Abstract
A longitudinal, observational prospective panel cohort study of 61 patients lasting one year was undertaken. Explanatory variables included sociodemographic factors along with factors related to the underlying pathology as well as the protocol used and the type of treatment received. These variables were analyzed both individually and in combination to account for confounding effects and model interactions. A generalized estimating equation (GEE) model was constructed for each adverse effect. Associations were calculated as odds ratios (OR). Confounding variables related to drug tolerability were identified. Follitropin-alpha and cetrorelix exhibited the poorest safety profile. With respect to local adverse drug reactions (ADEs), the results obtained in our study point to statistically significant tolerability improvements for menotropin when administered in insemination. For gastrointestinal ADEs, ganirelix was the drug that showed the highest tolerability in in vitro treatments whereas follitropin-alpha showed the lowest tolerability in insemination treatments. Diverse factors related to assisted reproduction techniques (ART) influence the incidence of adverse effects. Each drug has a different safety profile with possible interactions depending on the type of assisted reproduction therapy used.
Introduction
While clinical assays are designed to test the efficacy and safety of therapies to be introduced into clinical practice, well-designed observational studies, when not promoted by any commercial interest, may be better suited for detecting and evaluating the association of a given therapy with possible adverse effects. Currently, different initiatives are in process to promote the transparency of observational, non-randomized drug safety studies [Schneeweiss Citation2011].
After an exhaustive literature search in the main bibliographic databases (PubMed, EMBASE) using the term ‘infertility therapy’ combined with the names of the drugs most commonly used in these type of therapies (e.g., ‘gonal-f’, ‘synarel’, ‘orgalutran’, ‘cetrotide’, ‘puregon’ and ‘menopur’), we identified 443 articles. Most (414 articles), analyze the long-term safety and adverse effects of gonadotropins. Only 29 articles analyzed the short-term adverse effects of these drugs, focusing mostly on patient [Gibreel and Bhattacharya S 2009; Somkuti et al. Citation2006; Gocial et al. Citation2000] and nurse [Porter et al. Citation2008], satisfaction and administration comfort, as well as with drug convenience [Nahuis et al. Citation2009; Kettel et al. Citation2004; Pang et al. Citation2003; Platteau et al. Citation2003], drug tolerance [Rama et al. 2008; Christianson et al. Citation2007], incidence of allergic reactions [Harrison et al. Citation2000; Redfearn et al. Citation1995; Kinoshita et al. Citation1999; Citation2000; Harika et al. Citation1994; Dore et al. Citation1994], and local tolerability [Diedrich Citation2002; Feigenbaum et al. Citation2001; Alviggi et al. Citation2007; Giles et al. Citation2004; Nichols et al. Citation2001; Engmann et al. Citation1998; Olivennes et al. Citation2000; Van Hooren et al. Citation2001] associated with the drug presentation or means of administration. Only three articles describe the general effects profile [Gillies et al. Citation2000; Banz et al. Citation2002; Goa and Wagstaff Citation1998]. We did not find any article which established the predictor factors in currently available drugs for this indication or the adverse effects caused by them. In fact, only one study [Lin et al. Citation2010] mentions a combined effect of exclusively two drugs, cetrorelix and follitropin-alpha, with regard to local tolerability.
Despite this paucity of evidence, summary databases such as UptoDate [Uptodate 2011] assume the safety profile stated for this class of drugs in clinical research. It concludes that infertility drugs produce no short-term adverse effects apart from minor, transitory discomfort.
Our research group has thus undertaken a prospective observational study (from February 1, 2009 to February 1, 2010, both inclusive) to determine the short-term safety profile associated with the administration of different drugs in a group of women undergoing ART focusing on the relative risk associated with each drug.
Results
We recruited 61 patients with a median age of 34 years (s.d. ± 4.12, range: 21 - 41 years). provides a summary of the principal characteristics of the study subjects. Immigrants to Spain comprised only 9.80% of the study subjects, 25.40% were smokers, and 63% did not take any other medication previous to undergoing ART. The most frequent type of sterility was primary and due to male factor. The most common factor affecting female sterility was endometriosis. In vitro fertilization was most frequently used rather than artificial insemination.
Table 1. Main patient characteristics.
The confounding variables in the multivariate models are listed in . The most frequent confounders associated with a lower tolerance to drugs used in ART were 35 years and older, a smoker, suffering female sterility but not low ovarian reserve, undergoing in vitro fertilization rather than insemination, increased BMI, fewer years of sterility, and a sedentary lifestyle.
Table 2. Variables included in multivariate models for different types of adverse reactions.
The incidence of adverse drug effects (ADEs) are shown in . The final models for each type of ADE are presented in , classified according to the type of ADEs and in to the type of therapy (in vitro versus insemination). Most statistically significant values included ‘general’, ‘gastrointestinal’, and ‘injection site reactions’ ADEs.
Table 3. Incidence of adverse effects.
Table 4. Associations between drugs and the incidence of each type of adverse effects.
Table 5A. Associations between infertility drugs and the incidence of adverse effects according to administration (in monotherapy or in combination) and type of therapy (in vitro fertilization or artificial insemination).
Table 5B. Associations between infertility drugs and the incidence of adverse effects according to administration (in monotherapy or in combination) and type of therapy (in vitro fertilization or artificial insemination).
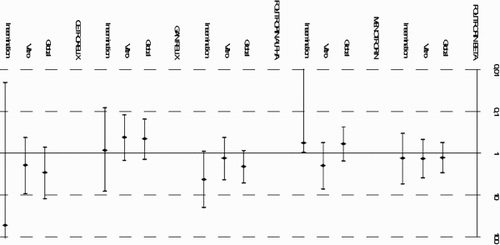
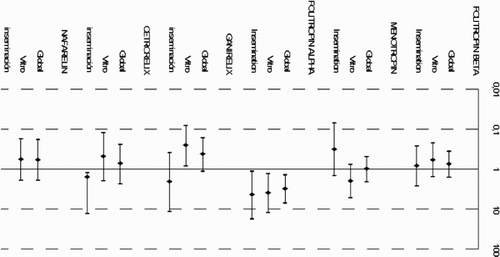
Some drugs, such as follitropin-alpha and cetrorelix, showed the poorest safety profile. The best profile was attributed to nafarelin and ganirelix for most of the ADEs (statistically significant for ganirelix ‘neuroendocrine’ ADEs, OR: 0.18; p = 0.037). Focusing on the therapy, ganirelix was the best tolerated drug in use for in vitro ART, although the differences were not statistically significant. The drug best tolerated in insemination treatments, for the most of ADE, was menopur. If we compare drugs, it becomes clear that the two enantiomers of follitropin present different safety profiles, with the beta form being more favorable than the alpha form. With respect to the two GnRH antagonists analyzed, ganirelix and cetrorelix, the latter was the least tolerated in all the ADEs studied except for ‘neuromuscular’ ADE. In fact, Ganirelix was the best tolerated drug in ‘general’ ADEs, especially when used in vitro therapy. Comparing GnRH antagonists with agonists, ganirelix was better tolerated than nafarelin (except in ‘general’ ADE), and nafarelin better tolerated than cetrorelix (except in ‘gastrointestinal’ ADE in in vitro ART).
Taking into account the ADE classification, we can conclude that with respect to ‘site injection reactions’ ADE, menotropin is the best tolerated drug in insemination. As for ‘gastrointestinal’ ADE, ganirelix was the best tolerated drug in in vitro, and follitropin-alpha the worst tolerated in insemination.
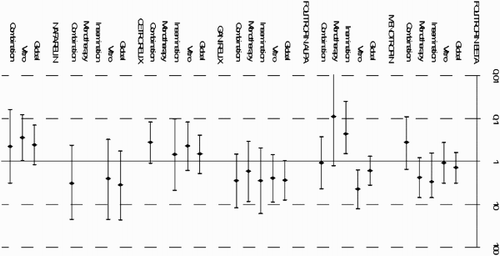
, , and show the estimates obtained for the groups, defined according to the type of therapy (artificial insemination or in vitro fertilization) as well as to the type of administration when used in monotherapy or in combination with other drugs. Menotropin exerted a different effect depending on the type of treatment used, with a statistically significant interaction (p < 0.001); the association was greater when administered in in vitro treatments (OR: 4.6) than when given in artificial insemination (OR: 0.5).
Figure 1. Association between infertility medication and general adverse drug effects stratified by assisted reproduction technique and type of administration (combination versus monotherapy) . Data shown as Odds Ratio (95% Confidence Interval) and estimated by multivariate Generalized Estimated Equation (GEE) models.
Discussion
In this study we have shown how several factors related to ART such as age, the underlying cause of sterility, and the drug prescribed are important determinants associated with the incidence of ADE. To reach this conclusion, we constructed a sufficiently complete causal model which takes into account the main factors implicated in drug safety in this type of treatment. We also considered the possible dynamic relationships between drug administration and the incidence of secondary effects, analyzing this association with the techniques suited for the longitudinal nature of the data.
In general, older patients, patients who have been sterile for a greater number of years, and those with low ovarian reserve seem to tolerate drug treatment better. In contrast, smokers have a lower tolerance for drug treatment. Other confounding variables which add to increased risk of lower tolerance include increased BMI, undergoing in vitro treatment rather than artificial insemination, and a sedentary life-style.
The literature includes few studies analyzing and comparing adverse reactions of different drugs employed during the course of ART. One study, for instance, indicated a higher tolerance for follitropin-alpha as compared to the beta form [Daya Citation2004], but did not account for confounding or interacting variables. As presented here, follitropin-beta was found to have a better safety profile than the alpha enantiomer, with the difference being somewhat dependent on the type of ART employed (in vitro fertilization or artificial insemination) and on whether the drugs were administered in monotherapy or in combination. In this context, recommendations for using one or the other should take into account the type of treatment required, and would suggest that follitropin-beta be used in cases of in vitro fertilization while its alpha form should be employed in artificial insemination therapies, since it tends to be used in monotherapy.
We have not identified studies which analyze whether the use of two or more drugs in combination could influence the incidence of ADEs. Only one descriptive study examines the safety risk of using a combination of FSH 150 UI with LH 75 UI [Burgues et al. 2001]. Nevertheless in the present study, we show that drug combination has an effect on a drug's safety profile. Combination interactions appear statistically significant for menotropin (p = 0.046) and follitropin beta (p = 0.035)
Focusing on the type of ADEs, reactions to the site injection is the only group which has been widely studied. The development of local intolerance symptoms produced by injection of gonadotropins has been traditionally attributed to product impurities associated with urinary gonadotropins [Kaplan et al. Citation2000; International Recombinant Human Chorionic Gonadotropin Study Group Citation2001; Al-Inany et al. Citation2005]. Therefore, improved methods of extraction and purification currently used in the production of human menopausal gonadotropins might explain the decreasing incidence of local reactions.
Several authors point out that the use of pen injectors and prefilled syringes facilitates drug administration and it could allow a more precise dosage administration [Pang and Kettel Citation2007]. In fact, in several studies in which a pen injector was used [Christianson et al. Citation2007], the hormone requirements were lower than for treatments which utilized vials and the treatment time was shortened accordingly [Platteau et al. Citation2003]. The reconstitution of the vials may also cause greater stress for the patients [Samkuti et al. 2006; Greco et al. Citation2005]. Interestingly, one study hypothesized that pen injectors may require greater education of the patients [Chen et al. Citation2009)]. This along with several other factors, such as the pen enabling self-administration of the drug [Pang 2003; Craenmeher et al. 2001] with greater convenience and comfort, especially for patients taking several drugs [Sedbon et al. Citation2006], suggests why many studies have observed a greater acceptance of the use of pen injectors on the part of the patients [Welcker et al. Citation2010; Gibreel and Bhattacharya Citation2010; Rama Raju et al. Citation2008; Aghssa et al. Citation2008; Weiss Citation2007; Kettle et al. 2004].
In contrast, to that described above, patients receiving either follitropin-beta or -alpha in pen devices exhibited a lower tolerance than those receiving menotropin in vials or ganirelix administered with a precharged syringe. Cetrorelix, which was administered in vials, was associated with the greatest risk of adverse effects. This could reflect other multiple factors related to local intolerance such as injection volume, speed of injection, osmolarity, excipients, part of body in which the injection is given, and needle size. In fact, some studies [Craenmehr et al. Citation2001; Voortman et al. Citation1999; Platteau et al. Citation2003] suggest that injector pens produce less site injection pain as a consequence of a smaller needle size and injection volume.
With respect to the administration route, all our patients received the drugs subcutaneously (with the exception of nafarelin inhalated). Two previous studies found no differences in adverse effects between subcutaneous and intramuscular injections of ART drugs [Nichols et al. Citation2001; Out et al. Citation1997]. In contrast, a third study found that subcutaneous injections allowed for self-administration and reduced injection pain [Kapplan et al. 2000].
This study has several limitations. The observational nature of the study did not allow the random assignment of drug treatment. This implies that we cannot assume or guarantee comparability among patients. Nevertheless, we have controlled its possible confounders by introducing into the model all those variables which could be related to the incidence of ADE in these types of patients. As presented, the analysis described in this communication was limited to the drugs administered to this type of patient in the reference clinic. As such, it did not include all possible ART drugs. However, drugs included in this meta-analysis are those most commonly used. Improving the knowledge of the drug safety profile should be useful in managing and selecting these drugs in clinical practice. Some drugs such as follitropin alpha versus beta or ganirelix versus cetrorelix could be considered therapeutic equivalents and the safety profile adds to the information to assist in their selection for use in treatment. This strategy could also be useful to predict tolerance and as a consequence optimize and individualize treatment. Adverse drug reactions constitute a clinical problem. It is clear that ADEs have an ever increasing economic, social, and public health repercussions.We cannot forget that adverse events are frequently responsible of non-compliance and abandoning treatment. This should emphasize that along with efficacy, drug safety constitutes a fundamental pillar of the therapeutic usefulness of treatment drugs.
Our analysis of adverse effects is noteworthy as the data were collected with a longitudinal, prospective protocol. This allowed us to ascertain the predictor variables by analyzing possible confounding effects between variables as well as a possible interaction effect among them. Moreover, the analysis presented was not influenced by patients with an elevated number of visits or with a tolerance to extreme treatment (either due to excess or defect). Nevertheless we must be cautious with the results of this study due to the low number of patients included and validation is required.
Materials and Methods
The project was approved by the International Research Board of La Mancha-Centro Hospital. All subjects gave their informed consent to participate in the study. This study was a longitudinal, observational prospective panel cohort study on the safety and tolerability of drugs used in ART. Study subjects were selected from patients receiving medication in the Pharmacy Service of the La Mancha-Centro Hospital, in Alcázar de San Juan (a Spanish province of Ciudad Real). The vast majority of immigrants who participated in this study were Romanians (population who usually learns Spanish). Despite this fact, a face-to-face interview was given to reassure the total comprehension of the questionnaire. The study lasted one year, from February 1, 2009 to February 1, 2010, both inclusive.
Patients included were women between 18 and 40 y of age undergoing assisted reproductive techniques with primary sterility or secondary sterility with a new partner or those between 18 and 38 y with secondary sterility and only one previous child. The study recruited all patients who consecutively refilled infertility medication in the hospital pharmacy in the study period; it excluded women who did not want to enroll in the study.
Follow-up was conducted through consecutive visits. Patients were interviewed using a structured questionnaire. It was administered by the hospital pharmacy personnel, who reviewed a list of possible adverse effects associated with the drugs prescribed. The list of adverse effects was elaborated according to the summary of product characteristics. Interviewers checked this reproductive list of adverse effects and those from other drug effects. The visit dates were recorded along with the drugs that the patients were taking at the time of the interview. To assure that the adverse effects were associated to the drugs, interviewers took into account: the association in time between drug administration and event, the drug pharmacology (including current knowledge of nature and frequency of adverse reactions), medical or pharmacological plausibility (signs and symptoms, mechanism), and the exclusion of other causes.
Patients who voluntarily dropped out were included in the analysis only up until the time for which data could be obtained. The classification of adverse effects was carried out with an organ- and system-based classification system according to the summary of product characteristics (SPC) of the drug's classification: central nervous system (headaches, mood swings, dizziness, drowsiness), cardiovascular system (shortness of breath, changes in blood pressure, palpitations), respiratory system (nasal irritation, respiratory infections, rhinitis, watery eyes), genital-urinary tract (vaginal pruritus, vaginal bleeding, breast tightness, urine infections), musculoskeletal system (back pain, muscle pain), skin (acne, seborrhea, hirsutism, baldness), endocrine-metabolic system (weight changes, liquid retention, swollen legs), gastrointestinal system (swelling, nausea, changes in appetite, diarrhea), site injection reactions, and other problems (bad taste in the mouth, temperature changes, miccional urgency). We included a category of general ADEs which we included general or global ADEs.
Explanatory variables were selected after reviewing the literature and databases using clinical and biological plausibility criteria, included sociodemographic factors (age, nationality, work situation), factors related to the underlying pathology (type of sterility, cause of sterility, duration of sterility in years, body mass index, analytical parameters lifestyle habits, and habitual medication), as well as the protocol and type of therapy prescribed. With regard to the latter, the drugs, the route of administration (inhalation, subcutaneous, intramuscular), drug presentation, drug combinations, number of cycles, and treatment results (pregnancy, no pregnancy, or cancellations) were considered. The variables were analyzed individually and together to account for confounders and interactions by means of an univariate study. We took in consideration for the final models those that were statistically significant.
The longitudinal nature of the data (repeated measures in the same patients carried out at different times) was considered in the multivariate analysis. Various generalized estimating equation (GEE) models were created with a logit data connection through an exchangeable-type correlation matrix. GEE represents a class of models that can be used for data in which the responses are correlated. GEE models can be used to account for the correlation of continuous or categorical outcomes. A GEE model was constructed for each adverse effect group. In each model, indicator variables were introduced for each drug, followed by any confounding variables. The dependent variables were introduced in the model in a similar way as a logistic model. Associations were summarized as odds ratios (OR) and 95% confidence intervals (95%CI). We selected a panel data analysis, because it considers repeated measures of several variables on a group of patients.
We also explored a possible modification of the effect (interaction) with regard to the type of ART (insemination or in vitro fertilization) and whether drugs were administered in monotherapy or in combination. Patients were stratified according to the type of ART and as a consequence, the study results were not affected by dosing or exposition to drug. Processing and analysis of the data was carried out with the STATA 10.0 statistics program. P values under 0.05 were considered to be significant.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Abbreviations
| GEE: | = | generalized estimating equation |
| OR: | = | odds ratios |
| ART: | = | assisted reproduction techniques |
| ADE: | = | adverse drug effects. |
| LH: | = | luteinizing hormone |
| FSH: | = | follicular stimulant hormone |
| NSAIDs: | = | nonsteroidal antiinflamatory drugs |
| SPC: | = | summary product characterics |
| BMI: | = | body mass index |
References
- Aghssa, MM. Azargoon, A. Ramezanzadeh, F. Bagheri, M. (2008) A comparison of the efficacy, tolerability, and convenience of two formulations of follitropin-alpha in Iranian woman undergoing intracytoplasmic sperm injection cycles. Fertil Steril 90(4):1043–1048.
- Al-Inany, H.G., Aboulghar, M., Mansour, R., and Proctor, M. (2005) Recombinant versus urinary human chorionic gonadotrophin for ovulation induction in assisted conception. Cochrane Database Syst Rev (2). Apr 18; (2): CD003719
- Alviggi, C., Revelli, A., Anserini, P., Ranieri, A., Fedele, L., Strina, I. (2007) A prospective, randomised, controlled clinical study on the assessment of tolerability and of clinical efficacy of Merional (hMG-IBSA) administered subcutaneously versus Merional administered intramuscularly in women undergoing multifollicular ovarian stimulation in an ART programme (IVF). Reprod Biol Endocrinol 5:45.
- Banz, C., Diedrich, K., & Ludwig, M. (2002) Ganirelix (Orgalutran). Tägliche Praxis 43(1):193.
- Beavers, N. (1997) Two new fertility drugs offer convenient self-administration. Drug Topics 141(21):30–32.
- Burgues and the Spanish collaborative group on female hypogonadotrophic hypogonadism (2001) The effectiveness and safety of recombinant human Lh to support follicular evelopment induced by recombinant human FSH in WHO groupI anovulation: evidence from a multicentre study in Spain. Hum Reprod 16(12):2525–2532.
- Chen, L.N., (2009) Clinical application of prefilled pen and conventional syringe during controlled ovarian stimulation for in vitro fertilization. Nan Fang Yi Ke Da Xue Xue Bao 29(1):100–104.
- Christianson, M.S., Barker, M.A., Schouweiler, C. and Lindheim, S.R. (2007) A retrospective comparison of clinical outcomes and satisfaction using reconstituted recombinant gonadotropins (rFSH) or cartridge rFSH with a pen device in donor oocyte cycles. Curr Med Res Opin 23(4):865–870.
- Craenmehr, E., Bontje, P.M., Hoomans, E., Voortman, G. and Mannaerts, B.M. (2001) Follitropin-beta administered by pen device has superior local tolerance compared with follitropin-alpha administered by conventional syringe. Reprod Biomed Online 3(3):185–189.
- Daya, S. (2004) Follicle-stimulating hormone in clinical practice: an update. Treat Endocrinol 3(3):161–171.
- Diedrich, K. (2002) Efficacy and safety of highly purified menotropin versus recombinant follicle-stimulating hormone in in vitro fertilization/intracytoplasmic sperm injection cycles: A randomized, comparative trial. Fertil Steril 78(3):520–528.
- Dickey, RP. Thornton, M. Nichols, J. Marshall, DC. Fein, SH. Nardi, RV. (2002) Comparison of the efficacy and safety of a highly purified human follicle-stimulating hormone (Bravelle(trademark)) and recombinant follitropin-(beta) for in vitro fertilization: A prospective, randomized study. Fertil Steril 77(6):1202–1208.
- Dore, P.C., Rice, C. and Killick, S. (1994) Human gonadotrophin preparations. May cause allergic reaction. BMJ. 308(6942):1509.
- Engmann, L. Shaker, A. White, E. Bekir, JS. Jacobs, HS. Tan, SL. (1998) Local side effects of subcutaneous and stimulation in in vitro fertilization: A prospective, randomized study. Fertil Steril 69(5):836–840.
- Feigenbaum, S. Miller, P. Kaufmann, R. Elkind-Hirsch, K. Fein, S. Marshall, H.D. (2001) A new highly purified human-derived FSH, Bravelle(trademark), is as effective and well tolerated as recombinant follitropin beta in ovulation induction in infertile women with ovulatory dysfunction. Today's Ther Trends 19(4):297–313.
- Gibreel, A. and Bhattacharya, S. (2010) Recombinant follitropin alfa/lutropin alfa in fertility treatment. Biologics 4:5–17.
- Giles, J., Requena, A., Guillen, A. and Garcia Velasco, J.A. (2004) Comparative, prospective, randomized study about the use of hp-HMG vs u-HMG in an egg donation program. Rev Iberoam Fertil Reprod Humana 21(5):313–319.
- Gillies, P.S. Faulds, D., Balfour, J.A. and Perry, CM. (2000) Ganirelix. Drugs 59(1):107–111.
- Goa, K.L. and Wagstaff, A.J. (1998) Follitropin alpha in infertility: A review. BioDrugs 9(3):235–260.
- Gocial, B., Keye, W.R., Fein, S.H. and Nardi, R.V. (2000) Subcutaneously administered repronex in female patients undergoing in vitro fertilization is as effective and well tolerated as intramuscular menotropin treatment. Fertil Steril 74(1):73–79.
- Greco, E. Polonio-Balbi, P. Ferrero, S. Baroni, E. Ubaldi, F. Rienzi, L. . (2005) Use of a fully automated injector for self-administration of follitropin alpha in an IVF/ICSI programme. Reprod Biomed Online 11(4):415–420.
- Harika, G., Gabriel, R., Quereux, C., Wahl, P. and Lavaud, F. (1994) Hypersensitization to human menopausal gonadotropins with anaphylactic shock syndrome during a fifth in vitro fertilization cycle. J Assisted Reprod Genet 11(1):51–53.
- Harrison, S., Wolf, T. and Abuzeid, M.I. (2000) Administration of recombinant follicle stimulating hormone in a woman with allergic reaction to menotropin: A case report. Gynecol Endocrinol 14(3):149–152.
- International Recombinant Human Chorionic Gonadotropin Study Group (2001) Induction of ovulation in World Health Organization group II anovulatory women undergoing follicular stimulation with recombinant human follicle-stimulating hormone: a comparison of recombinant human chorionic gonadotropin (rhCG) and urinary hCG. Fertil Steril 75(6):1111–1118.
- Kaplan, P.F. Austin, D.J. and Freund, R. (2000) Subcutaneous human menopausal gonadotropin administration for controlled ovarian hyperstimulation with intrauterine insemination cycles. Am J Obstet Gynecol 182(6):1421–1426.
- Kettel, LM. Scholl, G. Bonaventura, L. Pang, S. Sacks, P. Chantilis, S. (2004) Evaluation of a pen device for self-adminstration of recombinant human FSH in clomiphene citrate-resistant anovulatory women undergoing ovulation induction. Reprod Biomed Online 9(4):373–380.
- Kinoshita, T., Ootaka, K. and Ito, M. (1999) Delayed-type hypersensitivity reaction to human menopausal gonadotrophin. J Obstet Gynaecol Res 25(6):437–438.
- Kinoshita, T., Ootaka, K. and Itou, M. (2000) Adverse effects of injection site with hMG. Jpn J Fertil Steril 45(2):47–50.
- Lin, YH, Wen, YR., Chang, Y., Seow, KM., Hsieh, BC., Hwang, JL. (2010) Safety and efficacy of mixing cetrorelix with follitropin alfa: a randomized study. Fertil Steril 94(1):179–183.
- Nahuis, M. van der Veen, F. Oosterhuis, J. Mol, BW. Hompes, P. van Wely, M. (2009) Review of the safety, efficacy, costs and patient acceptability of recombinant follicle-stimulating hormone for injection in assisting ovulation induction in infertile women. Int J Womens Health 1(1):205–211.
- Nichols, J., Knochenhauer, E., Fein, S.H., Nardi, R.V. and Marshall, D.C. (2001) Subcutaneously administered repronex(registered trademark)a in oligoovulatory female patients undergoing ovulation induction is as effective and well tolerated as intramuscular human menopausal gonadotropin treatment. Fertil Steril 76(1):58–66.
- Olivennes, F. Ayoubi, JM. Fanchin, R. Rongières-Bertrand, C. Hamamah, S. Bouchard, P. (2000) GnRH antagonist in single-dose applications. Hum Reprod Update. 6(4):313–317.
- Out, H.J., Reimitz, P.E. and Bennink, H.J. (1997) A prospective, randomized study to assess the tolerance and efficacy of intramuscular and subcutaneous administration of recombinant follicle-stimulating hormone (Puregon). Fertil Steril 67(2):278–283.
- Pang, S. Kaplan, B. Karande, V. Westphal, LM. Scott, R. Givens, C. (2003) Administration of recombinant human FSH (solution in cartridge) with a pen device in women undergoing ovarian stimulation. Reprod BioMed Online 7(3):319–326.
- Pang, S. and Kettel, L.M. (2007) Clinical Experiences of Integrating the Follistim Pen® for the Self-administration of Follitropin Beta into Ovarian Stimulation Treatment Regimens. Touch Briefings 20–22. fertility treatement review
- Platteau, P. Laurent, E. Albano, C. Osmanagaoglu, K. Vernaeve, V. Tournaye, H. (2003) An open, randomized single-centre study to compare the efficacy and convenience of follitropin (beta) administered by a pen device with follitropin (alpha) administered by a conventional syringe in women undergoing ovarian stimulation for IVF/ICSI. Hum Reprod 18(6):1200–1204.
- Porter, R., Kissel, C., Saunders, H. and Keck, C. (2008) Patient and nurse evaluation of recombinant human follicle-stimulating hormone administration methods: Comparison of two follitropin injection pens. Curr Med Res Opin 24(3):727–735.
- Rama Raju, G.A., Suryanarayana, K., Prakash, G.J. and Krishna, K.M. (2008) Comparison of follitropin-(beta) administered by a pen device with conventional syringe in an ART programme - A retrospective study. J Clin Pharm Ther 33(4):401–407.
- Redfearn, A., Hughes, E.G., O'Connor, M. and Dolovich, J. (1995) Delayed-type hypersensitivity to human gonadotropin: Case report. Fertil Steril 64(4):855–856.
- Schneeweiss, S. (2011) Postmarketing studies of drug safety. BMJ 342:d342.
- Sedbon, E., Wainer, R. and Perves, C. (2006) Quality of life of patients undergoing ovarian stimulation with injectable drugs in relation to medical practice in France. Reprod Biomed Online 12(3):298–303.
- Somkuti, S.G., Schertz, J.C., Moore, M., Ferrande, L. and Kelly, E. (2006) Patient experience with follitropin alfa prefilled pen versus previously used injectable gonadotropins for ovulation induction in oligoanovulatory women. Curr Med Res Opin 22(10):1981–1996.
- Uptodate database (2011) Overview of treatment of female infertiity. Available at: External link http://www.uptodate.com/patients. Accessed January 2, 2011.
- Van Hooren, HG, Fischl, F- Aboulghar. Mares., Nicollet, B., Behre, HM. (2001) Comparable clinical outcome using the GnRH antagonist ganirelix or a long protocol of the GnRH agonist triptorelin for the prevention of premature LH surges in women undergoing ovarian stimulation. Hum Reprod 16(4):644–651.
- Voortman, G., van de Post, J., Schoemaker, R.C. and van Gerven, J.M. (1999) Bioequivalence of subcutaneous injections of recombinant human follicle stimulating hormone (Puregon) by pen-injector and syringe. Hum Reprod 14(7):1698–1702.
- Weiss, N. (2007) Gonadotrophin products: empowering patients to choose the product that meets their needs. Reprod Biomed Online 15(1):31–7.
- Welcker, J.T., Nawroth, F. and Bilger, W. (2010) Patient evaluation of the use of follitropin alfa in a prefilled ready-to-use injection pen in assisted reproductive technology: an observational study. Reprod Biol Endocrinol 8:111.