrif ugation technique. Fertil Steril 91(2):632–638.
- Rogers, B. J., Perreault, S. D., Bentwood, B. J., McCarville, C., Hale, R. W. and Soderdahl, D. W. (1983) Variability in the human-hamster in vitro assay for fertility evaluation. Fertil Steril 39:204–211.
- Sharma, R. K. and Agarwal, A. (1996) Sperm quality improvement in cryopreserved human semen. J Urol 156:1008–1012.
- Spano, M., Cordelli, E., Leter, G., Lombardo, F., Lenzi, A. and Gandini, L. (1999) Nuclear chromatin variations in human spermatozoa undergoing swim-up and cryopreservation evaluated by the flow cytometric sperm chromatin structure assay. Mol Hum Reprod 5:29–37.
- Stahl, O., Eberhard, J., Cavallin-Stahl, E., Jepson, K., Friberg, B.,Tingsmark, C., Spano, M. and Giwercman, A. (2008) Sperm DNA integrity in cancer patients: the effect of disease and treatment. Int J Androl 32:695–703.
- Steele, E. K., McClure, N. and Lewis, S. E. (2000) Comparison of the effects of two methods of cryopreservation on testicular sperm DNA. Fertil Steril 74:450–453.
- Subak, L. L., Adamson, G. D. and Boltz, N. L. (1992) Therapeutic donor insemination: a prospective randomized trial of fresh versus frozen sperm. Am J Obstet Gynecol 166:1597–1604.
- Thomson, L. K., Fleming, S. D., Aitken, R. J., De Iuliis, G. N., Zieschang, J. A. and Clark, A. M. (2009) Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod 24:2061–2070.
- Tomlinson, M., Moffatt, O., Manicardi, G., Bizzaro, D., Afnan, M. and Sakkas, D. (2001) Interrelationships between seminal parameters and sperm nuclear DNA damage before and after density gradient centrifugation: implications for assisted conception. Hum Reprod 16:2160–2165.
- Trummer, H., Tucker, K., Young, C., Kuala, N. and Meachum, R. B. (1998) Effect of storage temperature on sperm cryopreservation. Fertil Steril 70:1162–1164.
- Watson, P. F. (1995) Recent developments and concepts in cryopreservation of spermatozoa and the assessment of their post-thaw function. Reprod Fertil Dev 7:871–891.
- Woolley, D. M. and Richardson, D. W. (1978) Ultra-structural injury to human spermatozoa after freezing and thawing. J Reprod Fertil 53:389–394.
- World Health Organization. 1999. WHO laboratory manual for the examination of human semen and semen-cervical mucus interactions, 4th ed. Cambridge University Press, Cambridge, U K.
- Yogev, L., Gamzu, R., Paz, G., Kleiman, S., Botchan, A., Hauser, R. and Yavetz, H. (1999) Pre-freezing sperm preparation does not impair thawed spermatozoa binding to the zona pellucida. Hum Reprod 14:114–117.
- Younglai, E. V., Holt, D., Brown, P., Jurisicova, A. and Casper, R. F. (2001) Sperm swim-up technique and DNA fragmentation. Hum Reprod 16:1950–1953.