Abstract
A number of reports have suggested that the oxidative state of human albumin in serum and in some body fluids is associated with cell damage. However there are no reports on the redox state of human follicular fluid (FF) and its influence on oocyte viability. The aim of this study was to examine the relationship between the redox state of FF and serum on oocyte viability. The cytoplasmic condition of oocytes was evaluated microscopically at collection in 117 women. Deteriorating oocytes were recognized by degenerative changes in their cytoplasm. The redox state of FFs that yielded degenerated oocytes was evaluated and compared with fluids containing normal oocytes. The redox state of the corresponding FF and serum, at the time of oocyte retrieval, was analyzed by high performance liquid chromatography. The redox state of FF that contained degenerated oocytes was found to have a significantly elevated oxidized state compared with the FFs that yielded normal oocytes. Also the albumin in the FF of patients was found to be predominantly in the reduced state compared with that in their serum at the time of oocyte retrieval. In addition, increasing age and endometriosis were found to shift the redox of serum to the oxidative state. We propose that the reduced state of albumin in FF may play an important role in protecting oocytes from oxidative damage.
Introduction
Human serum albumin (HSA) plays an important role in the maintenance of the redox potential in extracellular fluids and in the osmotic regulation of the plasma in the vascular system that is involved in the transport of a wide variety of endogenous and exogenous molecules. One of the most important features of HSA structure is the presence of a highly reactive thiol group at position 34 (Cys-34). When the thiol group of HSA is in the free state, it is called mercaptalbumin (the reduced form; human mercaptalbumin, HMA) [King Citation1961]. In contrast, HSA in which the thiol group is bound to thiol-containing compounds is called nonmercaptalbumin (the oxidized form; human nonmercaptalbumin, HNA) [King Citation1961]. The major HNA compound is a mixed disulfide with cystine or oxidized glutathione (the reversible form of HNA, which is tentatively called HNA-1); whereas, others include oxidation products higher than the mixed disulfide, i.e., the sulfenic (-SOH), sulfinic (-SO2H), and sulfonic (-SO3H) states (the irreversible forms of HNA; called HNA-2) [Janatova et al. Citation1968]. Under physiological conditions in healthy subjects, the mean percentage of the HMA fraction of HSA in serum is usually around 75%. However, in patients with certain diseases such as kidney dysfunction, the level of the reduced form (HMA) decreases while the level of the oxidized form (HNA) is inversely increased [Terawaki et al. Citation2004].
On one hand, it has also been proposed that accumulated uremic toxins may induce oxidative stress. Some reports have also indicated that dialysis in patients with renal failure restored serum glucose-6-phosphate dehydrogenase, while superoxide dismutase and catalase activity rose to normal values [Chauhan et al. Citation1982], at the same time the proportion of oxidized albumin decreased [Sogami et al. Citation1984; Sogami et al. Citation1985]. Furthermore, it was found that uremic toxins such as BUN and creatinine were closely correlated with oxidized albumin [Matsuyama et al. Citation2009a]. Thus, the thiol-redox state of HSA is indicative of the systemic oxidative stress in the human body, probably because HSA is the most abundant extracellular protein and the Cys-34 (thiol) of HSA makes up approximately 80% of the total free thiol content in sera.
On the other hand, it has been reported that reactive oxygen species (ROS) in FF is higher in pregnant women than the levels in non-pregnant patients. High FF ROS levels were reported to be associated with negative outcomes in one study [Agarwal et al. Citation2003]. It has also been reported that a proper chromosomal alignment in oocytes was associated with high oxygen and antioxidant levels in FF. Although the significance of ROS levels in FF is still unclear and controversial, it is likely that a balanced oxygen and antioxidants level in FF is critical for the development of good quality oocytes. It is also possible that an elevated oxidative state could be a result of unbalanced oxygen and antioxidants.
At the time of oocyte retrieval we occasionally encounter degenerated oocytes that are readily distinguished by their complete cytoplasmic degeneration. Furthermore, it has recently been reported that oxidative stress in FF impairs oocyte quality [Tamura, et al. Citation2008]. In the experiments reported here our aim was to study the relationship between the redox state of albumin in human FF and the viability of oocytes in the corresponding aspirates. As the redox state of albumin in FF has not been reported previously, we also examined the related redox state of blood serum of patients at the time of oocyte retrieval. Furthermore, the correlation between the redox state of serum albumin associated with endometriosis and patients' age were analyzed.
Results
In total 193 FFs were analyzed. Ten oocytes had already degenerated (Aa) and the other 183 oocytes were considered to be morphologically normal (Ab) at the time of the oocyte retrieval. The mean percentage of each albumin fraction in the FFs that contained degenerated oocytes was 79.1 ± 5.3% in HMA, 19.1 ± 5.4% in HNA-1, and 1.8 ± 0.6% in HNA-2, while that of the FFs that contained viable oocytes was 84.5 ± 4.1% in HMA, 13.9 ± 3.8% in HNA-1, and 1.6 ± 0.6% in HNA-2. Thus, the redox state of FF that contained degenerated oocytes was found to have significantly shifted to the oxidized state compared with that of the FFs that yielded morphologically normal oocytes (B, HMA; P < 0.001, HNA-1; P < 0.001, HNA-2; NS).
Figure 1. A) A degenerated oocyte (a) and a morphologically normal oocyte (b). B) A comparison of the mean redox states of follicular fluid albumin that yielded morphologically normal oocytes and degenerated oocytes. HMA and HNA-1 of FF that contained degenerated oocytes were significantly shifted to the oxidized state compared with those of the FFs that yielded morphologically normal oocytes. However, there was no significant difference in NHA-2(HMA; P < 0.001, HNA-1; P < 0.001, HNA-2; NS). C) A comparison of the mean redox state of follicular fluid albumin and the corresponding sera. HMA, HNA-1 and HNA-2 in FF were all significantly reduced compared with those in serum (HMA; P < 0.001, HNA-1; P < 0.001, HNA-2; P < 0.001). HMA: human mercaptalbumin; HNA: human nonmercaptalbumin; FF: follicular fluid.
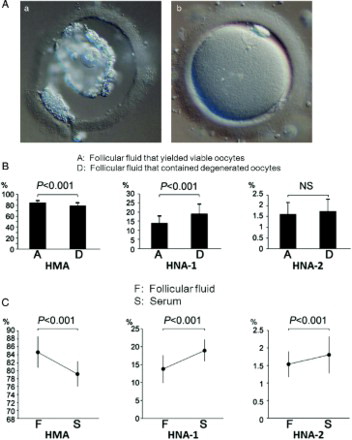
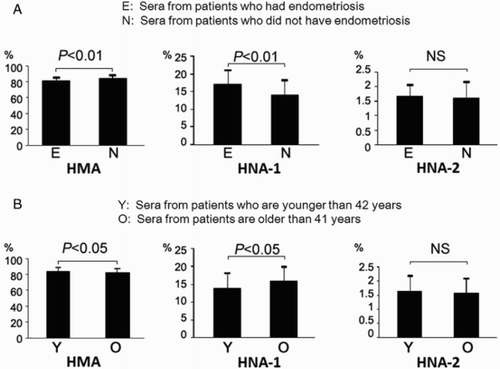
Among these 193 patients, sera were obtained from 117 at the time of ovum retrevial. Hence we examined the relationship between the redox state of albumin in the serum from 117 patients and that of albumin in their corresponding FF. The mean percentage of each albumin fraction in the FF from 117 patients was 84.6 ± 3.9% in HMA, 13.8 ± 3.9% in HNA-1, and 1.5 ± 0.4% in HNA-2, while that in the serum at the time of oocyte retrieval was 79.2 ± 3.2% in HMA, 19.0 ± 3.0% in HNA-1, and 1.8 ± 0.5% in HNA-2. Thus, the redox state of albumin in FF was found to be significantly reduced compared with that of serum (C, HMA; P < 0.001, HNA-1; P < 0.001, HNA-2; P < 0.001). Among the 117 patients, 12 women had endometriosis and the redox state of their sera (81.3 ± 4.4% in HMA, 17.0 ± 4.1% in HNA-1, and 1.7 ± 0.4% in HNA-2) was found to be significantly shifted to the oxidative state compared with the patients without endometriosis (84.4 ± 4.3% in HMA, 14.0 ± 4.0% in HNA-1, and 1.6 ± 0.6% in HNA-2; A, HMA; P < 0.01, HNA-1; P < 0.01, HNA-2; NS). Furthermore, the redox state of patients whose age was 42 and older (82.4 ± 4.4% in HMA, 16.0 ± 4.1% in HNA-1, and 1.6 ± 0.5% in HNA-2) was significantly shifted to the oxidative state compared with the redox state of patients who were younger than 42 (84.4 ± 4.3% in HMA, 14.0 ± 4.1% in HNA-1, and 1.6 ± 0.6% in HNA-2; B, HMA; P < 0.05, HNA-1; P < 0.05, HNA-2; NS).
Figure 2. A) The redox state of serum albumin from patients who had endometriosis. HMA and HNA-1 of sera from patients who had endometriosis were significantly shifted to the oxidative state compared to those without the disorder (HMA; P < 0.01, HNA-1; P < 0.01). Comparison of HNA-2 of sera showed that there was no significant difference between the two groups. B) The age of patients and the redox state of serum albumin. HMA and HNA-1 of patients whose age was 42 and older were significantly shifted to the oxidative state compared with women whose age was less than 42 years (HMA; P < 0.05, HNA-1; P < 0.05). HNA-2 of sera was not significantly different between the two groups. HMA: human mercaptalbumin; HNA: human nonmercaptalbumin.
Discussion
In the present study, we observed that the redox state of human FF albumin was predominantly in the reduced state. A highly reduced state of albumin was also reported in human lumbar cerebrospinal fluid albumin which is considered to participate in redox regulation in the central nervous system [Matsuyama et al. Citation2009b]. In contrast, it has been reported that the redox state of HSA shifts to the oxidative form in various pathophysiological conditions, such as hepatic [Sogami et al. Citation1985; Fukushima et al. Citation2007] and renal [Soejima et al. Citation2002; Soejima et al. Citation2004; Terawaki et al. Citation2007a; Terawaki et al. Citation2007b; Terawaki et al. Citation2010; Terawaki et al. Citation2011] disorders, as well as in diabetes [Suzuki et al. Citation1992;, Kawai et al. Citation2001], and other diseases [Hayakawa et al. Citation1997; Tomida et al. Citation2004; Kawai et al. Citation2010]. Moreover, the redox state is dynamic and may change under various physiological circumstances [Era et al. Citation1995; Imai et al. Citation2002].
In this study, we did not consider the redox status in serum to be a marker that can predict oocyte quality because redox state of different follicular fluids varies within an ovary (data not shown). Furthermore, there are a number of conditions that are associated with changes in oxidative stress. For example, as we found that age and endometriosis influence the redox state. These findings support the previous reports that suggested the occurrence of systemic oxidative stress in women with infertility associated with endometriosis [Andrade et al. Citation2010] and the redox stress hypothesis of aging [Sohal and Orr Citation2012]. Further research is needed to determine the influence of various conditions that may influence ovarian function and the associated oocyte morphological features. In consideration of the above, we propose that a favorable redox state of FF is required to protect oocytes from oxidative damage.
Materials and Methods
HPLC system for measuring the albumin redox state
Measurement of the albumin redox state was performed by a previously reported HPLC method [Matsuyama et al. Citation2009a; Matsuyama et al. Citation2009b]. Briefly, the HPLC system consisted of an AS-8010 auto-sampler (using an injection volume of 2 µL of sample) and a CCPM double-plunger pump in conjunction with an SC-8020 system controller (all from Tosoh, Tokyo, Japan). The chromatograph was obtained with a Finnigan UV6000LP photodiode array detector (detection range: 200-600 nm with 1-nm steps, Thermo Electron, Waltham, MA, USA). A Shodex-Asahipak ES-502N 7C column (10 × 0.76 cm I.D., DEAE-form for ion-exchange HPLC from Showa Denko, Tokyo, Japan; column temperature: 35 ± 0.5°C) was used in these studies. Elution was performed by linear gradient elution with ethanol (at the following concentrations: 0 to 1 min: 0%, 1 to 50 min: 0 → 10%, 50 to 55 min: 10 → 0%, 55 to 60 min: 0%) for specimens in a 0.05 M sodium acetate and 0.40 M sodium sulfate mixture (pH 4.85) at a flow rate of 1.0 mL/min. The buffer solution was de-aerated by bubbling helium through it. The HPLC profiles obtained from these procedures were subjected to numerical curve fitting with simulation software (PeakFit, version 4.05, SPSS Science, Chicago, IL, USA), and each peak shape was approximated by a Gaussian function. The values for the fractions of HMA [f(HMA)], HNA-1 [f(HNA-1)], and HNA-2 [f(HNA-2)] were obtained by the following equations, respectively:
f(HMA)(%) = [HMA/(HMA + HNA-1 + HNA-2)] × 100
f(HNA-1)(%) = [HNA-1/(HMA + HNA-1 + HNA-2)] × 100
f(HNA-2)(%) = [HNA-2/(HMA + HNA-1 + HNA-2)] × 100.
Follicular fluid and oocyte retrieval and morphological evaluation
All patients who donated FF and serum, for this study, signed an informed consent form and this study was approved by an ethics committee at the Nagai Clinic. Poor quality oocytes were recognized by morphological features known to be related to cytoplasmic degeneration. The redox state of FFs that yielded degenerated oocytes was evaluated and compared with fluids containing morphologically normal oocytes. Only the FFs that contained an oocyte in the first aspirate were included in this study to enable us to link each oocyte with its follicular fluid. Oocytes were evaluated using high resolution microscopy at the time of collection. Patients were divided into two groups: women 41 y or younger, and those 42 y and older. The average redox state of the serum albumin in both groups was calculated. The redox state of the serum albumin from patients who had endometriosis was also compared with those who did not have the disorder.
Statistical analysis
Comparisons of means were analyzed by the two-sample t-test using the statistical software StatMateIII (ATMS Inc., Tokyo, Japan). A correlation was considered to be significant when its P value was less than 0.05. Comparisons between means were analyzed by the paired t-test.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. Each author contributed equally to this paper.
Abbreviations
| HSA: | = | human serum albumin |
| HMA: | = | human mercaptalbumin |
| HNA: | = | human nonmercaptalbumin |
| FF: | = | follicular fluid; Cys-34: thiol group at position 34 of albumin |
| -SOH: | = | sulfenic form |
| -SO2H: | = | sulfinic form |
| -SO3H: | = | sulfonic form |
References
- Agarwal, A., Saleh, R.A. and Bedaiwy, M.A. (2003) Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 79:829–843.
- Andrade, A.Z., Rodrigues, J.K., Dib, L.A., Romão, G.S., Ferriani, R.A., Jordão Junior, A.A., (2010) Serum markers of oxidative stress in infertile women with endometriosis. Rev Bras Ginecol Obstet 32:279–285.
- Chauhan, D.P., Gupta, P.H., Nampoothiri, M.R., Singhal, P.C., Chugh, K.S. and Nair, C.R. (1982) Determination of erythrocyte superoxide dismutase, catalase, glucose-6-phosphate dehydrogenase, reduced glutathione and malonyldialdehyde in uremia. Clin Chim Acta 123:153–159.
- Era, S., Kuwata, K., Imai, H., Nakamura, K., Hayashi, T. and Sogami, M. (1995) Age-related change in redox state of human serum albumin. Biochim Biophys Acta 1247:12–16.
- Fukushima, H., Miwa, Y., Shiraki, M., Gomi, I., Toda, K., Kuriyama, S., (2007) Oral branched-chain amino acid supplementation improves the oxidized/reduced albumin ratio in patients with liver cirrhosis. Hepatol Res 37:765–770.
- Hayakawa, A., Kuwata, K., Era, S., Sogami, M., Shimonaka, H., Yamamoto, M., (1997) Alteration of redox state of human serum albumin in patients under anesthesia and invasive surgery. J Chromatogr B Biomed Sci Appl 698:27–33.
- Imai, H., Hayashi, T., Negawa, T., Nakamura, K., Tomida, M., Koda, K., (2002) Strenuous exercise-induced change in redox state of human serum albumin during intensive kendo training. Jpn J Physiol 52:135–140.
- Janatova, J., Fuller, J.K. and Hunter, M.J. (1968) The heterogeneity of bovine albumin with respect to sulfhydryl and dimer content. J Biol Chem 243:3612–3622.
- Kawai, K., Yoh, M., Hayashi, T., Imai, H., Negawa, T., Tomida, M., (2001) Effect of diabetic retinopathy on redox state of aqueous humor and serum albumin in patients with senile cataract. Tokai J Exp Clin Med 26:93–99.
- Kawai, K., Hayashi, T., Matsuyama, Y., Minami, T. and Era, S. (2010) Difference in redox status of serum and aqueous humor in senile cataract patients as monitored via the albumin thiol-redox state. Jpn J Ophthalmol 54:584–588.
- King, T.P. (1961) On the sulfhydryl group of human plasma albumin. J Biol Chem 236:PC5.
- Matsuyama, Y., Terawaki, H., Terada, T. and Era, S. (2009a) Albumin thiol oxidation and serum protein carbonyl formation are progressively enhanced with advancing stages of chronic kidney disease. Clin Exp Nephrol 13:308–315.
- Matsuyama, Y., Hayashi, T., Terawaki, H., Negawa, T., Terada, T., Okano, Y., (2009b) Human astrocytes and aortic endothelial cells actively convert the oxidized form of albumin to the reduced form: reduced albumin might participate in redox regulation of nerve and blood vessel systems. J Physiol Sci 59:207–215.
- Soejima, A., Kaneda, F., Manno, S., Matsuzawa, N., Kouji, H., Nagasawa, T., (2002) Useful markers for detecting decreased serum antioxidant activity in hemodialysis patients. Am J Kidney Dis 39:1040–1046.
- Soejima, A., Matsuzawa, N., Hayashi, T., Kimura, R., Ootsuka, T., Fukuoka, K., (2004) Alteration of redox state of human serum albumin before and after hemodialysis. Blood Purif 22:525–529.
- Sogami, M., Nagoka, S., Era, S., Honda., M, and Noguchi, K. (1984) Resolution of human mercapt- and nonmercaptalbumin by high-performance liquid chromatography. Int J Pept Protein Res 24:96–103.
- Sogami, M., Era, S., Nagaoka, S., Kuwata, K., Kida, K., Shigemi, J., (1985) High-performance liquid chromatographic studies on non-mercapt in equilibrium with mercapt conversion of human serum albumin. II. J Chromatogr 332:19–27.
- Sohal, R.S. and Orr, W.C. (2012) The redox stress hypothesis of aging. Free Radic Biol Med 52:539–555.
- Suzuki, E., Yasuda, K., Takeda, N., Sakata, S., Era, S., Kuwata, K., (1992) Increased oxidized form of human serum albumin in patients with diabetes mellitus. Diabetes Res Clin Prac 18:153–158.
- Tamura, H., Takasaki, A., Miwa, I., Taniguchi, K., Maekawa, R., Asada, H., (2008) Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res 44:280–287.
- Terawaki, H., Yoshimura, K., Hasegawa, T., Matsuyama, Y., Negawa, T., Yamada, K., (2004) Oxidative stress is enhanced in correlation with renal dysfunction: examination with the redox state of albumin. Kidney Int 66:1988–1993.
- Terawaki, H., Matsuyama, Y., Era, S., Matsuo, N., Ikeda, M., Ogura, M., (2007a) Elevated oxidative stress measured as albumin redox state in continuous ambulatory peritoneal dialysis patients correlates with small uraemic solutes. Nephrol Dial Transplant 22:968.
- Terawaki, H., Nakayama, K., Matsuyama, Y., Nakayama, M., Sato, T., Hosoya, T., (2007b) Dialyzable uremic solutes contribute to enhanced oxidation of serum albumin in regular hemodialysis patients. Blood Purif 25:274–279.
- Terawaki, H., Takada, Y., Era, S., Funakoshi, Y., Nakayama, K., Nakayama, M., (2010) The redox state of albumin and serious cardiovascular incidence in hemodialysis patients. Ther Apher Dial 14:465–471.
- Terawaki, H., Era, S., Nakayama, M. and Hosoya, T. (2011) Decrease in reduced-form albumin among chronic kidney disease patients: new insights in cardiovascular complications. Ther Apher Dial 15:156–160.
- Tomida, M., Ishimaru, J.I., Murayama, K., Kajimoto, T., Kurachi, M., Era, S., (2004) Intra-articular oxidative state correlated with the pathogenesis of disorders of the temporomandibular joint. Br J Oral Maxillofac Surg 42:405–409.