Abstract
Studies on elevated reactive oxygen species (ROS) levels in granulosa cells (GC) and its subsequent effect on fertilization are limited. Oxidative stress (OS) mediated alterations in GC of infertile women undergoing in vitro fertilization (IVF) and embryo transfer (ET) was investigated. GC were obtained from 28 women with endometriosis (Group A), 26 women with polycystic ovary syndrome (PCOS) (Group B), and 32 women with tubal factor infertility (Group C). GC characteristics including cell count, viability, morphology and number of oocytes retrieved, and oocyte quality were assessed. OS parameters such as ROS, mitochondrial membrane potential (MMP), and DNA fragmentation were also studied and IVF outcome parameters assessed. An ∼20 fold increase in GC ROS generation was observed in Group B as compared to Group C. Though not as high as Group B, Group A also showed significantly high ROS levels compared with Group C. More than 100-fold decrease in MMP in Group B compared with Group C was observed. A similar trend was observed in Group A, where MMP decreased 7 fold. Significant apoptosis was evident in Groups A and B supported by depolarization of MMP and significant increase in DNA damage. IVF outcome parameters including fertilization rate, good quality embryo formation rate, and pregnancy outcome were adversely affected in Group B. It is hypothesized that ∼20 fold increase in ROS generation in GC of PCOS women plays an adverse role in affecting the IVF success rate. It was of note that the IVF outcome parameters of women with endometriosis were not affected.
Introduction
Granulosa Cells (GC) are steroidogenic cells surrounding the oocyte and responsible for normal ovarian folliculogenesis. These cells are involved in follicular development [Clavero et al. Citation2003], oocyte maturation [Albertini and Barrett Citation2002], and atresia [Tilly et al. Citation1991]. GC proliferation is an important factor for the development of the follicle and provides a suitable microenvironment for oocyte maturation [Takekida et al. Citation2003]. Initiation of follicle growth is associated with GC division and transformation of GC morphology from flattened to cuboidal shapes [Stubbs et al. Citation2007]. It is now well established that GC plays an essential role in the process of follicular differentiation leading to the optimal conditions for oocyte development, ovulation, fertilization, and subsequent implantation [Adashi Citation1994].
It is evident that an excessive amount of reactive oxygen species (ROS) causes oxidative stress (OS) and damages oocytes and luteinized granulosa cells [Taniguchi et al. Citation2009]. OS reflects ROS production as by-products of rapid steroidogenesis in preovulatory GC [Hanukoglu Citation2006]. The preovulatory follicles are more likely to be susceptible to OS than the earlier stages of follicles [Spanel-Borowski Citation2010]. Two major sources of ROS production in GC are: i) leaky membranes of the mitochondrial respiratory chain and ii) a GC subtype without cytokeratin (CK) filaments, termed CK negative or (CK-) [Spanel-Borowski Citation2010]. Degenerating mitochondria in human GC from fresh follicle aspirates are indicative of ROS damage [Vilser et al. Citation2010]. It is also suggested that ROS is a by-product of a specific scavenger receptor for oxidized low density lipoprotein expressed by CK- GC [Serke et al. Citation2009].
Follicular atresia is a cyclic event which initiates in GC and is caused by apoptosis or programmed cell death [Tilly et al. Citation1991]. There is mounting evidence that the higher incidence of apoptosis in GC is associated with an increased rate of empty follicles and fewer oocytes retrieved [Nakahara et al. Citation1997], poor oocyte and embryo quality [Nakahara et al. Citation1997; Saito et al. Citation2002], and low conception and pregnancy rate [Nakahara et al. Citation1997; Oosterhuis et al. Citation1998]. There are limited reports correlating human GC apoptosis and ROS production and examining their impact on in vitro fertilization (IVF) outcome parameters [Jancar et al. Citation2007, Liu and Li 2010].
It is evident that GC dysfunction contributes to abnormal folliculogenesis in PCOS [Jakimiuk et al. Citation2001]. In a recent study, Das et al. [2008] observed significant differences in the rate of apoptosis and proliferation in GC of PCOS patients. Apoptosis in GC of women with endometriosis is reported to increase proportionately with the stage of the disease which, in turn, results in poor oocyte quality and reduced fertilization and pregnancy rates [Nakahara et al. Citation1997]. Saito et al. [2002] have reported that OS in GC reduces fertilization rate and might lead to decreased embryo quality. It is also evident that oocyte quality is impaired in endometriosis by the presence of 8-hydroxy-2'-deoxyguanosine (8-OHdG) in GC.
In a recent study, we observed increased ROS production and impaired antioxidant defense mechanism in follicular fluid (FF) of women with endometriosis and PCOS [Jana et al. Citation2010]. This motivated us to investigate the effect of morphological and ROS-mediated changes in GC of women with endometriosis and PCOS on oocyte and embryo quality. The possible relationship between ROS generation and changes in mitochondrial membrane potential (MMP) and extent of DNA fragmentation in GC was also explored.
Results
Oxidative stress in GC was studied for the first time by Seino et al. in 2002. They observed a significant decrease in fertilization rate and subsequent decrease in the quality of embryos in IVF cycles [Seino et al. Citation2002]. A recent study also suggests that OS may induce apoptosis in GC, resulting in low oocyte quality and poor outcome in IVF-ET [Liu and Li 2010]. However, a study on apoptosis and ROS generation in GC by Jancar et al. [2007] shows no significant effect of ROS on fertilization and blastocyst development in tubal factor infertility. The objective of the present study was to investigate morphological and ROS-associated changes in GC and correlate the observed changes with oocyte, embryo quality, and pregnancy outcome in women with endometriosis/PCOS undergoing IVF-ET.
Isolated GC characteristics such as cell count, viability, morphology, and oocyte parameters (number of oocytes formed, immature and mature oocyte formation rate) of Group A, endometriosis, and B, PCOS, were compared with Group C, controls (). Furthermore, final GC counts were determined from each women undergoing IVF. GC count was varied in each group of women and it was found to be 2.0 to 3.4x105 in Group A, 1.0 to 1.3x105 in Group B, and 2.1 to 4.9x105 in Group C. Significant decreases in GC cell count, viability, and morphology were observed in Group A and B as compared to Group C. Phase contrast and SEM images depicting typical morphological characteristics of GC from Group A, B, and C are shown in .
Figure 1. Morphological appearance of granulosa cells under phase contrast microscope. A1, B1, and C1) all phase contrast images are captured in 20X magnification and corresponding scanning electron microscopy images (A2, B2, and C2,individual GC) in the three groups. Group A represents endometriosis; Group B represents PCOS; and Group C represents controls. GC: granulosa cells.
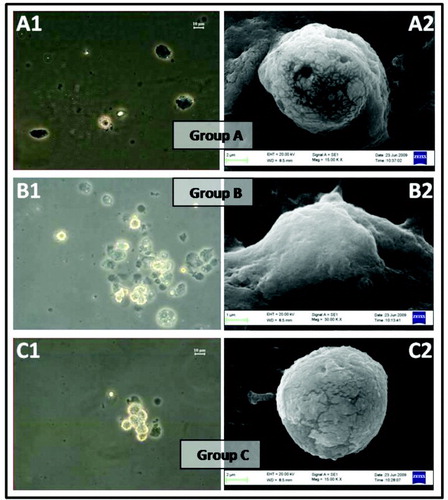
Table 1. Granulosa cell and oocyte characteristics in women undergoing IVF.
SEM images of Group A and Group B appeared to be morphologically abnormal when compared with the control group. SEM images confirmed the extracellular morphological changes of GC observed by bright field microscopy. Major morphological changes such as cell shape and abnormal membrane elongation were observed in Group A and Group B when compared with Group C under SEM (). On comparing Group B with Group C, statistically significant differences were observed in the number of oocytes. However, the number of oocytes retrieved was found to be comparable when Group A was compared with Group C. Immature and mature oocyte formation rates were significantly different in Group A and Group B compared with the controls (). OS markers (intracellular ROS, MMP, and DNA damage) and IVF outcome parameters (fertilization rate, embryo formation rate, and pregnancy outcome) are tabulated in .
Table 2. Changes in ROS, MMP, DNA fragmentation, and IVF outcome parameters in GC obtained from women with endometriosis (Group A) and PCOS (Group B) and compared with tubal factor infertility (controls; Group C).
Intracellular ROS generation in GC increased nearly 4-fold in Group A and nearly 20-fold in Group B when compared with Group C (). MMP was significantly less in both Groups A and B compared with Group C. This effect was again more pronounced in Group B indicating considerable mitochondrial dysfunction (). The percentage of DNA damage as estimated by TUNEL positivity in GC was significantly higher in both Groups A and B as compared to Group C (). Group B GCs were associated with a higher percentage of DNA damage when compared with the control group.
Figure 2. Flow cytometric histogram plot analysis has shown increased population in green (FL-1) channel indicates the generation of intracellular ROS (DCF-DA positive population) in GC of (A) endometriosis group, (B) PCOS group, and (C) control group. ROS: reactive oxygen species; GC: granulosa cells.
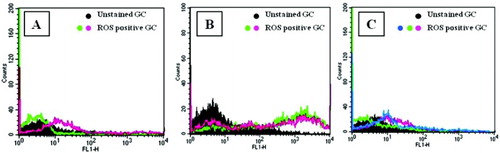
Figure 3. Flow cytometric dot plot analysis of mitochondrial membrane potential (∆Ψ) in GC as shown increased cells with reduced JC-1 fluorescence in the FL-2 (LR) channel in (A) endometriosis group, (B) PCOS group, and (C) control group. GC: granulosa cells.
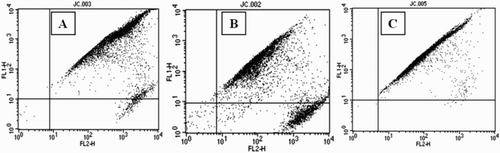
Figure 4. Granulosa cells phase contrast (Group A, B, and C), and DNA damaged TUNEL positive cells as shown in fluorescent microscopy (A1) Group A; (B1) Group B; (C1) Group C. Further, phase contrast images (D and E) and fluorescent microscopic images (D1 and E1) were positive and negative controls, respectively, used for TUNEL assay.
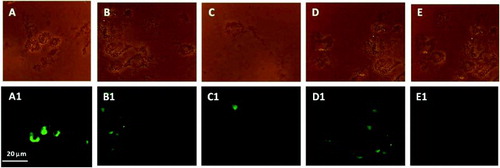
Figure 5. Percentage of mature oocyte formation rate (Grade III) and viable embryos formation rate (Grade I and II) in three groups. *P values were statistically significant (p < 0.05)
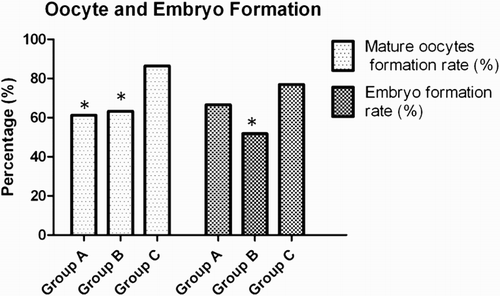
The IVF outcome parameters (fertilization rate, embryo formation rate, and pregnancy rate) were significantly less in Group B as compared to Group C (Table 2). However, there were no significant changes in fertilization rate, embryo formation rate, and pregnancy rate in Group A as compared to control groups. Clinical pregnancy was confirmed by the presence of gestational sac detected by ultrasound scan. Further, pregnancy rate was calculated based on the total number of positive pregnancies out of the total women who underwent IVF-ET. The rates of good quality oocytes and viable embryo formation were significantly less in Group A and B as compared to Group C (). However, the rate of embryo formation of Groups A and C did not differ significantly.
Discussion
Ovarian pathophysiology and oocyte development is normal in tubular infertility. Except for the mechanical blockage of the tube(s), there are no other associated pathological conditions of infertility. This group is, therefore, considered to be apparently GC normal and commonly considered as controls for comparison purposes with endometriosis and PCOS [Jana et al. Citation2010]. Endometriosis and PCOS are commonly associated with ovarian pathophysiology. Ovarian chocolate cyst develops in case of endometrioma (Stage-IV endometriosis) and immature follicular development and cyst formation are major pathological conditions in PCOS.
The GC count was found to be significantly less in Group B; this may be attributed to the increase in number of small antral follicles in PCOS [Mason et al. Citation1994]. The percentage of viable and morphologically normal GCs were observed to be significantly less in Groups A and B compared with Group C (). Further, the number of oocytes retrieved from Group B was significantly higher than Group C. This was due to a large number of immature follicles present in PCOS. The percentage of immature oocytes formed was significantly higher in Group A (38.7%) and Group B (36.73%) as compared to Group C (13.51%; p < 0.005). Again, formation of matured oocytes was significantly less in Group A (61.29%) and Group B (63.26%), when compared with Group C (86.48%; p < 0.005).
A nearly 20 fold increase in GC ROS generation was observed in Group B as compared to the other two groups (Table 2). The possible source of excessive ROS generation may be due to the morphologically abnormal GC in PCOS and impaired mitochondrial oxidative metabolism [Victor et al. Citation2009]. However, the mechanism involved in the generation of OS in PCOS still remains elusive [Duleba et al. Citation2004]. Though not as high as Group B, Group A also showed significantly high levels of ROS compared with Group C (P < 0.05). Our results are in good agreement with the findings of Saito et al. [2002], who have reported higher levels of oxidative modification in GC of endometriosis patients when compared with tubal factor, male infertility, and idiopathic infertility. In the present study, more than a 100-fold decrease in MMP in Group B compared with Group C was observed. This may reflect excessive ROS generation in this group, provoking the opening of the mitochondrial permeability pores, thereby causing apoptosis [Wang et al. Citation2009]. A similar trend was observed in Group A, where MMP decreased 7-fold.
It is evident that apoptosis is associated with depolarization of the MMP. Increased cell population with reduced JC-1 fluorescence in the present study, indicative of depolarized MMP (), indicates significant apoptosis in Groups A and B. Apoptotic changes in the present study are further supported by the significant increase in DNA damage observed in Group A and B compared to Group C. These findings are in agreement with reports of Saito et al. [2002] where they have documented increased levels of apoptotic cells in endometriosis. In another study, excessive luteinized DNA fragmentation in GC of PCOS patients undergoing IVF cycles is documented [Onalan et al. Citation2005]. IVF outcome parameters including fertilization rate, good quality embryo formation rate, and pregnancy outcome were adversely affected in Group B (). It was hypothesized that an ∼20 fold increase in ROS generation in GC of these women plays an adverse role in affecting the IVF success rate. It was interesting to note that the IVF outcome parameters of Group A were comparable with Group C.
Summarizing, the findings suggest that excessive ROS generation in GC of women with PCOS undergoing IVF-ET correlate with significant morphological abnormalities, decreased MMP, and higher levels of DNA fragmentation. Furthermore, oocyte and embryo quality are adversely affected. The toxic levels of ROS were relatively less in GC of women with endometriosis and the oxidative damage, though significantly higher than controls, did not affect the IVF outcome parameters considerably. It is evident from that poor IVF success rate in endometriosis is more due to oocytes/embryo impairment, endometrial defects, or defective endometrial/embryonic cross-talk than excessive ROS generation in GC.
Materials and Methods
The samples were collected at the Institute of Reproductive Medicine (IRM), Salt Lake, Kolkata, India and the study carried out at the Medical Biotechnology Unit, School of Medical Science and Technology, IIT, Kharagpur, India. Approval for this study was obtained from the Research Ethics Committee of the Institute. Written informed consent was obtained from all couples participating in this study.
Subject selection
GC were isolated from three different groups of IVF-ET by controlled ovarian stimulation. Twenty-eight women with endometriosis constituted Group A, while Group B and Group C consisted of 26 and 32 women with PCOS and tubal factor infertility, respectively. Group C, considered as controls, refers to women who had salpingectomy for ectopic pregnancy and proximal tubal obstruction because of low-grade infection or fimbrial occlusion with or without mild peritubal adhesions.
Male factor infertility was excluded from all three groups. Semen analysis was performed as per WHO guidelines [1999]. GC samples were obtained from only those women whose male partners had sperm count ≥ 20 million/ml, total motility ≥ 50%, morphology ≥ 35%, and ROS levels in the range of 0.02–0.05 count per min (cpm) × 106/10 million sperm cells. Semen samples were also subjected to hypo-osmotic swelling test (HOST; inclusion criteria ≥ 60%). Further, WHO [2010] guidelines were referred to, to evaluate semen parameters and also included research procedures such as semen ROS measurement.
All women were down-regulated with a GnRH agonist (Lupride, Sun Pharmaceuticals, Mumbai, India) from mid-luteal phase onwards and, when optimally down-regulated, were stimulated with recombinant FSH (Gonal F, Serono, Geneva, Switzerland). Based on ultrasound and serum estradiol assays, follicular size was monitored regularly. When size of the follicles reached 18 mm, hCG (Pregnyl, Organon, The Netherlands) was administered subcutaneously. The oocytes, following aspiration under transvaginal ultrasound guidance, were graded [Veeck Citation1999] and subsequently inseminated. Pronuclear (PN) scoring was done 16–18 h following insemination. Embryo quality was assessed before every embryo transfer [Veeck Citation1999], and a maximum of three embryos were transferred to all patients approximately 48 h (4-cell stage) after insemination.
Isolation of granulosa cells
The follicular aspirates were pooled from one patient and centrifuged at 300 × g for 5 min and the supernatant slowly removed using Pasteur pipette. Pellet was resuspended in 1 ml of Dulbecco's Modified Eagle Medium (DMEM) and gently layered on top of 2 ml 50% Percoll density gradient and centrifuged at 500 g for 20 min. The GC layer at the interface of the gradient and DMEM medium was collected. These cells were then washed once by resuspending in 3 ml of DMEM medium and centrifuged at 150 x g for 3 min. The final pellet was resuspended in 0.5 ml of DMEM medium. Further, RBCs were removed using hypo-osmotic lysis method. A 0.5 ml of cell suspension was placed in 15 ml conical bottomed centrifuged tube, 9 ml of sterile distilled water added, and the tube shaken gently. After 20s, 1 ml of 10x PBS, pH 7.4 was added and mixed thoroughly. The tube was then centrifuged at 150 x g for 3 min and the pellet resuspended in 0.5 ml of DMEM [Lobb and Younglai Citation2006; Mason et al. Citation1994]. To prepare individual GC, 80 IU/ml hyaluronidase (Cook, Brisbane, Australia) was added and the enzyme activity neutralized after 1 min by adding DMEM medium. Further, GC cell count, viability, and morphology were assessed.
Assessment of GC characteristics (count, viability, and morphology)
GC cell count was performed on hemocytometer. Isolated GC pellet was resuspended in 1 ml of DMEM medium and cell count, viability and morphology, were determined. GC counts were determined using a hemocytometer as described earlier by Seifer et al. [1996] with minor modifications. The results were reported in Mean ± SD cell counts in FF (). The GC cell suspension was mixed with an isotonic solution of trypan blue (0.2%; w/v) and viability determined by counting stained and unstained cells using a hemocytometer under a phase contrast microscope (Leica DMR, Germany). Morphological assessment of GC was done under phase contrast microscope. Morphological characteristics of GC including cell shape and cytoplasmic shrinkage were included. Morphologically normal and intact cells, having no cytoplasmic shrinkage was considered as normal. Results were reported as Mean ± SD of normal cells and experiment was performed in triplicate. Further GC membrane characteristic changes were assessed using scanning electron microscope (SEM). The cells were pre-fixed with glutaraldehyde (0.25%) in PBS at 4°C for 15 min and post-fixed with 1% osmium tetraoxide for 1hr. Then GCs were dehydrated in a series of 50%, 70%, 90%, and 100% alcohol gradient. Each step was carried out for 10 min. Finally, HMDS (1, 1, 1, 3, 3, 3-hexamethyl disilazane) was added drop wise to the GC, the cells were placed on glass cover slips, and air dried. These air dried samples were coated with gold and the cells examined under SEM (ZEISS, EVO 60) for assessing morphological changes in the GC membrane.
Measurement of intracellular ROS
H2DCF-DA (2',7'-dichlorofluorescein diacetate) is a cell-permeable peroxide-sensitive fluorescent probe and is used to detect intracellular free radicals. Once H2DCF-DA enters into the cells, it is hydrolyzed to DCF and trapped in the cell compartment. In the presence of free radicals like H2O2, peroxides, DCF is oxidized and gives the green fluorescent product 2',7-dichlorofluorescein which can be measured by flow cytometer or fluorescent microscope. The wavelength of the laser was 505-535 nm (excitation and emission, respectively) and green and red signals were detected using respective filters/channels.
Ten mM H2DCF-DA was added to GC suspended in 0.5 ml PBS and then incubated for 20 min at 37°C. After incubation, cells were washed with PBS twice and resuspended in 0.5 ml of PBS. GC generated green fluorescent intensity was measured in FL-1 channel using a flow cytometer (BD FACSCalibur cytometer, BD Biosciences, San Jose, CA, USA) and data analyzed using CellQuest Pro software.
Measurement of mitochondrial membrane potential
JC-1 (5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolcarbocyanine iodide), a lipophilic cation, was used to assess the mitochondrial status in cells. JC-1 changes its fluorescence reversibly from green (monomeric status) to orange (multimeric status) with change in MMP. JC-1 is rapidly taken up by normal polarized mitochondria forming JC-1 aggregates, which show a red spectral shift resulting in increased levels of red fluorescence emission. In apoptotic cells, the MMP collapses and the JC-1 cannot accumulate within the mitochondria. In these cells JC-1 remains in the cytoplasm in its monomeric form and subsequently reduces the red fluorescence. MMP assessment was performed by using JC-1, according to the manufacturer's (BD™ MitoScreen, BD Biosciences, UK) instructions. Briefly, JC-1 stock solution was prepared by mixing 125 µl of DMSO with one vial of lyophilized JC-1. This stock solution was further diluted with 12.375 ml of 1X assay buffer to make the final working solution. GC were added with 0.5 ml of JC-1 working solution and incubated for 10-15 min at 37°C in CO2 incubator. GC were washed twice at room temperature and centrifuged at 400 g for 5 min. Finally, the cells were resuspended in 0.5 ml of 1X assay buffer and analyzed GC by flow cytometry.
Assessment of DNA fragmentation
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay was performed as per the manufacturer's instructions in GC using ApoAlert DNA fragmentation assay kit. GC were fixed in 4% paraformaldehyde at 4°C for 25 min and then washed thrice in PBS. The cells were incubated in 50 µl permeabilization solution (0.2 % Triton X-100) for 5 min at room temperature (RT). This was followed by incubation in equilibration buffer for 10 min. GC were then transferred into 50 µl reaction mixture (45 µl equilibrium buffer + 5 µl nucleotide mixture + 1 µl TdT enzyme) and incubated in humidified chamber for 60 min in dark at 37°C. The reaction was terminated by incubating the GC in 2X SSC buffer for 15 min at RT. The cells were then washed three times with PBS/PVP following which they were treated with 0.5 µg/ml of DNase-free RNase. GC of Group C treated with DNAse1 served as positive controls. GC of Group C treated in the same manner except TdT labeling enzyme was not added in reaction mixture served as negative controls. The cells were analyzed under epifluorescence microscope (Leica DMR, Germany) and the apoptotic cells observed using green filter (520 ± 20 nm).
Statistical analysis
Data were analyzed using analysis of variance (one-way ANOVA) and t-test, as appropriate. All analyses were performed with Ky Plot version 2.0 beta 13 (Koichi Yoshioka, 1997–2000). Statistical significance was defined as P ≤ 0.05.
Abbreviations
| ROS: | = | reactive oxygen species |
| GC: | = | granulosa cells |
| OS: | = | oxidative stress |
| IVF: | = | in-vitro fertilization |
| ET: | = | embryo transfer |
| PCOS: | = | polycystic ovary syndrome |
| MMP: | = | mitochondrial membrane potential |
| CK: | = | cytokeratin |
| FF: | = | follicular fluid. |
Acknowledgments
The authors thank the IVF staff, Institute of Reproductive Medicine for support to successfully complete the study and the Government of India, Department of Biotechnology for financial support.
Declaration of interests: Financial support received from the Government of India, Department of Biotechnology.The authors report no conflicts of interest.
Author contributions: Experiment and writing manuscript: NBK; Experiment: RC; Concept: BN; Writing manuscript: KC.
References
- Adashi, E.Y. (1994) Endocrinology of the ovary. Hum Reprod 9:815–827.
- Albertini, D.F. and Barrett, S.L. (2002) Oocyte-somatic cell communication. Reprod Suppl 61:49–54.
- Clavero, A., Castilla, J.A., Núñez, A.I. García-Peña, M.L. Maldonado, V. Fontes, J., (2003) Apoptosis in human granulosa cells after induction of ovulation in women participating in an intracytoplasmic sperm injection program. Eur J Obstet Gynecol Reprod Biol 110:181–185.
- Das, M., Djahanbakhch, O., Hacihanefioglu, B., Saridogan, E., Ikram, M., Ghali, L., (2008) Granulosa cell survival and proliferation are altered in Polycystic Ovary Syndrome. J Clin Endocrinol Metab 93:881–887.
- Duleba, A.J., Foyouzi, N. and Karaca, M. (2004) Proliferation of ovarian theca-interstitial cells is modulated by antioxidants and oxidative stress. Hum Reprod 19:1519–1524.
- Hanukoglu, I. (2006) Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab Rev 38:171–196.
- Jakimiuk, A.J., Weitsman, S.R., Navab, A. and Magoffin, D.A. (2001) Luteinising hormone receptor, steroidogenesis acute regulatory protein and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab 86:1318–1323.
- Jana, S.K., Narendra Babu, K., Chattopadhyay, R., Chakravarty, B. and Chaudhury, K. (2010) Upper control limit of reactive oxygen species in follicular fluid beyond which viable embryo formation is not favorable. Reprod Toxicol 29:447–451.
- Jancar, N., Kopitar, A.N., Ihan, A., Klun, I.V. and Bokal, E.V. (2007) Effect of apoptosis and reactive oxygen species production in human granulosa cells on oocyte fertilization and blastocyst development. J Assist Reprod Genet 24:91–97.
- Liu, J.and Li, Y. (2010) Effect of oxidative stress and apoptosis in granulosa cells on the outcome of IVF-ET. Zhong Nan Da Xue Xue Bao Yi Xue Ban 9:990–994.
- Lobb, D.K. and Younglai, E.V. (2006) A simplified method for preparing IVF granulosa cells for culture. J Assist Reprod Genet 23:93–95.
- Mason, H.D., Willis, D.S., Beard, R.W.,Winston, R.M.L., Margara, R. and Franks, S. (1994) Estradiol production by granulosa cells of normal and polycystic ovaries: relationship to menstrual cycle history and to concentrations of sex steroids in follicular fluid. J Clin Endocrinol Metab 79:1355–1360.
- Nakahara, K., Saito, H., Saito, T., Ito, M., Ohta, N., Sakai, N., (1997) Incidence of apoptotic bodies in membrane granulosa of the patients participating in an in vitro fertilization program. Fertil Steril 67:302–308.
- Onalan, G., Selam, B., Baran, Y., Cincik, M., Onalan, R., Gunduz, U., (2005) Serum and follicular fluid levels of soluble Fas, soluble Fas ligand and apoptosis of luteinized granulosa cells in PCOS patients undergoing IVF. Hum Reprod 20:2391–2395.
- Oosterhuis, G.J.E., Michgelsen, H.W., Lambalk, C.B., Schoemaker, J. and Vermes, I. (1998) Apoptotic cell death in human granulosa-lutein cells: a possible indicator of in vitro fertilization outcome. Fertil Steril 70:747–749.
- Saito, H., Seino, T., Kaneko, T., Nakahara, K., Toya, M. and Kurachi, H. (2002) Endometriosis and oocyte quality. Gynecol Obstet Invest 53:46–51.
- Seifer, D.B., Gardiner, A.C., Ferreira, K.A. and Peluso, J.J. (1996) Apoptosis as a function of ovarian reserve in women undergoing in vitro fertilization. Fertil Steril. 66:593–598.
- Seino, T., Saito, H., Kaneko, T., Takahashi, T., Kawachiya, S. and Kurachi, H. (2002) Eight-hydroxy-2-deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in vitro fertilization embryo transfer program Fertil Steril 77:1184–1190.
- Serke, H., Vilser, C., Nowicki, M., Hmeidan, F.A., Blumenauer, V., Hummitzsch, K., (2009) Granulosa cell subtypes respond by autophagy or cell death to oxLDL-dependent activation of the oxidized lipoprotein receptor 1 and toll-like 4 receptor, Autophagy 5:991–1003.
- Spanel-Borowski, K. (2010) Ovulation as danger signaling event of innate immunity Mol Cell Endocrinol 333:1–7.
- Stubbs, S.A., Stark, J., Dilworth, S.M., Franks, S. and Hardy, K. (2007) Abnormal preantral folliculogenesis in polycystic ovaries is associated with increased granulosa cell division. J Clin Endocrinol Metab 92:4418–4426.
- Takekida, S., Matsuo, H. and Maruo, T. (2003) GnRH agonist action on granulosa cells at varying follicular stages. Mol Cell Endocrinol 202:155–164.
- Taniguchi, K., Taketani, T., Lee, L.M., Kizuka, F., Tamura, I. and Sugino, N. (2009) Melatonin Protects Granulosa Cells for Progesterone Production as an Antioxidant in Human Ovarian Follicles. Biol Reprod 81:378.
- Tilly, J.L., Kowalski, K.I., Johnson, A.L. and Hsueh, A.J.W. (1991) Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrinology 129:2799–2801.
- Veeck, L.L. (1999) An Atlas of Human Gametes and Conceptuses: An Illustrated Reference for Assisted Reproductive Technology. Parthenon Publishing Group Inc, New York, USA.
- Victor, V.M., Rocha, M., Banuls, C., Sanchez-Serrano, M., Sola, E., Gomez, M., (2009) Mitochondrial complex I impairment in leukocytes from polycystic ovary syndrome patients with insulin resistance. J Clin Endocrinol Metab 94:3505–3512.
- Vilser, C., Hueller, H., Nowicki, M., Hmeidan, F.A., Blumenauer, V. and Spanel-Borowski, K. (2010) The variable expression of lectin-like oxidized low-density lipoprotein receptor (LOX-1) and signs of autophagy and apoptosis in freshly harvested human granulosa cells depend on gonadotropin dose, age, and body weight. Fertil Steril 93:2706–2715.
- Wang, L.Y., Wang, D.H., Zou, X.Y., Xu, C.M. (2009) Mitochondrial functions on oocytes and preimplantation embryos. J Zhejiang Univ Sci 10:483–492.
- WHO. (1999) World Health Organization Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction. Cambridge University Press, New York, USA.
- WHO. (2010) WHO Laboratory Manual for the Examination and Processing of Human Semen, 5thed. Geneva: WHO Press 2010.