Abstract
Chromosomes in human spermatozoa are arranged non-randomly with the centromeres of non-homologous chromosomes forming a chromocenter. We have compared motile and immotile sperm populations in normozoospermic patients to determine if there is any dissimilarity in the formation of the chromocenter and the nuclear position of chromosome 17. Based on the differences between motile and immotile populations, we propose for the ‘optimal’ nuclear organization to be defined as containing 1 to 3 chromocenter(s) with central radial and median longitudinal position for the centromere of chromosome 17. By this definition, 42% of motile spermatozoa had ‘optima’ nuclei, in comparison to 25% of immotile spermatozoa (P < 0.05). Immotile spermatozoa exhibited a greater disruption in the formation of the chromocenter, altered position of the centromere of chromosome 17, and were more prone to chemical decondensation, resulting in higher nuclear and chromocenter volumes. The altered topology of the chromosomes might lead to the disruption of the sequence of events involved in fertilization and early embryonic development.
Introduction
The organization of nuclear chromatin has been intensively studied in somatic cells during the cell cycle, and was shown to be altered by pathological conditions and diseases (for review refer to [Fudenberg and Mirny Citation2012]). However, mature human spermatozoa have a unique, well arranged nuclear chromatin organization that is different from somatic cells [Zalensky et al. Citation1993; Wykes and Krawetz Citation2003]. The higher order of this chromatin spatial organization plays an important role in the compartmentalization of sperm nuclei and is characterized by the arrangement of the centromeres of non-homologous chromosomes into an inner chromocenter [Cremer et al. Citation1993; Zalenskaya and Zalensky Citation2004]. The chromocenter plays a fundamental role in the sperm nuclear architecture and is involved in proper intranuclear chromosome positioning with preferred chromosome territory (CT) localization [Cremer et al. Citation1993; Zalensky and Zalenskaya Citation2007]. It has been suggested that the distribution of CTs have functional significance in terms of gene expression in somatic sells [Takizawa et al. Citation2008]. It has also been shown that gene-rich chromosomes are localized more towards the interior of the nucleus, whereas the gene-poor chromosomes are found towards the periphery [Haaf and Ward Citation1995; Zalenskaya and Zalensky Citation2004]. Several studies have suggested that the order of chromosome deposition into the oocyte may be an epigenetic code that impacts chromatin re-modeling and activation of the male genome during the early stages of embryonic development [Zalensky et al. Citation1993; Hazzouri et al. Citation2000; McLay and Clarke Citation2003]. The improper organization of sperm chromatin may be one factor that can result in failed fertilization and/or a halt in zygote development [Zalenskaya et al. Citation2000; Wykes and Krawetz Citation2003].
The assessment of nuclear organization in spermatozoa is challenging due to the extreme compactness of chromatin that requires nuclear decondensation of deoxyribonucleic acid (DNA) as part of fluorescence in situ hybridization (FISH) analysis. Several chemical agents can be used for decondensation. Therefore, it must be taken into consideration that different chemicals and concentrations can considerably change the degree of decondensation, as well as the distribution of protamine/histones in spermatozoa [Mudrak et al. Citation2005]. Most previous studies are based on two-dimensional (2D) imaging to visualize chromosomal organization [Zalensky et al. Citation1995; Gurevitch et al. Citation2001; Zalenskaya and Zalensky Citation2004; Zalensky and Zalenskaya Citation2007; Tsend-Ayush et al. Citation2009; Ioannou and Griffin Citation2011; Vavouri and Lehner Citation2011]. While 2D imaging may be sufficient to understand basic nuclear structure, the cell nucleus has three-dimensional (3D) organization. Therefore, 3D imaging provides a more accurate assessment of nuclear structure by minimizing overlapping signal artefacts acquired during 2D imaging. The 3D evaluation of sperm nuclei is a labor intensive process, thus the published literature is limited to only two reports [Hazzouri et al. Citation2000; Manvelyan et al. Citation2008].
The quality and motility of spermatozoa in a given ejaculate is very heterogeneous, but only motile spermatozoa are capable of delivering DNA to the oocyte. During assisted reproductive techniques (ART) with in vitro fertilization (IVF), the motile fraction of spermatozoa is isolated from the rest of the ejaculate during sperm preparation prior to fertilization. However information about the differences in the nuclear organization of the isolated subpopulation of spermatozoa is lacking. The purpose of this study was to evaluate the structural differences between motile and immotile human spermatozoa in terms of chromocenter organization and the location of chromosome 17 using 3D digital morphometry and image analysis. The submetacentric chromosome 17 was chosen because it is one of the richest, in terms of gene density, in the human genome. It contains 16.2 genes per Mb, 78,839,971 bases, and is implicated in more than 70 recorded microdeletion/microduplication disorders, genetic conditions, and diseases [Zody et al. Citation2006].
Results
In total, more than 130,000 images of 563 spermatozoa were acquired and analyzed to validate descriptive 3D study results. The mean volume of sperm nuclei was 61.68 ± 22.54 µm3, with the volume occupied by the chromocenter being 2.66 ± 1.71 µm3, or approximately 4.5% of the sperm nuclei. The volume for the centromere of chromosome 17 was 0.27 ± 0.23 µm3. The volume measurements were normalized using the fold increase to adjust for the decondensation factor, resulting in the volumes of chromosome 17 and the chromocenter to be 0.22 ± 0.18 µm3 and 2.09 ± 1.21 µm3, respectively. The average distance from the tail attachment point to the centromere of chromosome 17 is 3.50 ± 1.07 µm or 2.92 ± 0.80 µm, after adjusting for the decondensation factor. The radial position of the centromere of chromosome 17 was confirmed by calculating the distance from the tail attachment (reference point) to the centromere signal (). Furthermore, based on the measurements of length of the long axis, short axis, and ellipsoidal shape of the cell, the sperm nuclear head was equally divided into two spheres: peripheral or central radial position (A). The longitudinal position of the centromere of chromosome 17 was defined by dividing the sperm nucleus into three equal segments along its long axis, from the tail attachment point to the tip of the acrosome. The three segments were labeled as tail, medial, and acrosomal regions. A schematic representation of the longitudinal position is provided in B.
Figure 1. Three-dimensional (3D) imaging of spermatozoon.Four different angles of a representative spermatozoon nucleus. For each cell, the following was measured: 1) the volume of the nucleus, 2) the volume of pan-centromeric signals, 3) the volume of the chromosome 17 centromere specific signal, 4) the number of observed chromocenter(s) (6 chromocenters for the depicted cell), 5) the distance from the tail attachment (reference point A) to the centromere signal (4.1 µm for the depicted cell), and 6) the long axis (8.4µm), (distance point from A to point B). Fluorescence in situ hybridization (FISH) probes: human chromosome pan-centromeric – green; α-satellite centromere specific chromosome 17 - red. Measurements from the tail attachment point to the centromere of chromosome 17.
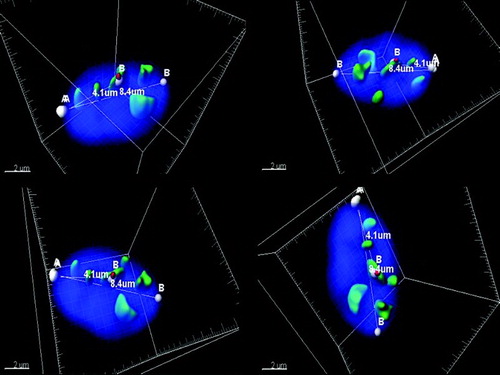
Figure 2. The schematic representation of the sperm nucleus. A) Radial cell division: P - peripheral, C – central (equally divided). B) Longitudinal cell division: A - acrosomal region, M - medial region, T - tail region (equally divided).
Figure 3. Descriptive evaluation of three variables in the motile and immotile spermatozoa. Three variables were taken into account (the number of chromocenters, the radial as well as the longitudinal positions of the centromere of chromosome 17 within the nucleus) to assess the variations of unique prototypes of spermatozoa. Localization of the centromere of chromosome 17: the radial position described as periphery (P) or central (C); the longitudinal position classified as acrosomal (A), medial (M), or tail region (T) and the numeral number represents the quantity of chromocenters in the sperm nucleus. Example: PA1: centromere of chromosome 17 located in the periphery (outer) acrosomal part of the nuclei with 1 observed chromocenter.
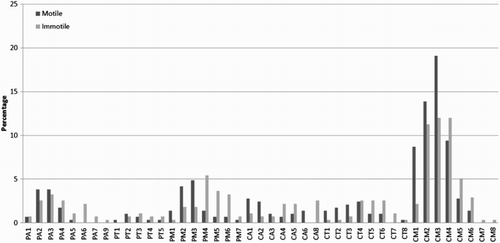
Significant differences were observed between the motile (n = 288) and the immotile (n = 275) spermatozoa in terms of the compaction of the sperm nucleus, the volume of the centromere of chromosome 17, and the volume of the chromocenter (P < 0.0001) (). Overall, spermatozoa were heterogeneous in terms of the localization and quantity of observed signals. When three variables were taken into account (the number of chromocenters, the radial and longitudinal positions of chromosome 17 within the nucleus) the motile spermatozoa had 35 different combinations of unique prototypes, while the immotile spermatozoa had 41 variations (). When the fourth factor, the direct involvement of chromosome 17 in the formation of the chromocenter was included, the motile spermatozoa had 58 different combinations while the immotile population had 65 variations.
Table 1. Compression between motile and immotile spermatozoa.
Only some spermatozoa had a single chromocenter. The majority of spermatozoa (90.6%) had multiple well defined clusters of signals (chromocenters). A maximum of eight such formations were observed in a few cells (< 1%). Moreover, the number of observed chromocenters was significantly less in the motile spermatozoa; 73% of nuclei contained 1 to 3 chromocenters, in comparison to 50% of the immotile population with 3 or less chromocenters (P < 0.001). Analysis of the longitudinal position of the centromere of chromosome 17 in the motile spermatozoa revealed that 68.4% of analyzed cells had chromosome 17 localized in the medial region; 12.8% were positioned in the acrosomal region, and 18.8% were located near the tail. Similar patterns of longitudinal positioning were observed in immotile spermatozoa. In 64% of the immotile spermatozoa the centromere of chromosome 17 was localized in the medial region, 13.8% in the acrosomal, and 22.2% in the tail region. When the radial position of the centromere of chromosome 17 was evaluated, central localization was present in 73.6% and 65% of the motile and the immotile spermatozoa, respectively. Furthermore, there was no significant difference between the motile and immotile spermatozoa when comparing the role of chromosome 17 in the formation of the chromocenter (54.9% and 56.4%, respectively). Based on the observed differences between these two populations, we propose for the ‘optimal’ nuclear organization to be defined as having 1 to 3 chromocenters, as well as a central radial and median longitudinal position of chromosome 17. Overall, the motile sperm population had significantly fewer numbers of cells with disrupted nuclear organization in the chromocenter and eccentric localization of the centromere of chromosome 17. Immotile spermatozoa were more prone to chemical decondensation, which resulted in higher nuclear and chromocenter volumes (). Almost 42% of motile spermatozoa were classified as having an ‘optimal’ nuclear organization in comparison to 25% of the immotile spermatozoa (P < 0.05).
Discussion
Centromeres are distinctive chromosomal regions, responsible for linking two sister chromatids to form a kinetochore and direct mitotic division. Centromeric regions of human chromosomes are comprised of highly repetitive non-transcribed α–satellite sequences that play an important structural role in nuclear chromosomal organization [Ugarkovic Citation2009].
It has been proposed that in mature human spermatozoa, the centromeres of non-homologous chromosomes cluster into chromocenter(s) which play a significant role in the configuration of the nuclear architecture [Zalensky et al. Citation1993; Zalensky et al. Citation1995]. Centromeres have been shown to have a mixture of protamines and histones in comparison to the histone-enriched telomeric sequences. This difference represents a non-random distribution of protamines in the spermatozoa [Wykes and Krawetz Citation2003]. Furthermore, centromere-centromere interactions are an important part of sperm nuclear organization. In earlier studies, Zalensky et al. reported the existence of a single chromocenter in the mildly decondensed sperm nuclei [Zalensky et al. Citation1993; Zalensky et al. Citation1995]. However, the same group later reported that the chromocenter is composed of several linear arrays of centromeres, with a specific order of chromosomes engaged in the formation of the chromocenters [Zalenskaya and Zalensky Citation2004]. In our study, we observed that less than 10% of spermatozoa contained a single chromocenter, while the majority of spermatozoa (90.6%) had multiple, well-defined clusters of signals. These findings are also in agreement with a study by Finch and colleagues, who reported numerous pan-centromeric signals in the sperm nucleus, with a common tendency to be located in the central region; however, no quantitative analysis of chromocenters was reported by that group due to an inability to differentiate between overlapping signals when utilizing 2D image data [Finch et al. 2008]. Acrocentric chromosomes (13-15, 21, and 22) are known to create clusters in the nucleolus of somatic cells; 20% of 86 analyzed spermatozoa showed similar positioning [Gurevitch et al. Citation2001]. While our results are in agreement with the study of Gurevitch et al. concerning the formation of chromocenters by clusters of non-homologous chromosomes, chromocenters were found to be more condensed in our study. We included analysis of the centromeres for all chromosomes, which occupied anywhere from 0.39% to 19.6% of the nucleus volume (mean 4.5%). However, in a study by Gurevitch et al. [2001] on the centromeres of five specific chromosomes, centromeres occupied anywhere from 8.3% to 87% of the sperm nucleus, which seems exceptionally high, as this leaves virtually no space in the nucleus for the rest of the chromatin. We propose that dissimilarity in the sample preparation, non-specific probe binding or image analyses methodology (2D vs. 3D) might account for the differences between these observations.
In human spermatozoa, the differential anchoring of centromeres to the nuclear matrix was demonstrated for several chromosomes. This supports the looped domain organization theory of chromatin organization in spermatozoa and results in the preferred localization of chromosomes [Yaron et al. Citation1998]. It has been proposed that this organization allows for the controlled unpacking, structural remodelling, and transfer of epigenetic information of the male genome after fertilization [Wu and Chu Citation2008]. Several studies have been carried out to analyze chromosome spatial organization in the sperm's nuclear head using a 2D approach [Gurevitch et al. Citation2001; Zalenskaya and Zalensky Citation2004; Zalensky and Zalenskaya Citation2007; Olszewska et al. Citation2008; Tsend-Ayush et al. Citation2009; Ioannou and Griffin Citation2011; Vavouri and Lehner Citation2011]. These studies concluded that each of the sperm chromosomes have distinct preferred territories, with the relative position of chromosomes, as well as the distances between selected pairs of centromeres being non-random. It was shown that this may be a result of the specific adherence between the centromeres of non-homologous chromosomes, which may ultimately result in a fixed and non-random nuclear localization of specific centromeres (2, 6, 7, 16, 17, X, and Y) in the chromocenter. For example, it was determined that chromosomes 7 and 17, on average, are located much closer to each other than chromosomes 16 to 17 [Zalenskaya and Zalensky Citation2004].
The nuclear positions of chromosomes have been shown to be based on chromosome size and/or gene density [Sun et al. Citation2000; Manvelyan et al. Citation2008]. There is evidence of strong correlations between gene expression and chromosomal nuclear positioning during pro-metaphase/metaphase and interphase stages [Cremer and Cremer Citation2001; Cremer et al. Citation2004; Zalenskaya and Zalensky Citation2004]. Non-random packaging of nuclear DNA with protein function and transcriptional gene activity was reported in human and murine spermatozoa by using array comparative genomic hybridization (CGH) analysis [Arpanahi et al. 2009]. Furthermore, gene silencing and activation is associated with chromosome repositioning [Kumaran et al. Citation2008].
Centromere position was well studied using 3D image analysis in a number of human cell types (lymphocytes, fibroblasts, lymphoblastoid, and neuroblastoma cells) as well as the different stages of cell cycling [Solovei et al. Citation2004]. Solovei and colleagues reported similar changes of centromere position during cell cycles with the formation of centromeric clusters during late G1 and early S phase of mitosis in all types of analyzed cells. Controversial results were reported in the nuclear organization of chromosomes 13, 15, 16, 18, 21, 22, X and Y in human blastomeres; while well defined nuclear CTs were observed in aneuploid embryonic cells, ‘healthy’ euploid blastomeres had random nuclear organization with chaotic CTs [Finch et al. 2008]. In contrast, well defined spatial organization of 13, 16, 18, 21, 22, X and Y chromosomes were observed in euploid human blastomeres, but were significantly altered in aneuploid embryonic cells [McKenzie et al. Citation2004].
Advanced 3D imaging has become more prevalent in understanding the cellular structures of various cell types. However, most published studies on human sperm have being carried out using 2D analysis, with the exception of only a couple of reports with somewhat conflicting results [Hazzouri et al. Citation2000; Manvelyan et al. Citation2008]. Confocal microscopy was performed on 30 spermatozoa in a study by Hazzouri and colleagues, who reported a preferred anterior position for chromosome X, but random positioning for chromosome 13. While they did observe centromere clusters, they found centromeres to be randomly dispersed throughout the sperm nucleus, which is in disagreement with several previous studies, as well as the findings reported above. In the study by Manvelyan and colleagues, a combination of 3D imaging, multicolor banding, and cell suspension fluorescence in situ hybridization (FISH) techniques were performed to characterize the position and orientation of all chromosomes in human sperm cells. The study was limited as it focused on only one ejaculate obtained from a healthy donor and only 30 analyzed spermatozoa. However, it did provide for the first time data regarding the topology and non-random positions of all chromosomes in human spermatozoa [Manvelyan et al. Citation2008]. While a different chromosome 17 visualization technique was used in that study, our results are comparable with Manvelyan et al. [2008], who reported that chromosome 17 was localized towards the central part of the nucleus in approximately 60% of cells, and that nearly 70% of cells had chromosome 17 localized in the middle part of the sperm. This is comparable to our results of 70% and 66%, respectively.
The arrangement of CTs in somatic cells can be altered during pathological processes such as laminopathies and various cancers [Ioannou and Griffin Citation2011]. Abnormal sperm chromosomal localization, using 2D image analysis, was reported in men with idiopathic infertility, elevated sperm aneuploidy, and reciprocal chromosome translocations [Finch et al. 2008; Olszewska et al. Citation2008; Wiland et al. Citation2008]. In contrast, modest differences in the nuclear organization of the (peri-) centromeric loci of 18 chromosomes (1-4, 6-12, 15-18, 20, and X/Y) were observed between patients with severe impairment of spermatogenesis and fertile controls by deriving 3D data from 2D images. [Ioannou et al. Citation2011].These discrepancies might be partially explained by the fact that the population of spermatozoa within a single ejaculate is highly heterogeneous; even within healthy, fertile men up to 25% inter-individual variations of nuclear organization has been reported [Wiland et al. Citation2008].
Reactive oxygen species (ROS) appear to affect the stability of human sperm DNA and sperm chromatin condensation, which eventually impact sperm function [Zubkova et al. Citation2005]. Furthermore, it has been shown that higher lipid peroxide levels in semen correlates to lower sperm motility [Kao et al. Citation2008]. This trend was also observed in the current study. Immotile cells were more prone to chemical decondensation, resulting in higher nuclear head and chromocenter volumes. When sperm DNA damage is present, abnormal chromatin condensation and disordered association of chromatin with the nuclear matrix has been observed [Yaron et al. Citation1998; Aoki and Carrell Citation2003].
Three-dimensional image analyses allow for the advanced understanding of sperm spatial nuclear organization, despite being labour intensive. Our data confirms that the centromere of chromosome 17 is generally localized in the medial and central part of the sperm nucleus. The results show that the motile group has a significantly higher number of cells with the preferred non-random position of chromosome 17. The immotile population has a higher number of cells with disrupted chromosome 17 localization and is more prone to decondensation, which may result in the disruption of chromatin architecture in the sperm nucleus. An altered topology of the chromosomes would presumably lead to the disruption of the structured sequence of events involved in fertilization and early embryonic development. Studies are now in progress to address this hypothesis.
Materials and Methods
Selection of Subjects
This study was approved by the Sunnybrook Health Sciences Centre Institutional Research Ethics Board. Semen samples were obtained with patient informed consent from three normozoospermic patients presenting to the Andrology Laboratory at the CReATe Fertility Centre. Each of these patients had a partner with an established cause of infertility.
Semen samples were collected by masturbation after 2 to 5 d of sexual abstinence. The semen was allowed to liquefy at 37°C and was analyzed within 60 min of collection. A routine semen analysis was performed with an evaluation of semen volume, sperm concentration, and sperm motility according to WHO guidelines [World Health Organization Citation1999]. Density gradient centrifugation was performed to separate motile from immotile sperm populations. Briefly, the semen sample was placed on top of 2 mL of 80% gradient medium (Nidacon Intl., Mölndal, Sweden) in a 15ml conical tube, and centrifuged at 1400g for 10 min which allowed the viable, motile sperm cells to move through the gradient medium, forming a pellet. The pellet (motile spermatozoa) and supernatant (immotile spermatozoa) were re-suspended separately in 3 mL of sperm washing medium, then centrifuged at 500g for 10 min, and were processed for FISH analysis.
Sperm nuclear organization assessment by FISH
The motile and immotile samples were processed separately, first by re-suspending in 1X phosphate buffer saline (PBS; Sigma-Aldrich Ltd., Oakville, ON, Canada) and fixed in -20°C Carnoy's solution (3:1 methanol/acetic acid). The fixed sample (10µl) was placed onto a glass slide which was then left on a heat block set at 50°C for 1 min. The slides were dehydrated in increasing concentrations of ethanol (70%, 85%, and 100%) for 2 min.
While nuclear decondensation is undesirable since it can alter the original intact sperm chromatin architecture, it is unavoidable. We have validated several decondensation protocols (unpublished data) and achieved uniform mild decondensation of sperm nuclei with NaOH. Slides were incubated in 0.5M NaOH solution at room temperature for 4 min and dehydrated again. The probe was prepared by mixing 1µl of Human Chromosome Pan-Centromeric probe (Cambio Ltd., Cambridge, UK) and 1µl of α-satellite Centromere Specific Chromosome 17 probe with 2µl of hybridizing solution (Cytocell Ltd., Cambridge, UK). The mixture was heated to 68°C for 5 min to denature the probes. After air drying the slides, 4µl of probe mixture was applied to the slide, covered with round glass cover slips, and sealed with rubber cement. The probe was hybridized at 37°C for 16 h in the Thermobrite slide processing system (Iris International Inc., Westwood, MA, USA) under controlled humidity levels. Two post hybridization washes with NP-40 and SSC buffer were performed to remove unbound probes. Finally, the cells were counter stained with 4',6-diamidino-2-phenylindole (DAPI; Abbott Laboratories, Abbott Park, IL, USA).
The slides were visualized using an Olympus BX61 fluorescent microscope (Olympus Corporation, Tokyo, Japan) with an oil immersion objective X100, equipped with a differential interference contrast (DIC; Chroma Technology Corp, Bellows Falls, VT, USA) and Hamamatsu C8484-15 digital camera (Hamamatsu Corporation, Hamamatsu City, Japan). For each sample, approximately 100 random spermatozoa were analyzed by acquiring 240 images per cell in X, Y, Z dimensions for each of the three fluorochromes and DIC using HCImage software (Hamamatsu Corporation, Bridgewater, NJ, USA). The images were deconvolved using Huygens Deconvolution & Analysis Software (Scientific Volume Imaging, Hilversum, The Netherlands) to recuperate signals from degrading, blurring, and noise and to produce 3D images of sperm nuclei (). Only the spermatozoa with a tail were visualized by DIC, and having an elliptical head shape were included in the analysis; overlapping cells and spermatozoa with no fluorescent signals (not hybridized) were excluded.
Image analyses were performed using IMARIS software (Bitplane Inc., South Windsor, CT, USA). For each cell, the volume of the nuclear head was measured, along with the volume of pan-centromeric signals (green) and the chromosome 17 centromere specific signal (red). The degree of nuclear decondensation was taken into consideration. The volume measurements were normalized using the fold increase to adjust for the decondensation factor. The fold increase was calculated based on the head volume of the average neat spermatozoa without decondensation, in comparison to the head volume of the decondensed spermatozoa. Only spermatozoa with mild decondensation of up to a 3 fold increase were included in the analyses.
Data was analyzed using the SPSS 19.0 statistical computer software (SPSS Inc., Chicago, IL, USA). One way ANOVA testing was used to compare differences in motile and immotile populations. Results were expressed as mean ± SD.
Declaration of interest: The material contained in the manuscript is original, has not been published, has not been submitted or is not being submitted elsewhere. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author contributions: Conceived and designed the experiments: NA, SIM, HR, CLL; Performed the experiments: NA, SIM; Analyzed the data: NA, SIM, SK, HR, CLL; Contributed reagents/ materials/ analysis tools: AG-ML; Wrote the manuscript: SIM.
Abbreviations
| 2D: | = | two-dimensional |
| 3D: | = | three-dimensional |
| ART: | = | assisted reproductive techniques |
| CGH: | = | comparative genomic hybridization |
| CT: | = | chromosome territory |
| DAPI: | = | 4',6-diamidino-2-phenylindole |
| DIC: | = | differential interference contrast |
| DNA: | = | deoxyribonucleic acid |
| FISH: | = | fluorescence in situ hybridization |
| IVF: | = | in vitro fertilization |
| PBS: | = | phosphate buffer saline |
| SD: | = | standard deviation |
| WHO: | = | World Health Organization. |
References
- Aoki, V.W. and Carrell, D.T. (2003) Human protamines and the developing spermatid: their structure, function, expression and relationship with male infertility. Asian J Androl 5:315–324.
- Arpanahi, A., Brinkworth, M., Iles, D., Krawetz, S.A., Paradowska, A., Platts, A.E. et al. (2009) Endonuclease-sensitive regions of human spermatozoal chromatin are highly enriched in promoter and CTCF binding sequences. Genome Res:1338–1349.
- Cremer, T. and Cremer, C. (2001) Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet 2:292–301.
- Cremer, T., Kupper, K., Dietzel, S. and Fakan, S. (2004) Higher order chromatin architecture in the cell nucleus: on the way from structure to function. Biol Cell 96:555–567.
- Cremer, T., Kurz, A., Zirbel, R., Dietzel, S., Rinke, B., Schrock, E., (1993) Role of chromosome territories in the functional compartmentalization of the cell nucleus. Cold Spring Harb Symp Quant Biol 58:777–792.
- Finch, K.A., Fonseka, K.G., Abogrein, A., Ioannou, D., Handyside, A.H., Thornhill, A.R., (2008) Nuclear organization in human sperm: preliminary evidence for altered sex chromosome centromere position in infertile males. Hum Reprod 23:1263–1270.
- Finch, K.A., Fonseka, G., Ioannou, D., Hickson, N., Barclay, Z., Chatzimeletiou, K., (2008) Nuclear organisation in totipotent human nuclei and its relationship to chromosomal abnormality. J Cell Sci 121:655–663
- Fudenberg, G. and Mirny, L.A. (2012) Higher-order chromatin structure: bridging physics and biology. Curr Opin Genet Dev 22:115–124.
- Gurevitch, M., Amiel, A., Ben-Zion, M., Fejgin, M. and Bartoov, B. (2001) Acrocentric centromere organization within the chromocenter of the human sperm nucleus. Mol Reprod Dev 60:507–516.
- Haaf, T. and Ward, D.C. (1995) Higher order nuclear structure in mammalian sperm revealed by in situ hybridization and extended chromatin fibers. Exp Cell Res 219:604–611.
- Hazzouri, M., Rousseaux, S., Mongelard, F., Usson, Y., Pelletier, R., Faure, A.K., (2000) Genome organization in the human sperm nucleus studied by FISH and confocal microscopy. Mol Reprod Dev 55:307–315.
- Ioannou, D., Meershoek, E.J., Christopikou, D., Ellis, M., Thornhill, A.R. and Griffin D.K. (2011) Nuclear organisation of sperm remains remarkably unaffected in the presence of defective spermatogenesis. Chromosome Res 19:741–753.
- Ioannou, D. and Griffin, D.K. (2011) Male fertility, chromosome abnormalities, and nuclear organization. Cytogenet Genome Res 133:269–279.
- Kao, S.H., Chao, H.T., Chen, H.W., Hwang, T.I., Liao, T.L. and Wei, Y.H. (2008) Increase of oxidative stress in human sperm with lower motility. Fertil Steril 89:1183–1190.
- Kumaran, R.I., Thakar, R. and Spector, D.L. (2008) Chromatin dynamics and gene positioning. Cell 132:929–934.
- Manvelyan, M., Hunstig, F., Bhatt, S., Mrasek, K., Pellestor, F., Weise, A., (2008) Chromosome distribution in human sperm - a 3D multicolor banding-study. Mol Cytogenet 1:25.
- McKenzie, L.J., Carson, S.A., Marcelli, S., Rooney, E., Cisneros, P., Torskey, S., (2004) Nuclear chromosomal localization in human preimplantation embryos: correlation with aneuploidy and embryo morphology. Hum Reprod 19:2231–2237.
- McLay, D.W. and Clarke, H.J. (2003) Remodelling the paternal chromatin at fertilization in mammals. Reproduction 125:625–633.
- Mudrak, O., Tomilin, N. and Zalensky, A. (2005) Chromosome architecture in the decondensing human sperm nucleus. J Cell Sci 118:4541–4550.
- Olszewska, M., Wiland, E. and Kurpisz, M. (2008) Positioning of chromosome 15, 18, X and Y centromeres in sperm cells of fertile individuals and infertile patients with increased level of aneuploidy. Chromosome Res 16:875–890.
- Pellestor, F., Andreo, B., Taneja, K. and Williams, B. (2003) PNA on human sperm: a new approach for in situ aneuploidy estimation. Eur J Hum Genet 11:337–341.
- Solovei, I., Schermelleh, L., Düring, K., Engelhardt, A., Stein, S., Cremer, C. and Cremer, T. (2004) Differences in centromere positioning of cycling and postmitotic human cell types. Chromosoma 112:410–423.
- Sun, H.B., Shen, J. and Yokota, H. (2000) Size-dependent positioning of human chromosomes in interphase nuclei. Biophys J 79:184–190.
- Takizawa, T., Meaburn, K.J. and Misteli, T. (2008) The meaning of gene positioning. Cell 135:9–13.
- Tsend-Ayush, E., Dodge, N., Mohr, J., Casey, A., Himmelbauer, H., Kremitzki, C.L., (2009) Higher-order genome organization in platypus and chicken sperm and repositioning of sex chromosomes during mammalian evolution. Chromosoma 118:53–69.
- Ugarkovic, D.I. (2009) Centromere-competent DNA: structure and evolution. Prog Mol Subcell Biol 48:53–76.
- Vavouri, T. and Lehner, B. (2011) Chromatin organization in sperm may be the major functional consequence of base composition variation in the human genome. PLoS Genet 7:e1002036.
- Wiland, E., Zegalo, M. and Kurpisz, M. (2008) Interindividual differences and alterations in the topology of chromosomes in human sperm nuclei of fertile donors and carriers of reciprocal translocations. Chromosome Res 16:291–305.
- World Health Organization (1999) WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction, Cambridge University Press, Cambridge, UK.
- Wu, T.F. and Chu, D.S. (2008) Epigenetic processes implemented during spermatogenesis distinguish the paternal pronucleus in the embryo. Reprod Biomed Online 16:13–22.
- Wykes, S.M. and Krawetz, S.A. (2003) The structural organization of sperm chromatin. J Biol Chem 278:29471–29477.
- Yaron, Y., Kramer, J.A., Gyi, K., Ebrahim, S.A., Evans, M.I., Johnson, M.P. and Krawetz S.A. (1998) Centromere sequences localize to the nuclear halo of human spermatozoa. Int J Androl 21:13–18.
- Zalenskaya, I.A., Bradbury, E.M. and Zalensky, A.O. (2000) Chromatin structure of telomere domain in human sperm. Biochem Biophys Res Commun 279:213–218.
- Zalenskaya, I.A. and Zalensky, A.O. (2004) Non-random positioning of chromosomes in human sperm nuclei. Chromosome Res 12:163–173.
- Zalensky, A.O., Allen, M.J., Kobayashi, A., Zalenskaya, I.A., Balhorn, R. and Bradbury, E.M. (1995) Well-defined genome architecture in the human sperm nucleus. Chromosoma 103:577–590.
- Zalensky, A.O., Breneman, J.W., Zalenskaya, I.A., Brinkley, B.R. and Bradbury, E.M. (1993) Organization of centromeres in the decondensed nuclei of mature human sperm. Chromosoma 102:509–518.
- Zalensky, A. and Zalenskaya, I. (2007) Organization of chromosomes in spermatozoa: an additional layer of epigenetic information? Biochem Soc Trans 35:609–611.
- Zody, M.C., Garber, M., Adams, D.J., Sharpe, T., Harrow, J., Lupski, J.R., (2006) DNA sequence of human chromosome 17 and analysis of rearrangement in the human lineage. Nature 440:1045–1049.
- Zubkova, E.V., Wade, M. and Robaire, B. (2005) Changes in spermatozoal chromatin packaging and susceptibility to oxidative challenge during aging. Fertil Steril 84:1191–1198.