Abstract
Semen from 33 patients were evaluated by light microscopy (LM) obtaining sperm concentration, percent motility, percentage of sperm with normal morphology (PAP staining), and percentage of dead sperm (Eosin Y stained). The samples were observed by polarizing microscopy (PM), that evaluates sperm morphology and the viability by birefringence of organelles, and it provides a PM index (percentage of birefringent, viable, motile sperm) and a percentage of dead, non-birefringent sperm. Sperm were processed for transmission electron microscopy (TEM) and TEM data were elaborated with a mathematical formula able to provide a fertility index (FI, number of sperm free of structural defects) and percentages of sperm immaturity and necrosis (dead sperm). To test the reliability of these techniques, the values of normal acrosome, nucleus, midpiece, and tail and the presence of cytoplasmic residues obtained with the three methods were compared. With the exception of cytoplasmic residues (P = 0.40), significant differences in the evaluation of each organelle were observed and TEM analysis resulted as the most stringent screening. In addition, relationships among relevant sperm variables were investigated. Motility showed positive correlations with the percentage of normal tail, midpiece, and PM index (P < 0.01), but it exhibited negative correlations with indices of sperm death (non-birefringent sperm: P < 0.05; percentage of eosin Y stained sperm: P < 0.05; necrosis: P < 0.01), which were positively correlated with each other (P < 0.01). Positive correlations were found between indices expressing normal sperm morphology: FI with PM index (P < 0.01) and with the percentage of normal sperm (PAP staining) (P < 0.01), which in turn were correlated with the PM index (P < 0.001). Sperm immaturity showed positive correlations (P < 0.01) with the presence of cytoplasmic residues detected with the three methods. In conclusion, LM, PM, and TEM are reliable techniques in evaluating sperm quality. PM appears to offer several advantages ‘midway’ between LM and TEM and it should be considered in sperm analysis.
Introduction
Sperm analysis has been used to determine the fertilizing potential of sperm [Chemes and Rawe Citation2003; Aitken Citation2006; Visco et al. Citation2010]. In specialized laboratories, semen evaluation is generally performed by light microscopy (LM) and sperm morphology is assessed in smeared and stained glass slides prepared according to World Health Organization (WHO) [1992; 1999] guidelines. The staining procedures recommended by WHO guidelines are the Papanicolaou (PAP), Shorr, or Diff-Quick stains.
Although the analysis of sperm by LM, allows for examining viable samples, it has obvious technical limitations in terms of resolution. The best method for exploring sperm morphology is ultrastructural analysis performed by transmission electron microscopy (TEM). This technique permits the detailed study of different organelles rigorously characterising sperm abnormalities [Chemes Citation2000; Baccetti et al. Citation2001] although it is limited to fixed samples. The main problem with sperm analysis performed by TEM is quantization of the data, since only sections of cells can be examined. Several years ago, our group suggested a probability based mathematical model to elaborate TEM data and to provide a fertility index, represented by the number of sperm with normal structure, and the percentages of sperm pathologies [Baccetti et al. Citation1995; Collodel and Moretti Citation2008]. A different method of quantification of TEM analysis was applied by Bartoov et al. [1999] in order to find a synthetic indicator of sperm quality.
An alternative method for semen evaluation has recently been proposed [Collodel et al. Citation2010], the polarizing microscope (PM) allows for analyzing, in a single step, the motility, viability, morphology, and obviously, the concentration of spermatozoa. This approach is based on the birefringence characteristics of the spermatozoa due to anisotropic properties of their protoplasmic texture [Baccetti Citation2004], avoiding the staining of cells smeared on a slide. For example, in the mature sperm nucleus the natural birefringence depends on the presence of nucleoprotein filaments that are ordered in rods and longitudinally oriented. Most of the tail is also birefringent due to the organization of axoneme and mitochondria in the midpiece [Baccetti Citation2004]. A comparison of the same human semen samples examined by LM and PM suggested the clinical utility of PM in semen analysis [Collodel et al. Citation2010]. The main advantage is the possibility of in vivo analysis of sperm morphology, concomitant with a more detailed examination of sperm cells than that obtained by LM. These three methods showed strengths and weaknesses in the evaluation of human sperm morphology.
The aim of the present study was to statistically compare the evaluation of normal sperm organelles, the acrosome, nucleus, midpiece, tail, and the presence of cytoplasmic residues, performed with the LM, PM, and TEM methods. Linear correlations of the most relevant sperm variables were also carried out in order to assess the reliability of these techniques.
Results and Discussion
In current laboratory practice, LM is the routine technique to evaluate sperm morphology, examined in semen stained smears with the main criteria for normalcy based on morphometric parameters of the sperm head, midpiece, and flagellum as suggested by WHO guidelines [1992; 1999; 2010]. The information acquired by LM is rather incomplete due to the technical limitations of LM, although different methods have been suggested to improve morphological evaluation [Coetzee et al. Citation1999; Akl et al. Citation2011; Singh et al. Citation2011]. These limitations are overcome by using TEM that enables an exploration of the ‘ultrastructure world’. This approach goes beyond a descriptive morphology of the appearance of spermatozoa, and it plays a pivotal role in the definition and the study of sperm pathology [Baccetti et al. Citation1988; Chemes and Rawe Citation2003].
Recently, our group proposed the use of PM as a valid alternative to conventional semen analysis performed by LM [Collodel et al. Citation2010]. It is based on the natural birefringent properties of sperm. This method enables the discrimination of sperm morphology and viability in vivo as a function of birefringence, a typical property of living sperm that disappears in dead cells.
The main goal of this study was the statistical determination of the relationship between LM, PM, and TEM in the definition and classification of normal sperm organelles. By performing all three examinations using the same samples, it was possible to investigate whether the tests were equivalent, although different powers of resolution were used. The sperm characteristics of the 33 patients evaluated by LM, PM, and TEM are summarized in . Median values showed that sperm concentration, progressive motility, and morphology were lower than the values of normality reported in WHO guidelines [1992; 1999]. The PM index (indicating the pool of birefringent sperm with motility) and the FI ( mathematically elaborated by TEM analysis) were reduced in the study group compared to normal values [Collodel and Moretti Citation2008; Collodel et al. Citation2010]. The percentages of dead sperm (eosin Y stained) and sperm pathologies (necrosis and immaturity, mathematically elaborated by TEM analisis) were higher, particularly necrosis, than the values observed in samples of men of proven fertility [Collodel and Moretti 2008]. The percentage of non-birefringent sperm, representing dead sperm obtained by PM analysis, is also reported in . The median percentages of normal acrosome, normal nucleus, normal midpiece, normal tail and presence of cytoplasmic residue evaluated with the three methods, LM (PAP staining), PM and TEM, were reported in Figure 1. Acrosome: PAP (LM) = 42%, PM = 34% and TEM = 20%; nucleus: PAP (LM) = 45%, PM = 32% and TEM = 18%; midpiece: PAP (LM) = 60%, PM = 54% and TEM = 30%; tail: PAP (LM) = 63%, PM = 48% and TEM = 28%; cytoplasmic residues: PAP (LM) = 12%, PM = 12% and TEM = 12%. An example of PM birefringent property of spermatozoa is shown in . As reported by Collodel et al. [2010], a normal spermatozoon examined using the PM method shows a non-luminous acrosome and a luminous and normal sized compact nucleus and flagellum (A). A non-birefringent acrosome is quickly recognized since it is not bright and therefore its presence or absence, its shape, its dimension, and the possible presence of vacuoles can also be easily assessed. The subacrosomal region, not covered by the acrosome, is uniformly birefringent in normal spermatozoa, since the chromatin probably shows a normal structure. Therefore, when a nucleus is well shaped but not birefringent, it is considered to be anomalous because the chromatin has lost its typical nucleoprotein texture. A totally birefringent sperm head is devoid of the acrosome, which could be reacted or absent (B). The tail of viable, motile spermatozoa must show positive birefringence, reflecting the microtubular organization of the axoneme. The normal mitochondrial helix is birefringent, since it is characterized by a particular filamentous texture. When an organelle loses its typical structure, the birefringence disappears and the spermatozoon is considered dead (non-birefringent sperm, (C). PAP staining is shown in .
In Figure 1 significant differences among the percentages of each considered organelle, obtained with the three methods, were observed; TEM is the most accurate technique for the evaluation of the different sperm regions, whereas LM was, as expected less informative due to its resolution. The presence of cytoplasmic residues is the only variable detected by all three methods that does not show a significant difference (P = 0.40).
Figure 1. The median percentages of normal acrosome, nucleus, midpiece, tail, and cytoplasmic residue evaluated with the three methods, light microscopy (LM; Papanicolaou staining, PAP), polarizing microscope (PM), and transmission electron microscopy (TEM), are reported. Significant differences were detected among the values of each organelle obtained by the three methods. * P < 0.01, ** P < 0.001: PAP vs. PM; ° P < 0.001: PAP vs TEM; # P < 0.001: PM vs. TEM.
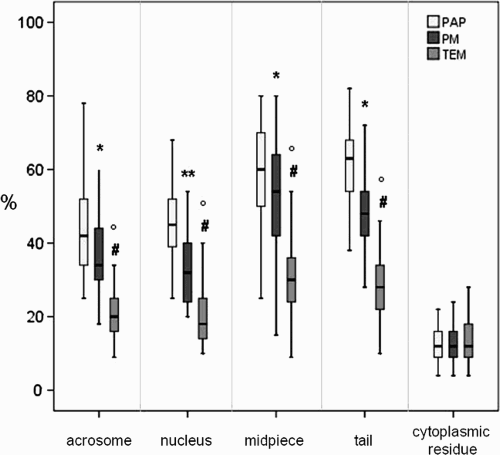
Figure 2. Sperm observed using polarizing microscope (PM). A) two sperm are shown. The lower sperm (arrow) is a normal sperm with a well shaped nucleus, not birefringent acrosome covering the anterior part of the head. The region of the nucleus devoid of acrosome and the tail are birefringent. The upper sperm presents a head with normal nucleus and acrosome but a tail of reduced length. B) sperm with absent or reacted acrosomes are shown. The nuclei appear totally birefringent (arrows). C) a dead, abnormal sperm is present. The head (h) and the tail (t) are not birefringent. A, B, C) Bar 12 µm.
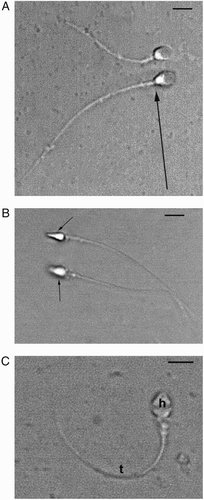
Figure 3. Light microscopy (LM) micrograph (Papanicolaou staining, PAP) showing normal sperm (arrow), sperm with normal acrosomes, and nuclei but altered tails (*), sperm with altered nuclei and/or tails (°). Bar 12 µm.
Table 1. Median and interquartile difference (ID) of sperm characteristics of 33 consecutive patients obtained by light, polarizing, and TEM microscopy.
It is noteworthy that PM appears to be a technique that performs ‘midway’ between LM and TEM in the evaluation of normal sperm yet is more manageable than TEM. It is a classical optical microscope equipped with a polarizing lens and in term of cost-benefit could easily be part of laboratory practice for semen analysis since it provides more information than LM. As previously reported [Collodel et al. Citation2010], the most relevant advantage of PM over LM is the possibility to discriminate viable immotile sperm from dead sperm based on birefringence. In addition, PM enables one to evaluate, in a single step, sperm number, motility, viability, and morphology avoiding the staining of cells smeared on a slide, reducing the cost of the materials. PM is quick and the data interpretation is easy as the organelles show different colors. Moreover, the information provided by this system permits the assessment of the appearance and shape of sperm, in addition to regular protoplasmic structures in the acrosome and tail, closely approaching ultrastructural analysis. For example, sperm chromatin can be evaluated in vivo using PM birefringence since when chromatin loses its texture, brightness disappears. Gianaroli et al. [2008; Gianaroli et al. Citation2010] investigated the characteristics of birefringence in the human sperm head and they applied PM for the choice of the sperm to be injected in the oocyte during intracytoplasmic sperm injection. They found that these natural properties of sperm examined under polarizing light could provide a diagnostic tool and a novel method for sperm selection.
Comparative studies between LM and TEM techniques are rare. To the best of our knowledge only Visco et al. [2010] have compared sperm defects assessed by both LM and TEM and then correlated them with sperm motility. They concluded that TEM might be an additional diagnostic tool in the presence of asthenozoospermia or the absence of motility, which is important since the pattern of axoneme and periaxonemal structure can be visualized in the longitudinal and cross sections with TEM. In addition, TEM is absolutely required in the diagnosis of sperm alterations with a possible genetic origin, known as systematic defects, since there is a common sperm phenotype that predominates in a patient and it resembles similar defects in other individuals suffering from the same condition [Chemes Citation2000; Baccetti et al. Citation2001]. The most common examples of these defects are ‘dysplasia of the fibrous sheath’ [Chemes et al. Citation1987; Rawe et al. Citation2001], ‘globozoospermia’ [Larson et al. Citation2001; Moretti et al. Citation2005], and ‘primary ciliary dyskinesia’ [Moretti and Collodel Citation2006; Roomans et al. Citation2006].
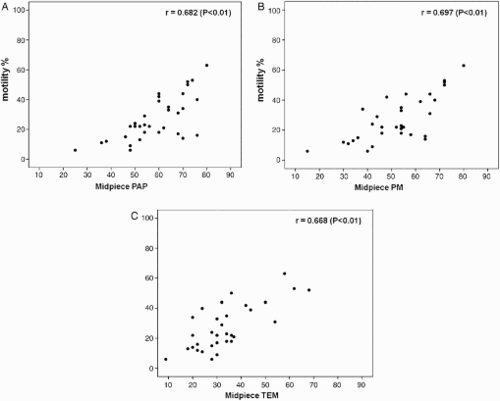
In this study, linear correlations of most relevant sperm variables were considered. Sperm motility positively correlated with normal tail ( A, B, C; P < 0.01) and normal midpiece (A, B, C; P < 0.01), evaluated by the three methods, confirming the validity of the techniques and the reliability of these analyses. The higher concentration of the points (lower left, C and 5C) in the scatter plots confirmed the stringency of TEM evaluation. In addition, correlations between sperm motility and the variables specifying viable (PM index) and dead sperm (assessed by the three methods) were calculated. A positive significant correlation (r = 0.871; P < 0.01) was detected between motility and the PM index. Negative significant correlations were observed between motility and indices of sperm death, i.e., non-birefringent sperm (r = -0.358; P < 0.05), percentages of dead sperm (eosin Y stained; r = -0.382; P < 0.05), and necrosis (r = -0.433; P < 0.01).
Figure 4. Scatter plots of linear correlations between sperm motility and normal sperm tail percentages evaluated by A) light microscopy (LM; Papanicolaou staining, PAP), B), polarizing microscope (PM), and C) transmission electron microscopy (TEM).
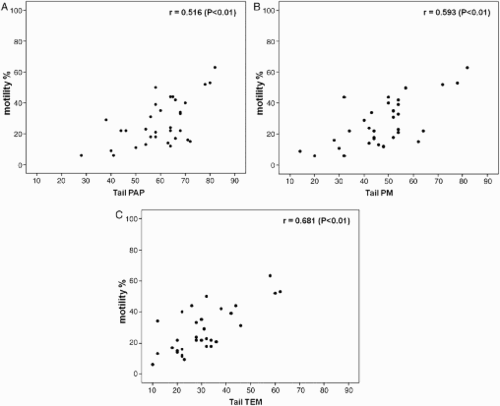
Figure 5. Scatter plots of linear correlations between sperm motility and normal sperm midpiece percentages evaluated by A) light microscopy (LM; Papanicolaou staining, PAP), B), polarizing microscope (PM), and C) transmission electron microscopy (TEM).
Positive correlations were also observed between indices of sperm death, i.e., percentage of eosin Y stained dead sperm, percentage necrosis (r = 0.961; P < 0.01) and with percentage non-birefringent sperm (r = 0.960; P < 0.01) and between percentage sperm necrosis with percentage non-birefringent sperm (r = 0.960; P < 0.01). Concerning the indices related to normal sperm morphology, FI, obtained by TEM analysis (), showed a positive correlation with the PM index (r = 0.829; P < 0.01) and with the percentage of sperm with normal morphology evaluated by LM (PAP; r = 0.742; P < 0.01) which in turn positively correlated with the PM index (r = 0.636; P < 0.001). Finally, immature sperm showed a significant positive correlation (P < 0.01) with the presence of cytoplasmic residues evaluated with LM (PAP method; r = 0.736), PM (r = 0.736), and TEM (r = 0.809).
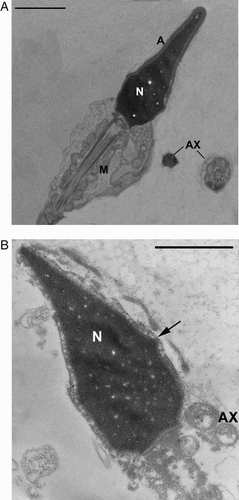
Figure 6. TEM micrographs of longitudinal sections of sperm. A) sperm with a normally shaped acrosome (A) with regular size and compact content present in a normal position and with a regularly shaped nucleus (N) with condensed chromatin. Mitochondria (M) have a regular shape and are organized in a normal helix. Cross sections of normal flagella with a 9 + 2 axonemal pattern (AX) are also present. B) necrotic sperm with broken plasma membrane (arrow), nucleus (N) with disrupted chromatin and without acrosome. The axoneme (AX) and periaxonemal structures of the tail are degraded. Bar 1 µm.
In conclusion, this study compared TEM and PM analysis versus LM examination, which is the most widely used method for semen analysis. While the LM and TEM techniques are well established in the analysis of seminal fluid, PM should be recognized as an important tool. Every approach aimed at amplifying the knowledge of sperm analysis deserves to be further investigated.
Materials and Methods
Semen samples
Semen samples were obtained from 33 men (aged 20 to 36 y) recruited in January 2010 at the Interdepartmental Centre for Research and Therapy of Male Infertility, University of Siena. Patients were informed of and gave written consent for the procedures related to the study. Semen samples were collected by masturbation after 3–5 d of sexual abstinence and examined after liquefaction for 30 min at 37°C. Volume, pH, sperm concentration, and progressive motility were quantified according to World Health Organization guidelines [WHO Citation1999].
Light microscopy (LM)
Sperm viability was assessed by 0.5% Eosin Y staining in 0.9% NaCl. One drop of fresh semen was mixed with one drop of eosin solution on a microscope slide, covered with a cover slip and examined after 30 s at 400x with a Leitz Aristoplan Microscope (Leica, Wetzlar, Germany). Slides were assessed immediately. Viable spermatozoa were unstained (white) and dead cells were stained (red). Two hundred sperm were evaluated from each sample.
Sperm morphology was analyzed by Papanicolaou staining (PAP, Fig. 3). Ejaculated human spermatozoa were washed twice in Phosphate Buffer Saline (PBS), smeared on pre-cleaned glass slides, air dried, and fixed in an equal volume of ethanol 95% and ether for 5-15 min. Classification of sperm morphology was performed following WHO guidelines. A percentage of sperm morphology of ≥ 30% was considered normal [WHO Citation1992]. Two hundred sperm were evaluated from each sample using a Leitz Aristoplan microscope (Leica, Wetzlar, Germany) at 100x bright field objective and 10x ocular.
Polarizing microscope (PM)
Samples were analyzed in a Burker counting chamber using a polarizing microscope (63x objective and a 10x ocular, Leica Microsystems DM EP). Spermatozoa were categorized following the different motility grades [WHO Citation1999], and viability and morphology were assessed by the natural birefringence of nucleus and flagellum. Normal sperm nucleus and normal tail are birefringent and show positive anisotropy according to Wiener [1912]. The PM evaluation allows for obtaining a PM index expressing the percentage of birefringent sperm with normal morphology and with progressive motility. A PM index equal to 20% was considered as the cut off point for identifying sperm quality [Collodel et al. Citation2010].
Transmission electron microscopy analysis (TEM)
Ejaculates were fixed in cold Karnovsky fluid at 4°C for 2 h, then centrifuged at 3,000 x g for 15 min. The pellet was washed in 0.1 M cacodylate buffer (pH 7.2) for 12 h, post-fixed for 1 h at 4°C in 1% buffered osmium tetroxide, dehydrated, and embedded in Epon–Araldite. The sections cut with an ultramicrotome Supernova (Reickert Jung,Vienna, Austria) were collected on copper grids, stained with uranyl acetate and lead citrate, and observed and photographed with a Philips EM208 electron microscope (Philips Scientifics, Eindhoven, The Netherlands).
For each sample 300 sperm sections were examined considering 16 characteristics of sperm organelles that may be present in a normal state (A) or in one or more different abnormal states, or defects. The obtained TEM data has been elaborated by a mathematical formula [Baccetti et al. Citation1995] based on Bayesian technique that calculates the number of sperm without structural defects (FI). The lowest fertility index assuring normal fertility was slightly lower than 2 x 106 of sperm in an ejaculate [Collodel and Moretti Citation2008]. In addition, this method enables obtaining percentages of sperm pathologies; in this study we considered immaturity and necrosis [Collodel and Moretti Citation2008], immature sperm show altered acrosomes, round or elliptical nuclei with uncondensed chromatin, and cytoplasmic droplets. In necrotic (dead) sperm (B) the plasma membrane is broken, the nuclei are altered, the chromatin disrupted, and the acrosomes, when present, are reacted. Mitochondria are swollen and disorganized as well as the cytoskeletal structures of the tail.
In the screening of the different sperm regions, we considered a ‘normal acrosome’ to be a normally shaped acrosome with regular size and compact content present in a normal position; a ‘normal nucleus’ was a regularly shaped nucleus with condensed chromatin; a ‘normal midpiece’ had mitochondria with a regular shape and with evident cristae assembled in a well-organised helix; a ‘normal tail’ showed flagella with a 9 + 2 axonemal pattern, with normal dynein arms, and well organized periaxonemal structure, i.e., outer dense fibers and a fibrous sheath. Every examined section showed an intact plasma membrane.
Statistical analysis
The data are reported as median and interquartile differences (ID: 75°-25° centile). The normality of the distributions was calculated by the Kolmogorov-Smirnov test. The differences between PAP, PM, and TEM evaluations, concerning acrosome, nucleus, midpiece, tail, and cytoplasmic residue percentages, were performed by the Friedman test for repeated measures. Dunn's multiple comparison test was applied when the Friedman test was significant. The correlations were calculated by the Spearman correlation coefficient (r). A P value < 0.05 (two-tailed) was considered statistically significant. For analyses, we used SPSS version 20 statistical software (SPSS Inc., Chicago, Illinois, USA) and GraphPad software Inc. version 5.00 for Windows.
Declaration of interest: The authors report no declarations of interest
Author contributions: Substantial contributions to conception, design, and interpretation of the data, PM and TEM analysis, manuscript writing: GC; Statistical analysis: FI; LM, PM analysis and TEM preparation: LM, GT; TEM analysis and interpretation of the data: NAP; Substantial contributions to conception, design, and interpretation of the data, PM and TEM analysis, manuscript writing: EM. All the authors revised the manuscript and gave their final approval.
Abbreviations
| LM: | = | light microscopy |
| PM: | = | polarizing microscope |
| TEM: | = | transmission electron microscopy |
| PAP: | = | Papanicolaou staining |
| WHO: | = | World Health Organization |
| FI: | = | fertility index. |
References
- Aitken, R.J. (2006) Sperm function tests and fertility. Int J Androl 29:69–75.
- Akl, L.D., Oliveira, J.B., Petersen, C.G., Mauri, A.L., Silva, L.F., Massaro, F.C., (2011) Efficacy of the motile sperm organelle morphology examination (MSOME) in predicting pregnancy after intrauterine insemination. Reprod Biol Endocrinol 23:9:120.
- Baccetti, B., Burrini, A.G., Collodel, G., Magnano, A.R., Piomboni, P. and Renieri, T. (1988) Immunocytochemistry and sperm pathology. J Submicrosc Cytol Pathol 20:209–224.
- Baccetti, B., Bernieri, G., Burrini, A.G., Collodel, G., Crisà, N., Mirolli, (1995) Notulae seminologicae. 5. Mathematical evaluation of interdependent submicroscopic alterations. J Androl 16:356–371.
- Baccetti, B., Capitani, S., Collodel, G., Di Cairano, G., Gambera, L., Moretti, E., (2001) Genetic sperm defects and consanguinity. Hum Reprod 16:1365–1371.
- Baccetti, B. (2004) Microscopical advances in assisted reproduction. J Submicrosc Cytol Pathol 36:333–339.
- Bartoov, B., Eltes, F., Reichart, M., Langzam, J., Lederman, H. and Zabludovsky, N. (1999) Quantitative ultramorphological (QUM) analysis of human sperm: diagnosis and management of male infertility. Arch Androl 42:161–177.
- Chemes, H.E., Brugo, S., Zanchetti, F., Carrere, C. and Lavieri, J.C. (1987) Dysplasia of the fibrous sheath: an ultrastructural defect of human spermatozoa associated with sperm immotility and primary sterility. Fertil Steril 48:664–669.
- Chemes, H.E.J. (2000) Phenotypes of sperm pathology: genetic and acquired forms in infertile men. J Androl 21:799–808.
- Chemes, H.E.J. and Rawe, V.Y. (2003) Sperm pathology: a step beyond descriptive morphology. Origin, characterization and fertility potential of abnormal sperm phenotypes in infertile men. Hum Reprod Update 9:405–428.
- Coetzee, K., Kruger, T.F. and Lombard, C.J. (1999) Repeatability and variance analysis on multiple computer-assisted (IVOS) sperm morphology readings. Andrologia 31:163–168.
- Collodel, G. and Moretti, E. (2008) Morphology and Meiotic Segregation in Spermatozoa from Men of Proven Fertility. J Androl 29:106–114.
- Collodel, G., Capitani, S., Federico, M.G., Pascarelli, N.A., Geminiani, M. and Moretti, E. (2010) Natural sperm birefringence can be used to estimate sperm viability and morphology. Syst Biol Reprod Med 56:465–472.
- Gianaroli, L., Magli, M.C., Collodel, G., Moretti, E., Ferraretti, A.P. and Baccetti, B. (2008) Sperm head's birefringence: a new criterion for sperm selection. Fertil Steril 90:104–112.
- Gianaroli, L., Magli, M.C., Ferraretti, A.P., Crippa, A., Lappi, M., Capitani, S., (2010) Birefringence characteristics in sperm heads allow for the selection of reacted spermatozoa for intracytoplasmic sperm injection. Fertil Steril 93:807–813.
- Larson, K.L., Brannian, J.D., Singh, N.P., Burbach, J.A., Jost, L.K., Hansen, K.P., (2001) Chromatin structure in globozoospermia: a case report. J Androl 22:424–431.
- Moretti, E., Collodel, G., Scapigliati, G., Cosci, I., Sartini, B. and Baccetti, B. (2005) “Round head” sperm defect. Ultrastructural and meiotic segregation study. Submicrosc Cytol Pathol 37:297–303.
- Moretti, E. and Collodel, G. (2006) Three cases of genetic defects affecting sperm tail: a FISH study. J Submicrosc Cytol Pathol 38:137–141.
- Rawe, V.Y., Galaverna, G.D., Acosta, A.A., Olmedo, S.B. and Chemes, H.E. (2001) Incidence of tail structure distortions associated with dysplasia of the fibrous sheath in human spermatozoa. Hum Reprod 16:879–886.
- Roomans, G.M., Ivanovs, A., Shebani, E.B. and Johannesson, M. (2006) Transmission electron microscopy in the diagnosis of primary ciliary dyskinesia. Ups J Med Sci 111:155–168.
- Singh, S., Sharma, S., Jain, M. and Chauhan, R. (2011) Importance of Papanicolaou staining for sperm morphologic analysis: comparison with an automated sperm quality analyzer. Am J Clin Pathol 136:247–251.
- Visco, V., Raffa, S., Elia, J., Delfino, M., Imbrogno, N., Torrisi, M.R., (2010) Morphological sperm defects analyzed by light microscopy and transmission electron microscopy and their correlation with sperm motility. Int J Urol 17:259–266.
- Wiener, O. (1912) Die Theorie des Mischkorpers fur das Feld der stationaren Stomung. Erste abandlung. Die mittel Wertsatze fur Kraft, Polarisation und Energie. 32Bd, I-IV, pp. 509–604.
- WHO (1992) World Health Organization (WHO) laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge: Cambridge University Press, 1–107.
- WHO (1999) World Health Organization (WHO) laboratory manual for the examination of human semen and semen-cervical mucus interaction. 4th ed. Cambridge: Cambridge University Press.
- WHO (2010) World Health Organization (WHO) laboratory manual for the examination and processing of human semen 5th ed. Geneva, Switzerland, WHO Press.