Abstract
Cystic fibrosis is the most frequent autosomal recessive disease in the Caucasian population, with an incidence of 1:2500 newborn and a frequency of 1:25. The associated gene is Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) and it encodes the CFTR protein that functions as a chloride (Cl−) channel. It is found in the apical membrane of exocrine epithelial cells, responsible for the regulation of the movement of water and solutes through biological membranes. To our knowledge, there are no studies on protein localization in the different cell types of the seminiferous epithelium with different pathologies. The aim of the present study was to analyze the expression of the CFTR protein in the human seminiferous epithelium of infertile males with different pathologies. CFTR protein expression was studied by immunohistochemistry in paraffin sections of testicular biopsies of six infertile men: Sertoli cell only syndrome, maturation arrest, secondary obstructive azoospermia, primary obstructive azoospermia due to congenital bilateral absence of the vas deferens (CBAVD), severe oligozoospermia, and retrograde ejaculation. All cell types of the seminiferous epithelium were studied: Sertoli cells, spermatogonia, primary spermatocytes at the leptotene/zygotene and at the pachytene stages, secondary spermatocytes, round, elongating and elongated spermatids, and spermatozoa. With the exception of sperm, all cells were labeled in the cytoplasm and in the cytoplasmic membrane. In the patient with CBAVD labeling was light at the cell membrane and absent in the cytoplasm of Sertoli cells and diploid germ cells. Generally, labeling was stronger after the diploid stage, which is probably related to cell volume reduction during spermiogenesis. The results obtained also suggest that the CFTR protein may impact CBAVD spermatogenesis and other pathologies.
Introduction
The Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene is localized in the long arm of chromosome 7 and consists of 27 exons and several long introns (250 kb). It encodes the chloride (Cl−) channel CFTR protein, located in the apical cytoplasmic membrane of exocrine epithelial cells, responsible for transepithelial movement of water and solutes. The channel is a 1,480 aminoacid glycoprotein and a member of the ABC transporter superfamily [ Kerem et al. Citation1989; Riordan et al. Citation1989; Rommens et al. Citation1989; Zielenski et al. Citation1991].
The CFTR protein has two similar motifs, each containing a membrane-spanning domain (MSD) composed of six transmembrane segments (TMD) and a cytoplasmatic nucleotide-binding domain (NBD) with ATP interacting motifs [Riordan et al. Citation1989]. The two motifs are linked by a cytoplasmatic hydrophilic regulatory (R) domain that contains multiple consensus phosphorylation sites for protein kinase A (PKA) and protein kinase C (PKC) [Riordan et al. Citation1989; Luo et al. Citation1998; Grangeia et al. Citation2008]. CFTR protein is synthesised in the endoplasmic reticulum, glycosylated in the Golgi complex, and then transported to the cytoplasmic membrane [Kopito Citation1999; Turnbull et al. Citation2007].
Mutations in the CFTR gene are the cause of Cystic Fibrosis (CF), one of the most severe genetic diseases in the Caucasian population [Welsh et al. Citation2001]. Depending on the severity of the mutations, different clinical manifestations are observed, such as chronic lung disease, pancreatic insufficiency, and infertility [Castellani et al. Citation2008]. The most frequent mutation, F508del, occurs in about 70% of the CF cases. The abnormal F508del protein is retained in the endoplasmic reticulum and then degraded in the proteasome [Kopito Citation1999; Turnbull et al. Citation2007].
Male infertility occurs in about 95% of the cases with CF due to congenital bilateral absence of the vas deferens (CBAVD). In CBAVD, the transport of testicular sperm is obliterated, with dilation of the epididymis and obstructive azoospermia [Tizzano and Buchwald Citation1995]. CFTR mutations may cause CBAVD in the absence of CF. Patients with CBAVD but without CF, present one severe CFTR mutation in one allele and one mild CFTR mutation in the other, or two mild CFTR mutations in the two alleles [Grangeia et al. Citation2004; Grangeia et al. Citation2007]. Functional sperm are produced, since testicular sperm can be recovered for intracytoplasmic sperm injection (ICSI) treatment cycles by testicular sperm extraction, with very satisfactory results [Larriba et al. Citation1998; Wong et al. 1998; Sousa et al. Citation2002; Grangeia et al. Citation2004; Grangeia et al. Citation2007].
To our knowledge, this is the first report of CFTR protein expression in the different cell types of the human seminiferous epithelium (SE) from infertile patients. The results showed different CFTR labeling patterns in the Sertoli cell only syndrome (SCOS), maturation arrest (MA), secondary obstructive azoospermia (OAZsec), CBAVD, and severe oligozoospermia (SOZ). Albeit preliminary, the data presented is consistent with an involvement of the CFTR protein in volume reduction during spermiogenesis. This may also aid in the design of a hypothesis for the mechanism of male infertility by inactive or misdirected CFTR protein.
Results
CFTR protein expression was studied by immunohistochemistry in the SE of six different pathologies summarized in . The general architecture of the ST and SE at a lower magnification of testis sections per pathology can be observed in .
Figure 1. Immunohystochemical detection of the CFTR protein in different pathologies of infertile patients. General architecture of the seminiferous tubules at low magnification. A) Sertoli cell only syndrome (SCOS), B) maturation arrest (MA), C) secondary obstructive azoospermia (OAZsec), D) congenital bilateral absence of the vas deferens (CBAVD), E) severe oligozoospermia (SOZ), and F) retrograde ejaculation (RE). Bars: 100 µm (A, E), 50 µm (B, C, D, F).
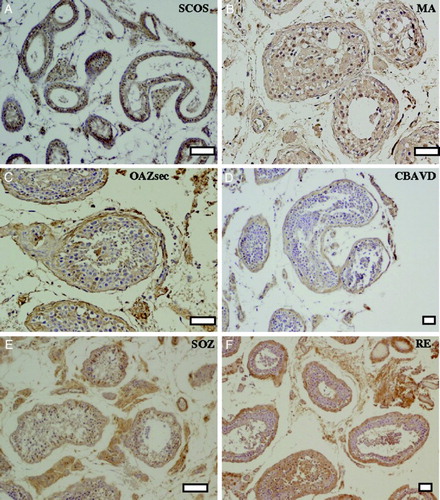
Table 1. Localization of CFTR protein in the seminiferous epithelium of infertile men with different pathologies.
In the controls (absence of primary antibody) and in spermatozoa no labeling was found, as expected. In SCOS, labeling in Sertoli cells (SC) was intense in the cytoplasmic membrane and in the basal region of the cytoplasm, being mild in the apical region of the cytoplasm (A,B). In MA, labeling of Sertoli cells and diploid germ cells was mild in the cytoplasmic membrane and light in the cytoplasm (C-E).
Figure 2. Immunohystochemical detection of CFTR protein in different pathologies of infertile patients. Sertoli cell only syndrome (SCOS): A) reaction, B) negative control. Maturation arrest (MA): C,D) reaction, E) negative control. Secondary obstructive azoospermia (OAZsec): F,G) reaction, H,K) negative control. Congenital bilateral absence of the vas deferens (CBAVD): I,J) reaction, H,K) negative control. Sertoli cells (SC), spermatogonia A dark (Ad), spermatogonia A pale (Ap), primary spermatocytes at the zygotene stage (Z), primary spermatocytes at the pachitene stage (P), secondary spermatocytes (ST2), round spermatids (Sa), early elongating spermatids (Sb), late elongating spermatids (Sc), elongated spermatids (Sd), spermatozoa (Sz). Bars: 10 µm (A,B), 5 µm (C-K).
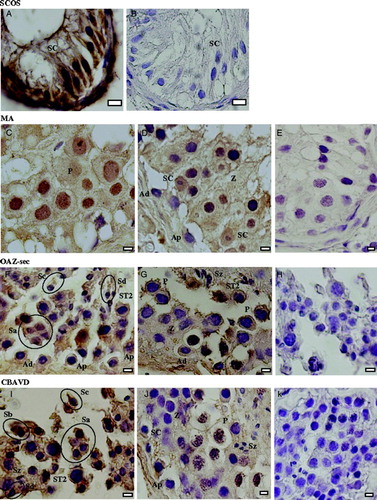
In OAZsec, SC, spermatogonia (SG), and primary spermatocytes at the leptotene/zygotene stage (Lep-Zyg) showed a mild labeling both in the cytoplasmic membrane and in the cytoplasm. In primary spermatocytes at the pachitene stage (Pac), secondary spermatocytes (ST2), and round spermatids, labeling was intense in the cytoplasmic membrane and mild in the cytoplasm. In elongating and elongated spermatids, labeling was mild in the cytoplasmic membrane and light in the cytoplasm (F-H).
In CBAVD, SC and diploid germ cells showed a light labeling in the cytoplasmic membrane, being negative in the cytoplasm. ST2 and haploid germ cells presented an intense labeling in the cytoplasmic membrane and a mild labeling in the cytoplasm (I-K).
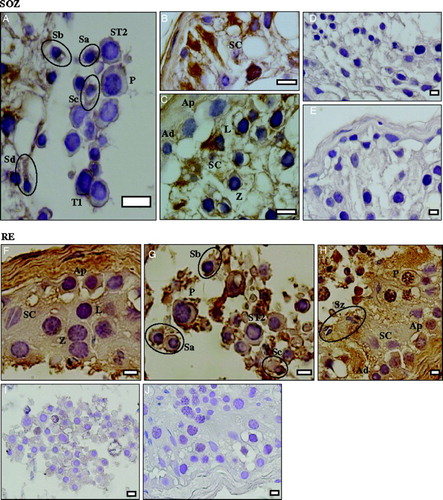
In SOZ, labeling in SC was intense in the cytoplasmic membrane and in the basal region of the cytoplasm, being mild in the apical region of the cytoplasm. In SG, labeling was light in the cytoplasmic membrane and in the cytoplasm. In Lep-Zyg, labeling was mild in the cytoplasmic membrane and negative in the cytoplasm. In Pac, ST2 and spermatids, labeling was intense in the cytoplasmic membrane and mild in the cytoplasm (A-E). In retrograde ejaculation (RE), SC, SG, and Lep-Zyg showed a mild labeling in the cytoplasmic membrane and in the cytoplasm. In Pac, ST2 and spermatids labeling was intense in the cytoplasmic membrane and mild in the cytoplasm (F-J).
Figure 3. Immunohystochemical detection of the CFTR protein in different pathologies of infertile patients. Severe oligozoospermia (SOZ): A-C) reaction, D,E) negative control. Retrograde ejaculation (RE): F-H) reaction, I,J) negative control. Sertoli cells (SC), spermatogonia A dark (Ad), spermatogonia A pale (Ap), primary spermatocytes at the leptotene stage (L), primary spermatocytes at the zygotene stage (Z), primary spermatocytes at the pachitene stage (P), telophase I (T1), secondary spermatocytes (ST2), round spermatids (Sa), early elongating spermatids (Sb), late elongating spermatids (Sc), elongated spermatids (Sd), spermatozoa (Sz). Bars: 10 µm (A-C), 5 µm (D-J).
Due to the lack of synchrony in the development of germ cells, the different orientations in the paraffin sections and the low number of cases in the present report, it is very difficult to establish with certainty in figures to which spermatogenic-stage they belong. In Sertoli cell only syndrome and maturation arrest, there is no classification. For obstructive azoospermia, CBAVD, severe oligozoospermia, and retrograde ejaculation, the seminiferous tubules were different between each other and several presented shedding of cells into the lumen (signal of tubular obstruction). As in all of the latter there was presence of all cell-stages, according to Clermont [1963] we would classify these samples as in spermatogenic-stages V-VI.
Discussion
CFTR protein presents two mechanisms of action, it acts as a primary Cl− cAMP dependent channel [Riordan et al. Citation1989; Luo et al. Citation1998], and it regulates sodium [Reddy and Quinton Citation2003] and water [Schreiber et al. Citation1999] channels. In the rat testis, CFTR mRNA expression was negative in SC, mild in diploid germ cells and strong in round spermatids [Trezise and Buchwald Citation1991; Trezise et al. Citation1993]. In contrast SC, a functional CFTR protein was found in rat SC cultures [Boockfor et al. Citation1998].
The flow of water and other small molecules through membranes is fundamental during spermatogenesis. In rodents, CFTR protein expression was observed in round and elongated spermatids and detected with the simultaneous expression of the water channel, suggesting that their interaction could be involved in volume reduction observed during spermiogenesis [Gong et al. Citation2001]. In accordance with those findings, mouse water channel mRNA expression was found both in the cytoplasmic membrane and cytoplasm for aquaporin 7 [Suzuki-Toyota et al. 1999] and aquaporin 8 [Huang et al. Citation2006].
There are only a few studies on CFTR gene expression in human SE, but none in patients with CBAVD. Absence of CFTR mRNA expression was described in human SE of cases with conserved spermatogenesis [Tizzano et al. Citation1994]. On the contrary, expression of the CFTR protein, also in normal samples, was observed in the cytoplasm and cytoplasmic membrane of primary spermatocytes and elongating spermatids [Hihnala et al. Citation2006]. The CFTR protein has been observed in SC and all types of germ cells in normal samples, but with decreased apparent intensity in non-obstructive azoospermic patients [Xu et al. Citation2011].
To our knowledge, this is the first report on the immunohystochemical localization of the CFTR protein in different cell types of the human SE from infertile patients with distinct pathologies, including CBAVD. All cells presented labeling in the cytoplasmic membrane and in the cytoplasm, although with different intensities in relation to different types of SE cells and pathologies. These results are in accord with the observations in rodents, concerning a more intense labeling of the cytoplasmic membrane after the second meiosis, at the round spermatid stage, with absence of staining in testicular sperm [Trezise and Buchwald 1991; Trezise et al. 1993]. An intense labeling in the cytoplasmic membrane was observed in pachytene primary spermatocytes of OAZsec and SOZ, and in secondary spermatocytes in OAZsec, CBAVD, and SOZ. A special mention should be given to the CBAVD case that showed a light labeling at the cytoplasmic membrane of Sertoli cells and diploid germ cells and absence of staining in the cytoplasm.
In conclusion, the present data corroborates previous findings in rodents and humans regarding the possible involvement of the CFTR protein in volume reduction during spermiogenesis. Further, it suggests that the CFTR protein might have other functions, alone or through its interaction with other transporters, since all cells displayed CFTR staining in the cytoplasmic membrane. Albeit preliminary, the present study showed different patterns of labeling in distinct pathologies, namely in CBAVD, reinforcing the hypothesis that CFTR gene expression in the SE has an impact in infertility. Further studies should be conducted to increase the population studied, using stereological tools, to clarify these observations.
Materials and Methods
This work did not involve human or animal experiments. According to the National Law on Medically Assisted Procreation (PMA, Law 32/2006) and the guidelines of the National Council on Medically Assisted Procreation (CNPMA, 2008), patient databases and human seminiferous tubule (ST) fragments were obtained, after patients' informed and written consent. Patients were involved in infertility treatment cycles with TESE-ICSI [Sousa et al. Citation2002].
We have a small library of testicular biopsies of different pathologies. Several biopsies were analyzed by Hematoxylin-Eosin to verify the integrity and the amount of the seminiferous tubules. Of several analyzed, the present six cases were selected based on a clear morphology related to the diagnosis with enough tubules and Sertoli cell and germ cells present. We are aware that individual variation might significantly alter the results. However, the present work only represents a clinical case report and not a stereological study aimed to analyze individual variation.
Six cases were selected: SCOS, MA, OAZsec, CBAVD, SOZ, and RE. The later was used as control for conserved spermatogenesis. The male mean age was 37.5 (28-51) y and the mean time of infertility was 3.5 (1-7) y. Four cases presented normal and two cases elevated (SCOS: 16.9 mIU/ml, SOZ: 19.79 mIU/ml) serum follicular stimulating hormone (FSH) levels, and all had normal karyotypes, absence of Y chromosome microdeletions, and a normal CF screening (except case with CBAVD). The first four cases showed total absence of sperm in the ejaculate. The patient with CBAVD presented reduced semen volume (0.4 ml) and pH (6.4), and the patient with RE had mild oligozoospermia. The testicular volumes were normal except in the patient with SCOS (hypotrophyc). The epididymis were normal, except in patients with CBAVD (globus major) and OAZsec (dilated). The vas deferens were absent in the patient with CBAVD and were not possible to evaluate in the case with OAZsec.
The patient with OAZsec had left cryptorchidism (orchydectomy at age 13) and a right surgical correction for inguinal hernia (age 15). In the patient with CBAVD, CF screening revealed two mutations, F508del (severe) and R117H (mild), with a normal polymorphism of the 7/9T alleles. The patient with SOZ showed evolutive oligozoospermia, with 0.240 x 106/ml sperm (2002) and 0.052 x 106/ml sperm (2007). The case with RE was due to lesion of the hypogastric nerves during colon surgery.
Cases with SCOS and MA performed TESE at both testicles (5-7 fragments of 1.2 mm3), but no spermatozoa was found after extensive searching. They underwent a program of donor semen. All other cases had TESE in only one testicle (4-7 fragments), with numerous germ cells and in situ mobile testicular sperm. They underwent ICSI and attained a successful pregnancy with birth of healthy children, except for the case with SOZ.
Light Microscopy
Fragments of ST were fixed in 4% paraformaldehyde (DAC, Merck, Darmstadt, Germany) in PBS (Phosphate Buffered Saline, Sigma, Saint Louis, Missouri, USA), pH 7.3, during 24 h, at 4°C, washed with 70% ethanol, dehydrated using an increasing sequence of ethanol concentrations, cleared with xylene, impregnated, and embedded in paraffin (Merck). Glass slides (Industrial Quality, Germany) were coated with Vectabond Reagent Solution (Vector Laboratories, Burlingame, USA).
We did not study seminiferous tubules per spermatogenic stage. We just identified all cell types according to Holstein and Roosen-Runge [1981], Sousa et al. [2002], and Sá et al. [2008]. Ten sequential sections (3 µm thick) were obtained from each block, and the first was stained with Hematoxylin-Eosin (Merck) to verify the integrity and the amount of the ST. To find the different cell types, all ST (more or less 20) in each section were analyzed.
Immunostaining
Immunohistochemistry was performed with the Novo Link Min Polymer Detection System kit (Leica Biosystems Ltd., Newcastle, United Kingdom). Sections were dewaxed and rehydrated, slides placed in a dark, moistened chamber, incubated with Peroxidase Block, to block endogenous peroxidases (30 min), and washed in PBS (2 x 5 min). Sections were then incubated in a drop of Protein block (15 min), to block non-specific reactive sites, washed in PBS, drained, and incubated with a primary monoclonal antibody anti–human CFTR R Domain (R&D Systems, Minneapolis, USA), at 1:100 in PBS (30 min). After washing in PBS, sections were incubated in a drop of Post Primary Block (30 min), washed in PBS, incubated in a drop of Polymer (30 min), washed in PBS, and labeled with 50 µl of a solution containing 3,3'diaminobenzidine (47.5 µl DAB Substrate Buffer + 2.5 µl DAB Chromogen) for 5-20 sec. Sections were then drained, washed in distilled water (5 min), contrasted with Vector Hematoxylin QS Counterstain (Vector) (15-20 sec), washed in distilled water, dehydrated in ethanol, cleared with xylene, and embedded with Vectamount Mounting Medium (Vector). As negative control, incubation with the primary antibody was omitted. Image analysis was performed in a light microscope (Olympus BX41) and images obtained through a camera Olympus DP70 and software DP Controller (Olympus Corporation, Tokyo, Japan).
Semiquantitative (subjective) evaluation of cell labeling was taken from hundreds of images, evaluated from high quality prints, taken as reference the strongest labeling of the cytoplasmic membrane and cytoplasm of Sertoli cells found in SCOS.
Abbreviations
| CBAVD: | = | congenital bilateral absence of the vas deferens |
| CF: | = | cystic fibrosis |
| CFTR: | = | Cystic Fibrosis Transmembrane Conductance Regulator |
| FSH: | = | follicular stimulating hormone |
| ICSI: | = | intracytoplasmic sperm injection |
| Lep-Zyg: | = | primary spermatocytes at the leptotene-zygotene stage |
| MA: | = | maturation arrest at the primary spermatocyte stage |
| MSD: | = | membrane-spanning domain |
| NBD: | = | nucleotide-binding domain |
| OAZsec: | = | secondary obstructive azoospermia |
| Pac: | = | primary spermatocytes at the pachitene stage |
| PBS: | = | phosphate buffered saline |
| PKA: | = | protein kinase A |
| PKC: | = | protein kinase C |
| R: | = | regulatory domain |
| RE: | = | retrograde ejaculation |
| SCOS: | = | Sertoli cell only syndrome |
| SC: | = | Sertoli cells |
| SE: | = | seminiferous epithelium |
| SG: | = | spermatogonia |
| SOZ: | = | severe oligozoospermia |
| ST: | = | seminiferous tubules |
| ST2: | = | secondary spermatocytes |
| TESE: | = | testicular sperm extraction |
| TMD: | = | transmembrane domain. |
Acknowledgments
We would like to acknowledge to: Jorge Beires, MD, PhD, Gynecologist (Director, Department of Obstetrics, Hospital of S. João, E.P.E., Porto, Portugal) and José Manuel Teixeira da Silva, MD, Gynecologist for oocyte retrieval; José Correia, MD, Anesthetist (Department of Anesthesiology, Hospital of S. João, E.P.E., Porto, Portugal); Paulo Viana, BSc and Mariana Cunha, BSc, Clinical Embryologists-ESHRE, for biopsy preparation (CGRA.Barros); Ana Gonçalves, BSc, Cláudia Osório, MSc and Nuno Barros, BSc, for spermatology assistance (CGRA.Barros); Teresa Barandela, Technician, and Elsa Oliveira, 1st Class Technical Specialist of Pathology, Cytology and Thanatology in the Area of Diagnosis and Therapy, for microscopy assistance (ICBAS).
Declaration of interest: Supported in part by Foundation for Science and Technology (FCT) for a PhD grant to RS (SFRH/BD/23616/2005). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author contributions: Performed the experiments, analyzed the data, and reviewed the manuscript prior to submission: ST; Supervised the experiments, analyzed the data, and reviewed the manuscript prior to submission: RS; Conceived the experiments and reviewed the manuscript prior to submission: AG; Supervised the IVF work: JS; Performed female patient evaluation, controlled ovarian hyperstimulation, embryo transfer and patient follow-up: CO; Performed male patient evaluation and testicular biopsies: LF; Performed experiments assistance: ÂA; Reviewed the manuscript prior to submission: SP; Performed patient recruitment and supervised the clinical and embryological work: AB; Conceived and designed the experiments, analyzed the data, contributed with reagents/materials/analysis tools and wrote the manuscript: MS.
References
- Boockfor, F.R., Morris, R.A., DeSimone, D.C., Hunt, D.M. and Walsh, K.B. (1998) Sertoli cell expression of the cystic fibrosis transmembrane conductance regulator. Am J Physiol Cell Physiol 274:C922–C930.
- Castellani, C., Cuppens, H., Macek, M., Jr., Cassiman, J.J., Kerem, E., Durie, P., (2008) Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros 7:179–196.
- Clermont, Y. (1963) The Cycle of the seminiferous epithelium in man. Am J Anat 112:35–51.
- Gong, X.D., Li, J.C., Cheung, K.H., Leung, G.P., Chew, S.B. and Wong, P.Y. (2001) Expression of the cystic fibrosis transmembrane conductance regulator in rat spermatids: implication for the site of action of antispermatogenic agents. Mol Hum Reprod 7:705–713.
- Grangeia, A., Niel, F., Carvalho, F., Fernandes, S., Ardalam, A., Girodon, E., (2004) Characterization of cystic fibrosis conductance transmembrane regulator in gene mutations and IVS8 poly(T) variants in Portuguese patients with congenital absence of the vas deferens. Hum Reprod 19:2502–2508.
- Grangeia, A., Sá, R., Carvalho, F., Martins, J., Girodon, E., Silva, J., (2007) Molecular characterization of the cystic fibrosis transmembrane conductance regulator gene in congenital absence of the vas deferens. Genet Med 9:163–172.
- Grangeia, A., Barro-Soria, R., Carvalho, F., Dama, A.M., Maurício, A.C., Kunzelmann, K., (2008) Molecular and functional characterization of CBAVD-causing mutations located in CFTR nucleotide-binding domains. Cell Physiol Biochem 22:79–92.
- Hihnala, S., Kujala, M., Toppari, J., Kere, J., Holmberg, C. and Hoglund, P. (2006) Expression of SLC26A3, CFTR and NH3 in the human male reproductive tract: role in male subfertility caused by congenital chloride diarrhoea. Mol Hum Reprod 12:107–111.
- Holstein, A.F. and Roosen-Runge, E.C. (1981) Atlas of Human Spermatogenesis. Grosse Verlag, Berlin, Germany.
- Huang, H.F., He, R.H., Sun, C.C., Zhang, Y., Meng, Q.X. and Ma, Y.Y. (2006) Function of aquaporins in female and male reproductive systems. Hum Reprod 12:785–795.
- Kerem, B-S., Rommens, J.M., Buchanan, J.A., Markiewicz, D., Cox, T.K., Chakravarti, A., (1989) Identification of cystic fibrosis gene: genetic analysis. Science 245:1073–1080.
- Kopito, R.R. (1999) Biosynthesis and Degradation of CFTR. Physiol Rev 79:167–173.
- Larriba, S., Bassas, L., Gimenez, J., Ramos, M.D., Segura, A., Nunes, V., (1998) Testicular CFTR splice variants in patients with congenital absence of the vas deferens. Hum Mol Genet 7:1739–1743.
- Luo, J., Pato, M.D., Riordan, J.R. and Hanrahan, J.W. (1998) Differential regulation of single CFTR channels by PP2C, PP2A, and others phosphatases. Am J Physiol 274:C1397–1410.
- Reddy, M.M. and Quinton, P. (2003) Functional interaction of CFTR and ENaC in sweat glands. Pflugers Arch 445:499–503.
- Riordan, J.R., Rommens, J.M., Kerem, B-S., Alon, N., Rozmahel, R., Grzelczak, Z., (1989) Identification of cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066–1073.
- Rommens, J.M., Iannuzzi, M.C., Kerem, B-S., Drumm, M.L., Melmer, G., Dean, M., (1989) Identification of cystic fibrosis gene: chromosome walking and jumping. Science 245:1059–1065.
- Sá, R., Neves, R., Fernandes, S., Alves, C., Carvalho, F., Silva, J., (2008) Cytological and expression studies and quantitative analysis of the temporal and stage-specific effects of follicle-stimulating hormone and testosterone during cocultures of the normal human seminiferous epithelium. Biol Reprod 79:962–975.
- Schreiber, R., Nitschke, R., Greger, R. and Kunzelmann, K. (1999) The cystic fibrosis transmembrane conductance regulator activates aquaporin 3 in airway epithelial cells. J Biol Chem 274:11811–11816.
- Sousa, M., Cremades, N., Silva, J., Oliveira, C., Ferráz, L., Teixeira da Silva, J., (2002) Predictive value of testicular histology in secretory azoospermic subgroups and clinical outcome after microinjection of fresh and frozen-thawed sperm and spermatids. Hum Reprod 17:1800–1810.
- Suzuki–Toyota, F., Ishibashi, K. and Yuasa, S. (1999) Immunohistochemical localization of a water channel, aquaporin 7 (AQP-7), in the rat testis. Cell Tissue Res 295:279–285.
- Tizzano, E.F. and Buchwald, M.M. (1995) CFTR expression and organ damage in cystic fibrosis. Ann Intern Med 123:305–308.
- Tizzano, E.F., Silver, M.M., Chitayat, D., Benichou, J.C. and Buchwald, M. (1994) Differential cellular expression of cystic fibrosis transmembrane regulator in human reproductive tissues. Clues for the infertility in patients with cystic fibrosis. Am J Pathol 144:906–914.
- Trezise, A.E.O. and Buchwald, M. (1991) In vivo cell specific expression of the cystic fibrosis transmembrane conductance regulator. Nature 253:434–437.
- Trezise, A.E.O., Linder, C.C., Grieger, D., Thompson, E.W., Meunier, H., Griswold, M.D., (1993) CFTR expression is regulated during both the cycle of the seminiferous epithelium and oestrous cycle of rodents. Nat Genet 3:157–164.
- Turnbull, E.L., Rosser, M.F.N. and Cyr, D.M. (2007) The role of the UPS in cystic fibrosis. BMC Biochem 8 ( Suppl 1):S1–S11.
- Welsh, M.J., Ramsey, B.W., Accurso, F. and Cutting, G.R. (2001) Cystic fibrosis. In The Metabolic and Molecular Basis of Inherited Disease. Vol 1, 8th edition, ed Scriver, C.L, Beaudet, A.L., Sly, W.S. and Valle., D., McGraw–Hill, New York, USA pp.5121–5188.
- Wong, P.Y.D. (1998) CFTR gene and male fertility. Mol Hum Reprod 4: 107–110.
- Xu, W.M., Chen, J., Chen, H., Diao, R.Y., Fok, K.L., Dong, J.D., (2011) Defective CFTR-dependent CREB activation results in impaired spermatogenesis and azoospermia. PLoS One 6:e19120.
- Zielenski, J., Rozmahel, R., Bozon, D., Kerem, B., Grzelczak, Z., Riordan, J.R., (1991) Genomic DNA sequence of the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Genomics 10:214–228.