Abstract
Testicular function, specifically, production of testosterone by Leydig cells, diminishes during aging. Estradiol is also produced by the testis and potentially acts in an autocrine or paracrine manner to help regulate spermatogenesis. However, changes in estradiol concentration or receptor expression within the testis during aging remain unclear. This study was designed to test the hypothesis that the estrogen environment of the testis is altered during aging and that these changes are associated with declining sperm production. Sprague Dawley rats were examined at three, 15, 18, and 21 months of age to detail changes in sperm production and testicular concentration of testosterone and estradiol; five rats were used at three and 21 months and three rats were used at 15 and 18 months. Daily sperm production declined 49% from 15 to 21 months of age. Testicular concentrations of estradiol declined 53% from 15 to 21 months of age; testosterone concentrations were not significantly different. These results suggest that declines in intra-testicular estradiol may contribute to declining sperm production. We further tested our hypothesis by treating rats once every third day with a subcutaneous injection of estradiol valerate (1 µg/kg) from 15 to 18 months of age. Estradiol was increased 54% in treated animals while testosterone was unaffected. Estrogen receptor alpha (ESR1) expression was significantly reduced from 15 to 18 months and expression in estrogen-treated animals was significantly higher than age-matched controls. Additionally, ESR1 expression in 18 month treated animals was not different from 15 months of age. Importantly, daily sperm production in 18 month treated animals was 22% higher than age-matched controls; thus, treatment prevented approximately half of the decline observed in control animals. Collectively, our results suggest that estrogen is involved in maintaining optimum spermatogenesis in adult rats and that estrogen treatment may attenuate the age-associated loss in sperm production.
Introduction
Classically, testosterone and other androgens have been associated with the male while estradiol and other estrogens have been associated with the female. However, the testes express functional aromatase [Carpino et al. Citation2001; Carreau et al. Citation2001; Conley et al. Citation1996] and produce estradiol in addition to testosterone [Free and Jaffe Citation1979; Pearl et al. Citation2007; Setchell and Cox Citation1982; Setchell et al. Citation1983; Waites and Einer-Jensen Citation1974]. Additionally, estrogen receptor alpha (ESR1) and beta (ESR2) are expressed within the testis and epididymis of multiple species, including humans and rats [Carreau and Hess Citation2010; Hess et al. Citation2011; Zaya et al. Citation2012], suggesting that the male reproductive tract is both a source and target for estrogen regulation. Data from the estrogen receptor alpha (αERKO) and aromatase (ArKO) knockout mice initially demonstrated that estrogens are important for male reproductive function. Aromatase knockout mice appear to develop normally but become infertile due to a disruption of spermatogenesis [Robertson et al. Citation1999]. The efferent ducts of αERKO mice fail to properly reabsorb testicular fluid creating a back pressure leading to testicular atrophy and infertility [Hess et al. Citation1997]. Mice and rats treated with ICI 182780, an estrogen receptor antagonist, also fail to properly reabsorb fluid from the lumen of the efferent ducts suggesting that the infertility of αERKO mice is due to the lack of estrogen action, rather than developmental problems [Cho et al. Citation2003; Lee et al. Citation2000; Oliveira et al. Citation2001]. Cauda sperm numbers in both αERKO and ICI-treated mice are reduced and the sperm have lower motility and fertilizing ability [Cho et al. Citation2003]. Sperm defects in αERKO mice were recently shown to result from the inability of the epididymal epithelium to maintain an adequate luminal environment [Joseph et al. Citation2010]. Estrogen receptors and aromatase are also expressed by sperm throughout the reproductive tract indicating that the sperm themselves are a potential source and target for estrogen regulation. Interestingly, a correlation was found between sperm aromatase expression and the percentage of sperm with morphological abnormalities [Carreau et al. Citation2009]. In humans, cases of men with aromatase deficiency have been reported and deficiencies in germ cell numbers and motility are indicated in these men [Carani et al. Citation1997; Herrmann et al. Citation2002; Jones et al. Citation2006; Maffei et al. Citation2007].
Testicular steroidogenesis is reduced in aging rats aged 18 months and older leading to decreased concentrations of testosterone in the testis and circulation [Banerjee et al. Citation1998; Zirkin and Chen Citation2000; Zirkin et al. Citation1997]. In humans, free testosterone in the serum declines steadily after 40 years of age [Perheentupa and Huhtaniemi Citation2009; Tenover Citation2003]. Additionally, sex hormone binding globulin increases during aging in humans, likely further reducing the levels of bioavailable steroids [Carreau et al. Citation2004; Perheentupa and Huhtaniemi Citation2009]. Low testosterone is associated with a number of symptoms including decreased energy, decreased libido, reduced muscle mass, and erectile dysfunction (see review by [Zirkin and Tenover Citation2012]). Testicular function is regulated in part by the gonadotropins, LH and FSH, which are secreted from the anterior pituitary. However, changes in pituitary function are likely not responsible for the lower steroidogenic capacity of the Leydig cells suggesting that some mechanism within the testis, or the Leydig cells themselves, is primarily responsible for reduced testosterone production [Chen et al. Citation2002; Wang et al. Citation1999]. Recent data suggest that oxidative stress increases with age and interferes with the activity and expression of steroidogenic enzymes in the Leydig cells, leading to decreased testosterone production [Beattie et al. Citation2013]. Estradiol is also a product of testicular steroidogenesis. Therefore, it is possible that aging of the testis may cause a decrease in estrogen production, but, whether this occurs, remains unclear. Determining whether testicular estradiol declines with age was one of the aims of this study.
In addition to declining steroidogenesis, sperm production is decreased in the human testis with aging [Johnson et al. Citation1984a; Neaves et al. Citation1984; Petersen and Pakkenberg Citation2000]. A number of structural changes have been described that may account for this decrease including fewer premeiotic cells [Johnson et al. Citation1984b], sloughing of germ cells [Paniagua et al. Citation1987a], increased apoptosis [Kimura et al. Citation2003], and decreased number of Sertoli cells [Johnson et al. Citation1984c; Paniagua et al. Citation1987b]. Estradiol stimulates cell proliferation and inhibits apoptosis of immature rat Sertoli cells [Lucas et al. Citation2011; Royer et al. Citation2012], but potential contributions to declining testicular function during aging remains unknown.
While estrogen appears physiologically relevant for male reproduction, its role in spermatogenesis remains ill-defined, especially in adults. Therefore, this study was designed to test the hypothesis that the estrogen environment of the testis is altered during aging and that these changes are associated with declining sperm production. Specifically, the following aims were addressed: (1) does the concentration of estradiol decline during aging; (2) does estrogen receptor expression change during aging; and (3) does treatment with estradiol during aging affect sperm production.
Results
Sperm production
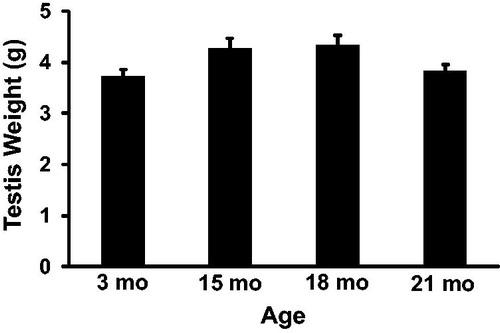
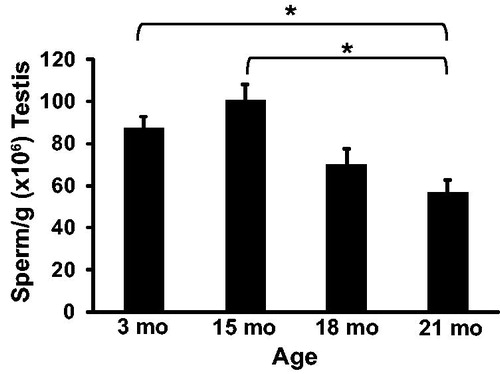
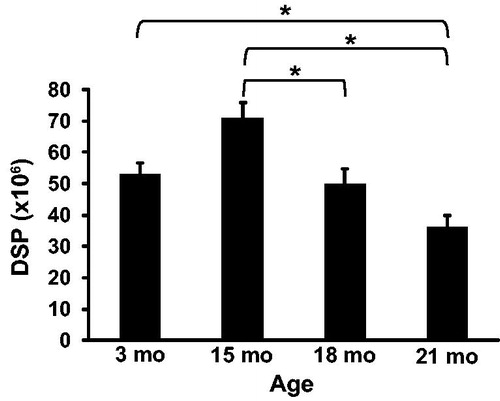
To determine the effect of aging on rat spermatogenesis, detergent resistant spermatids were counted and used to calculate sperm per gram of testis and daily sperm production at 3, 15, 18, and 21 months of age. Daily sperm production () was reduced from 70.98 ± 4.88 million at 15 months of age to 49.82 ± 4.88 million at 18 months of age (p = 0.04) and to 36.15 ± 3.78 million at 21 months of age (p < 0.01). Daily sperm production was also lower at 21 months when compared to 3 months of age (52.89 ± 3.78 million; p = 0.04). Fifteen, 18, and 21 month old rats weighed 571 ± 29, 597 ± 29, and 580 ± 22 grams, respectively. Each of these weights was significantly greater than 3 month old rats (395 ± 22 g), which was expected for Sprague Dawley rats, but were not different from each other. Paired testis weight () was not significantly different between any age group. Sperm per gram of testis () was 56.83 ± 5.81 million at 21 months which was significantly lower than sperm per gram at 3 months (87.12 ± 5.81 million; p = 0.01) and 15 months (100.69 ± 7.5 million; p < 0.01).
Figure 1. Daily sperm production (DSP) in rats. DSP was significantly lower at 18 and 21 months compared to 15 months of age. Additionally, DSP at 21 months was significantly lower than 3 months of age. n = 5 animals per group at 3 and 21 months; n = 3 animals per group at 15 and 18 months. *Indicates p < 0.05.
Testicular steroid concentrations
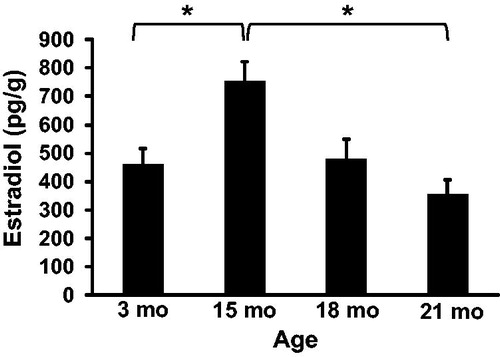
To determine the effect of aging on steroid hormone production intra-testicular concentrations of testosterone () and estradiol () were quantified at 3, 15, 18, and 21 months of age by ELISA. The concentration of testosterone in the testis was 33.1 ± 10.8, 44.7 ± 13.9, 21.6 ± 13.9, and 21.9 ± 10.8 ng/g at 3, 15, 18, and 21 months of age, respectively. While testosterone was numerically highest at 15 months of age, there were no statistical differences between any ages. The concentration of estradiol in the testis increased from 461 ± 54 pg/g at 3 months to 751 ± 70 pg/g at 15 months of age (p = 0.03) and then decreased to 480 ± 70 pg/g at 18 months of age and 354 ± 54 pg/g at 21 months of age. Estradiol trended lower at 18 months of age (p = 0.07) and was significantly lower at 21 months of age (p < 0.01).
Figure 4. Intra-testicular testosterone concentrations. Testosterone concentration was not significantly different between rats at 3, 15, 18, and 21 months of age. n = 5 animals per group at 3 and 21 months; n = 3 animals per group at 15 and 18 months.
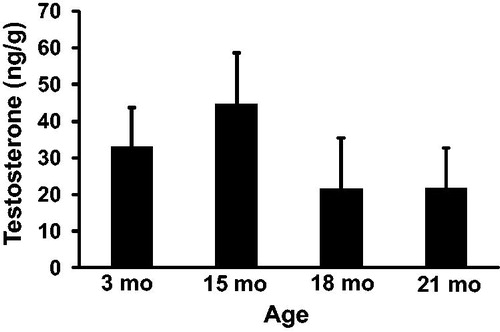
Figure 5. Intra-testicular estradiol concentrations. Estradiol concentration was significantly higher at 15 months compared to 3 months of age. Estradiol concentration was also significantly lower at 21 months compared to 15 months of age. n = 5 animals per group at 3 and 21 months; n = 3 animals per group at 15 and 18 months. *Indicates p < 0.05.
Effects of estrogen treatment
The above data suggest that both daily sperm production and estradiol concentrations decrease during aging. As an initial study to investigate the contribution of estradiol to declining sperm production, we treated animals with estradiol valerate from 15 to 18 months of age. We chose 15 to 18 months because the larger percentage drop in daily sperm production occurred during this time period. Intra-testicular estradiol was 740 ± 97 pg/g in treated animals (). This concentration was significantly higher than age-matched controls (p = 0.04) and numerically similar to the concentration observed at 15 months of age. The concentration of testosterone in the testis of treated animals was not different from age-matched controls (). Daily sperm production was 60.6 ± 0.7 million in estrogen-treated animals which was significantly higher than age-matched controls (p = 0.01; ). Additionally, estrogen treatment appeared to prevent 50% of the decline normally observed from 15 to 18 months of age. Small numerical increases were observed in both paired testis weight and sperm per gram of testis in 18 month treated animals compared with age-matched controls, but these were not statistically significant ().
Table 1. Testicular parameters of 18 month control and estrogen-treated rats (n = 3 per group).
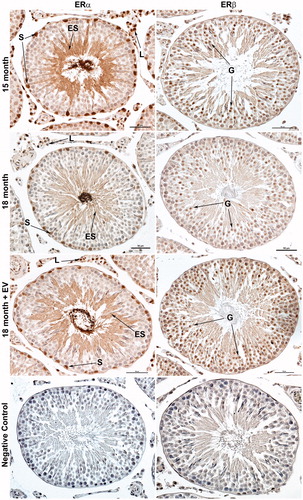
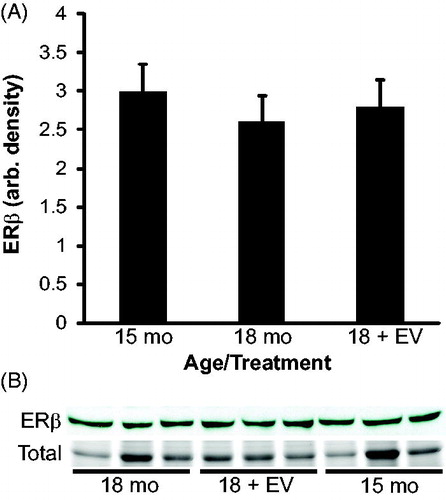
We next asked if expression of estrogen receptor alpha (ESR1) and beta (ESR2) were altered by age or treatment. Estrogen receptor alpha expression was observed in Sertoli cell nuclei, Leydig cell nuclei, and elongating spermatids in all animals (). Expression appeared to be reduced at 18 months compared to 15 months. Estrogen receptor alpha expression also appeared higher in 18 month treated animals when compared to age-matched controls and similar to 15 month animals. Estrogen receptor beta expression was observed primarily in the developing germ cells in all animals () and appeared to be of similar intensity in both ages and treatments. No immunostaining was observed in sections incubated with pre-absorbed antibodies for ESR1 or ESR2.
Figure 6. Immunolocalization of estrogen receptor alpha (ESR1/ERα) and estrogen receptor beta (ESR2/ERβ) in rat testis. Positive immunostaining for ESR1 was observed in Sertoli cell nuclei (S), Leydig cell nuclei (L), and elongated spermatids (ES). The intensity of ESR1 immunostaining appeared weaker in 18 month control animals compared to 15 month or 18 month estrogen-treated (EV) animals. Positive immunostaining for ESR2 was observed primarily in developing germ cells (G) prior to the elongated spermatid stage. The intensity of ESR2 immunostaining appeared similar between all groups. No immunostaining was observed in negative control sections incubated with pre-absorbed primary antibody. Bar = 50 µm.
To confirm the expression results observed by immunohistochemsitry (IHC), western blots were performed. A band corresponding to ESR1 was observed at 66 kD in all animals which is the appropriate molecular weight for ESR1. Results of densitometry analysis for ESR1 agree with the results suggested by immunostaining (). Estrogen receptor alpha expression was reduced from 15 to 18 months of age (p = 0.01). Expression of ESR1 in 18 month treated animals was significantly higher than age-matched controls (p = 0.02) and not different from 15 months of age (p = 0.9). These results suggest that ESR1 expression is reduced during aging and that estrogen treatment prevented the decline in ESR1 expression. A band corresponding to ESR2 was observed at 55 kD in all animals which is the appropriate molecular weight for ESR2 (). Densitometry of ESR2 indicated that expression was similar between ages and treatments.
Figure 7. Densitometry of estrogen receptor alpha (ESR1/ERα) expression in rat testis. A band corresponding to ESR1 (66 kD) was present in each animal. Coomassie staining of membranes (total) confirmed equal protein loading for each animal. Densitometry indicated that ESR1 expression was significantly reduced in 18 month animals compared to either 15 month controls or 18 month estrogen-treated (EV) animals. n = 3 animals per group. a vs. b indicates p < 0.05.
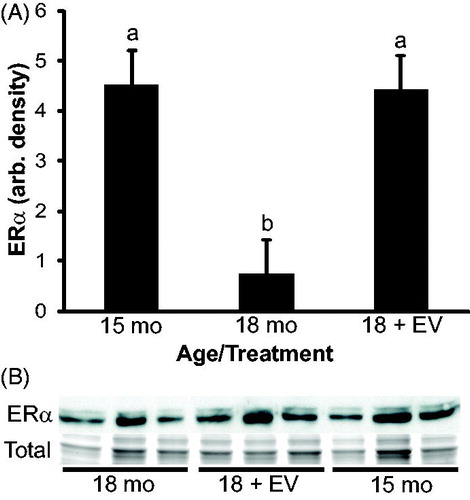
Figure 8. Densitometry of estrogen receptor beta (ESR2/ERβ) expression in rat testis. A band corresponding to ESR2 (55 kD) was present in each animal. Coomassie staining of membranes (total) confirmed equal protein loading for each animal. Densitometry of ESR2 indicated that ESR2 expression was not significantly different between ages or treatments. n = 3 animals per group.
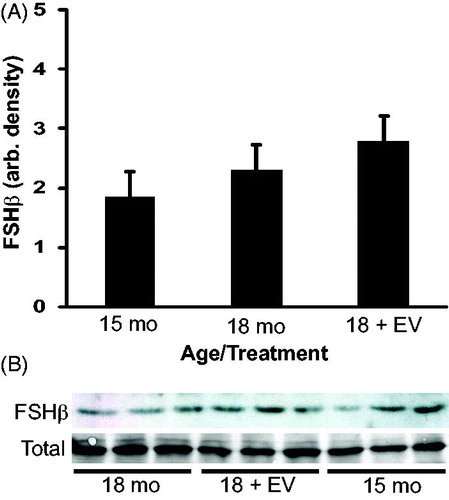
Follicle stimulating hormone (FSH) is important for spermatogenesis and is known to be regulated by estradiol. To investigate the effect of treatment on FSH production the relative amount of FSHβ subunit within the pituitary was quantified by densitometry of western blots. The FSHβ subunit expression was similar between both ages and treatments () suggesting that estrogen treatment from 15 to 18 months did not change FSH production.
Figure 9. Densitometry of follicle stimulating hormone (FSH)β subunit expression in rat pituitary. A band of appropriate molecular weight corresponding to FSHβ was present in each animal. Coomassie staining of membranes (total) confirmed equal protein loading for each animal. Densitometry indicated that FSHβ expression was not significantly different between ages or treatments. n = 3 animals per group.
Discussion
The results reported herein suggest that the estrogen environment of the testis is altered during aging in rats and that these changes likely contribute to declining sperm production. Low dose treatment with estrogen during aging prevented the decline in intra-testicular estradiol concentrations and expression of ESR1 observed in aging rats. Additionally, estrogen supplementation during aging prevented approximately 50% of the decline in daily sperm production observed during aging. Collectively, these results suggest that a normal estrogen environment likely contributes to maintaining optimum levels of spermatogenesis in adults. Additionally, these results provide further support for the hypothesis that in addition to testosterone, estradiol, produced by aromatase in the testis, is critical for male fertility. The 15, 18, and 21 month old rats used in this study correspond to human ages of approximately 47, 57, and 66 years of age, respectively. Bioavailable estradiol has been suggested to decline with age in humans [Carreau et al. Citation2004] and the human testis expresses ESR1 and ESR2 [Filipiak et al. Citation2012; Saunders et al. Citation2002]. Therefore, results from our study could be applicable to aging in humans.
Leydig cell steroidogenesis is impaired during aging leading to decreased testosterone production in rats [Chen et al. Citation2002; Luo et al. Citation2005; Zirkin et al. Citation1993]. While intra-testicular testosterone was numerically reduced in our animals, this reduction was not statistically significant. Importantly, our results suggest that intra-testicular concentrations of estradiol are reduced during aging in Sprague-Dawley rats. Treatment with estradiol valerate prevented the decline in intra-testicular estradiol concentrations but had no effect on testosterone concentrations, suggesting that treatment effects were solely the result of estrogen action and not indirect via androgens. Equally important as serum or tissue concentration for hormone action is the expression of its receptor(s) within the target tissue. Most cell types within the testis express one or both forms of the estrogen receptor but the exact expression pattern appears to vary between, and sometimes within a species (see review by [Carreau and Hess Citation2010]). In our rats, ESR1 was expressed in Sertoli cells, Leydig cells, and elongated spermatids while ESR2 was expressed primarily in pre-elongated spermatid germ cells. Interestingly, our results suggest that ESR1 protein expression was reduced during aging but that ESR2 expression was unaltered. Our results for ESR1 are consistent with previous observations that ESR1 mRNA in the testis is reduced during aging [Hamden et al. Citation2008], but to our knowledge, we are the first to report a change in protein expression in rats. Decreases in ESR1 expression during aging have also been reported in other tissues such as the kidney [Pereira-Simon et al. Citation2012; Sharma and Thakur Citation2004]. Treatment with estradiol valerate during aging prevented the decline in ESR1 expression, but had no effect on ESR2 expression. These results suggest that ESR1 expression is regulated by estradiol and support the notion that ESR2 is constitutively expressed.
Our results for sperm production are consistent with previous reports that have documented decreased sperm numbers in aged humans [Johnson et al. 1984], rats [Lucio et al. Citation2013; Wang et al. Citation1993; Wang et al. Citation1999] and other species such as horses [Johnson and Neaves Citation1981]. Coincident with reduced sperm per gram of testis and daily sperm production were decreased estradiol concentrations and ESR1 expression during aging. In rodent models lacking complete estrogen production such as aromatase knockout (ArKO) mice [Robertson et al. Citation1999], or when estrogen production is reduced by aromatase inhibitors [Kondarewicz et al. Citation2011], spermatogenesis is severely impaired. Treatment with estradiol valerate from 15 to 18 months of age prevented approximately 50% of the normal decline in daily sperm production. Future studies will address if higher doses of estrogen treatment are capable of greater recovery in sperm production. Nevertheless, the current results indicate that alterations in the estrogen environment of the testis during aging are, at least in part, responsible for the reduction in daily sperm production which suggests estrogen is involved in maintaining optimum spermatogenesis in adult males.
Results in the hypogonadal (hpg) mouse [Baines et al. Citation2008] and gonadotropin releasing hormone (GnRH) immunized rat [Meachem et al. Citation2005] models suggest that estrogen acts to increase spermatogenesis by increasing FSH and not directly at the testis. However, this is paradoxical since estradiol inhibits FSH secretion in GnRH intact animals [Ebling et al. Citation2000; Finkelstein et al. Citation1991; Pitteloud et al. Citation2008] suggesting that the stimulatory effect of estrogen on FSH secretion is unique to animals lacking GnRH signaling. While it is possible serum FSH concentrations were increased in our animals, we believe this to be unlikely because our animals had functional GnRH signaling and the dynamics of FSH synthesis and secretion. Follicle-stimulating hormone β subunits assemble very efficiently with the alpha subunit and once the heterodimer is formed, secretion of FSH proceeds via the constitutive pathway with very little storage within the pituitary [Jablonka-Shariff et al. Citation2008; Muyan et al. Citation1994; Pearl et al. Citation2010]. Thus for an increase in serum FSH to occur, expression of the FSHβ subunit would need to be increased. Expression of the FSHβ subunit was not upregulated in our treated animals and thus it is likely serum FSH was unaffected. Therefore, estrogen is likely acting directly at the testis to influence spermatogenesis and not indirectly via FSH in our model.
Germ cell apoptosis appears to occur at a higher rate in the aged testis [Barnes et al. Citation1998; Kimura et al. Citation2003; Wang et al. Citation1999] which is one mechanism by which daily sperm production could be reduced. Estradiol appears to regulate apoptosis of rat Sertoli cells [Royer et al. Citation2012] and inhibited apoptosis of human germ cells in vitro [Pentikainen et al. Citation2000]. Thus, it is possible that reduced estradiol within the testis during aging leads to increased germ cell apoptosis and lower daily sperm production. Estrogen receptor beta knockout (ERβKO) mice are fertile with no apparent defects in spermatogenesis [Zhao et al. Citation2008] and no change was observed in ESR2 expression during aging or after treatment in our animals. Estrogen receptor α expression was reduced during aging and maintained by estrogen treatment in our animals suggesting improved sperm production in our animals is linked to ESR1 expression. Observations in ERα knock-in (ENERKI) mice, which have a mutated form of ESR1, recently indicated that ligand-independent ERα signaling was essential for fluid reabsorption by the efferent ducts, but ligand dependent ERα signaling was involved with germ cell viability [Sinkevicius et al. Citation2009]. Additionally, the testis phenotype observed in ENERKI mice was observed in adult animals suggesting ESR1 is involved with regulating adult spermatogenesis and not a developmental defect. Our results support the hypothesis that estrogen acts via ESR1 ligand-dependent pathways to maintain optimum levels of spermatogenesis in the adult testis and to attenuate the age-associated loss in sperm production.
Collectively, our results suggest that the estrogen environment of the testis is altered with aging and likely contributes to declining sperm production in rats. However, many of the cellular and molecular mechanisms by which estrogen maintains spermatogenesis in rats and humans remain to be elucidated. Most of the current knowledge about estrogen action in the male has been derived from models with complete estrogen removal (e.g. ArKO mice), pharmacologic interventions to block receptor action (e.g. ICI 182780), or administration of supraphysiological doses to normal animals (see reviews by [Carreau and Hess Citation2010; O'Donnell et al. Citation2001]. While these models have established that estrogen is essential for optimum male fertility, an aging model, as described herein, may provide a unique system, with more normal physiologic properties, to elucidate the role of estrogen in adult spermatogenesis.
Materials and Methods
Animals
Male Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA, USA). A total of 19 rats were used for this study and were individually housed with ad libitum access to food and water in the Western Michigan University animal facility until they reached the appropriate age. Animals in each age group were from different litters to account for genetic diversity. Tissues were collected from five animals at three and 21 months of age, and from three animals at 15 and 18 months of age. An additional three animals were injected with estradiol valerate and collected at 18 months of age. Fifteen, 18, and 21 months of age in rats were chosen because they are equivalent to human ages of 47, 57, and 66 years, respectively. Three month old rats are approximately 18–25 years of age in humans. Human age equivalents were calculated as previously described [Quinn Citation2005] assuming a life expectancy of two years for Sprague Dawley rats [Cameron et al. Citation1982] and 76 years for humans [Arias 2008].
Injections were given subcutaneously on the dorsal side once every third day in the early afternoon. Three rats were injected with 1 µg/kg estradiol valerate dissolved in isotonic saline with 1% ethanol from 15 to 18 months of age. Another three rats (vehicle controls) were injected with isotonic saline containing 1% ethanol from 15 to 18 months of age. Estradiol valerate has a slow-release mechanism so that the estrogen was released over time during the three days between injections. Additionally, once in the circulation estradiol valerate is converted to 17β-estradiol [Dusterberg and Nishino Citation1982]. All procedures were conducted with the approval of the Western Michigan University animal care and use committee.
When animals reached the appropriate age, testes were removed and weighed individually; values reported are paired testis weights. One testis from each rat was bisected and each half weighed. One half of the divided testis was frozen in liquid nitrogen and stored at −80 °C while the other half was processed for sperm counts. The other testis was fixed in 4% paraformaldehyde. Tissues from all animals were collected in the early afternoon and tissues from treated animals were collected 24 h after the final injection.
Sperm counts
Sperm production was evaluated by a determination of detergent-resistant spermatids as previously described [Amann and Lambiase Citation1969; Robb et al. Citation1978; Wicks et al. Citation2013]. Briefly, one gram of frozen testicular parenchyma was homogenized in 10 ml of sterile, aqueous 0.9% NaCl/0.05% Triton X-100 at room temperature for 2 min using a OmniTip tissue homogenizer. This homogenate was poured into a 50 ml sterile tube, the homogenization vessel rinsed, and the total volume brought to 20 ml. All homogenates were immediately refrigerated at 4 °C and the number of detergent-resistant spermatids determined 24 h later using a hemocytometer (two counts within 10% for each sample). Daily sperm production was calculated by multiplying sperm/g of testis by the paired testis weight and dividing by the time divisor of 6.1 d. The time divisor is based on the number of days during one cycle of the seminiferous epithelium in which spermatids are in a stage of maturation which makes them resistant to both homogenization and detergent [Robb et al. Citation1978].
Protein and steroid concentrations
Frozen testis samples (≈1 g) were thawed at room temperature and minced into small pieces. These pieces were homogenized in a buffer consisting of 50 mM Tris-Base, 10 mM EDTA, 150 mM NaCl, 0.1% Tween 20 and protease inhibitors (CompleteMini; Roche, Indianapolis, Indiana) at pH 7.6 for approximately 2 min using a OmniTip tissue homogenizer (Bioexpress, Kaysville, UT). This homogenate was centrifuged for 15 min at 2271×g to remove any large portions of unsolubilized tissue. The remaining supernate was then centrifuged at 13,500×g to remove any remaining unsolubilized tissue. Total protein concentration of each homogenate was determined by the BCA Protein Assay (EMD Millipore; Billerica, MA). Testosterone and estradiol concentrations of each testis homogenate were determined using competitive ELISAs following the manufacturer’s instructions (ENZO Life Sciences, Farmingdale, NY; ADI-901-065; ADI-901-174) and normalized to weight of tissue homogenized. The range of the estradiol standard curve was 15.6 to 1,000 pg/ml and assay sensitivity was 13.9 pg/ml. The range of the standard curve for testosterone was 7.81 to 2,000 pg/ml and assay sensitivity was 5.7 pg/ml.
Tissue immunohistochemistry
The localization of ESR1 and ESR2 in the testis was investigated by immunohistochemistry using a protocol previously validated in our lab for rats [Zaya et al. Citation2012]. Based on previous results demonstrating cytoplasmic and nuclear immunostaining of epididymal epithelial cells, we suspect that these antibodies bind to both ligand-bound and ligand-free receptor [Zaya et al. Citation2012]. Tissue was paraffin embedded and sectioned at a thickness of 5 µm. Antigen retrieval was performed by submerging slides in a citric acid based antigen unmasking solution (Vector Laboratories, Inc., Burlingame, CA) in Coplin jars and steam heated to 93 °C for 5 min after which they were allowed to cool to room temperature. After a blocking step, tissues were incubated overnight at 4 °C with antibodies to ESR1 (1:250; MC-20; rabbit anti-mouse ESR1; Santa Cruz Biotechnology, Santa Cruz, CA) or ESR2 (1:250; H-150; rabbit anti- human ESR2; Santa Cruz Biotechnology). Following primary antibody incubation, sections were incubated with goat anti-rabbit biotinylated secondary antibody followed by an avidin–biotin horseradish peroxidase complex (Vector Laboratories). Immunostaining was visualized using DAB chromagen (Vector Laboratories). Pre-absorbed primary antibodies were substituted for primary antibody as a negative control. All slides were counterstained with Immunomaster Hematoxylin and evaluated by light microscopy.
Western blotting
Western blots for ESR1 and ESR2 were performed using a procedure previously validated in our lab for rats [Zaya et al. Citation2012] with the same antibodies used for immunohistochemistry. Western blots for FSHβ were performed using a previously validated antibody from the National Hormone Peptide Program (anti-rat FSHβ IC-1). Equal amounts of protein from each homogenate, determined by the BCA assay, were loaded and separated on a SDS-polyacrylamide gel with a MiniProtean Tetra System (BioRad; Hercules, CA, USA) using Precision Plus Protein molecular weight standards (BioRad) and transferred to an Immobilon–FL membrane (Millipore, Bedford, MA, USA). Membranes were incubated with 5% non-fat dry milk for one h at room temperature to block non-specific binding. Primary antibodies (ESR2, 1:200; ERα, 1:250; FSHβ, 1:5,000) were incubated for two h at room temperature. Membranes were washed and incubated with alkaline phosphatase-conjugated goat anti-rabbit IgG (1:10,000; Applied Biosystems) for one h at room temperature. All membranes were visualized with chemiluminescence (Tropix CSPD with Nitro Block; Applied Biosystems) and exposed on Blue Basic AutoRad film (Bioexpress). Equal protein loading of lanes was confirmed by Coomassie staining of PVDF membranes after protein transfer.
Data analysis
Differences between 3, 15, 18, and 21 month animals were determined by ANOVA using the general linear model statement with SAS statistical analysis software (SAS Release 9.2, SAS Institute, Cary, NC). Data was tested for normality using the Shapiro-Wilk test and was found to be normally distributed. If the overall ANOVA was significant, differences between groups were determined using Tukey’s multiple comparison test. Differences between 18 month control and 18 month estrogen-treated animals were determined using a t-test. Differences in ESR1, ESR2, and FSHβ expression were determined by ANOVA and Tukey’s multiple comparison test. Values are reported as least squares means ± SEM. Significance of data was determined at p < 0.05 for all analyses.
Declaration of interest
The authors report no declarations of interest.
Author contributions
Conceived and designed the experiments, performed the experiments, and analyzed the data: MC, CAP; Wrote the manuscript: CAP.
| Abbreviations | ||
| ESR1 | = | estrogen receptor alpha |
| ESR2 | = | estrogen receptor beta |
| αERKO | = | estrogen receptor knockout |
| ERβKO | = | estrogen receptor beta knockout |
| ArKO | = | aromatase knockout |
| ENERKI | = | estrogen receptor alpha knock in |
| DSP | = | daily sperm production |
| IHC | = | immunohistochemsitry |
Acknowledgments
This work was supported in part by funds from the Faculty Research and Creative Activities Award, Western Michigan University. We thank Lynn Plew for assistance with animal care and Jennifer Long for help performing hormone assays.
References
- Arias, E. (2008) United States Life Tables, 2008. National Vital Statistics Report. 61:1–64
- Amann, R.P., and Lambiase, J.T., Jr. (1969) The male rabbit. 3. Determination daily sperm production by means of testicular homogenates. J Anim Sci 28:369–74
- Baines, H., Nwagwu, M.O., Hastie, G.R., Wiles, R.A., Mayhew, T.M., and Ebling, F.J. (2008) Effects of estradiol and FSH on maturation of the testis in the hypogonadal (hpg) mouse. Reprod Biol Endocrinol 6:1–10
- Banerjee, P.P., Banerjee, S., Lai, J.M., Strandberg, J.D., Zirkin, B.R., and Brown, T.R. (1998) Age-dependent and lobe-specific spontaneous hyperplasia in the brown Norway rat prostate. Biol Reprod 59:1163–70
- Barnes, C.J., Covington, B.W.t., Cameron, I.L., and Lee, M. (1998) Effect of aging on spontaneous and induced mouse testicular germ cell apoptosis. Aging (Milano) 10:497–501
- Beattie, M.C., Chen, H., Fan, J., Papadopoulos, V., Miller, P., and Zirkin, B.R. (2013) Aging and luteinizing hormone effects on reactive oxygen species production and DNA damage in rat leydig cells. Biol Reprod 88:1–7
- Cameron, T.P., Lattuada, C.P., Kornreich, M.R., and Tarone, R.E. (1982) Longevity and reproductive comparisons for male ACI and Sprague-Dawley rat aging colonies. Lab Anim Sci 32:495–9
- Carani, C., Qin, K., Simoni, M., Faustini-Fustini, M., Serpente, S., Boyd, J., et al. (1997) Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 337:91–5
- Carpino, A., Pezzi, V., Rago, V., Bilinska, B., and Ando, S. (2001) Immunolocalization of cytochrome P450 aromatase in rat testis during postnatal development. Tissue Cell 33:349–53
- Carreau, S., Bourguiba, S., Lambard, S., Galeraud-Denis, I., Genissel, C., Bilinska, B., et al. (2001) Aromatase expression in male germ cells. J Steroid Biochem Mol Biol 79:203–8
- Carreau, S., Bourguiba, S., and Marie, E. (2004) Testicular and blood steroid levels in aged men. Reprod Biol 4:299–304
- Carreau, S., Delalande, C., and Galeraud-Denis, I. (2009) Mammalian Sperm Quality and Aromatase Expression. Microscopy Research and Technique 72:552–7
- Carreau, S., and Hess, R.A. (2010) Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci 365:1517–35
- Chen, H., Hardy, M.P., and Zirkin, B.R. (2002) Age-related decreases in Leydig cell testosterone production are not restored by exposure to LH in vitro. Endocrinology 143:1637–42
- Cho, H.W., Nie, R., Carnes, K., Zhou, Q., Sharief, N.A., and Hess, R.A. (2003) The antiestrogen ICI 182,780 induces early effects on the adult male mouse reproductive tract and long-term decreased fertility without testicular atrophy. Reprod Biol Endocrinol 1:1–17
- Conley, A.J., Corbin, C.J., Hinshelwood, M.M., Liu, Z., Simpson, E.R., Ford, J.J., et al. (1996) Functional aromatase expression in porcine adrenal gland and testis. Biol Reprod 54:497–505
- Dusterberg, B., and Nishino, Y. (1982) Pharmacokinetic and pharmacological features of oestradiol valerate. Maturitas 4:315–24
- Ebling, F.J., Brooks, A.N., Cronin, A.S., Ford, H., and Kerr, J.B. (2000) Estrogenic induction of spermatogenesis in the hypogonadal mouse. Endocrinology 141:2861–9
- Filipiak, E., Suliborska, D., Laszczynska, M., Walczak-Jedrzejowska, R., Oszukowska, E., Marchlewska, K., et al. (2012) Estrogen receptor alpha localization in the testes of men with normal spermatogenesis. Folia Histochem Cytobiol 50:340–5
- Finkelstein, J.S., O'Dea, L.S., Whitcomb, R.W., and Crowley, W.F., Jr. (1991) Sex steroid control of gonadotropin secretion in the human male. II. Effects of estradiol administration in normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab 73:621–8
- Free, M.J., and Jaffe, R.A. (1979) Collection of rete testis fluid from rats without previous efferent duct ligation. Biol Reprod 20:269–78
- Hamden, K., Silandre, D., Delalande, C., El Feki, A., and Carreau, S. (2008) Age-related decrease in aromatase and estrogen receptor (ERalpha and ERbeta) expression in rat testes: protective effect of low caloric diets. Asian J Androl 10:177–87
- Herrmann, B.L., Saller, B., Janssen, O.E., Gocke, P., Bockisch, A., Sperling, H., et al. (2002) Impact of estrogen replacement therapy in a male with congenital aromatase deficiency caused by a novel mutation in the CYP19 gene. J Clin Endocrinol Metab 87:5476–84
- Hess, R.A., Bunick, D., Lee, K.H., Bahr, J., Taylor, J.A., Korach, K.S., et al. (1997) A role for oestrogens in the male reproductive system. Nature 390:509–12
- Hess, R.A., Fernandes, S.A., Gomes, G.R., Oliveira, C.A., Lazari, M.F., and Porto, C.S. (2011) Estrogen and its receptors in efferent ductules and epididymis. J Androl 32:600–13
- Jablonka-Shariff, A., Pearl, C.A., Comstock, A., and Boime, I. (2008) A carboxyl-terminal sequence in the lutropin beta subunit contributes to the sorting of lutropin to the regulated pathway. J Biol Chem 283:11485–92
- Johnson, L., and Neaves, W.B. (1981) Age-related changes in the Leydig cell population, seminiferous tubules, and sperm production in stallions. Biol Reprod 24:703–12
- Johnson, L., Petty, C.S., and Neaves, W.B. (1984a) Influence of age on sperm production and testicular weights in men. J Reprod Fertil 70:211–8
- Johnson, L., Petty, C.S., Porter, J.C., and Neaves, W.B. (1984b) Germ cell degeneration during postprophase of meiosis and serum concentrations of gonadotropins in young adult and older adult men. Biol Reprod 31:779–84
- Johnson, L., Zane, R.S., Petty, C.S., and Neaves, W.B. (1984c) Quantification of the human Sertoli cell population: its distribution, relation to germ cell numbers, and age-related decline. Biol Reprod 31:785–95
- Jones, M.E., Boon, W.C., Proietto, J., and Simpson, E.R. (2006) Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol Metab 17:55–64
- Joseph, A., Hess, R.A., Schaeffer, D.J., Ko, C., Hudgin-Spivey, S., Chambon, P., et al. (2010) Absence of estrogen receptor alpha leads to physiological alterations in the mouse epididymis and consequent defects in sperm function. Biol Reprod 82:948–57
- Kimura, M., Itoh, N., Takagi, S., Sasao, T., Takahashi, A., Masumori, N., et al. (2003) Balance of apoptosis and proliferation of germ cells related to spermatogenesis in aged men. J Androl 24:185–91
- Kondarewicz, A., Kolasa, A., Zawislak, B., Baranowska-Bosiacka, I., Marchlewicz, M., Wenda-Rozewicka, L., et al. (2011) Testis morphology in rats chronically treated with letrozole, an aromatase inhibitor. Folia Histochem Cytobiol 49:677–84
- Lee, K.H., Hess, R.A., Bahr, J.M., Lubahn, D.B., Taylor, J., and Bunick, D. (2000) Estrogen receptor alpha has a functional role in the mouse rete testis and efferent ductules. Biol Reprod 63:1873–80
- Lucas, T.F., Pimenta, M.T., Pisolato, R., Lazari, M.F., and Porto, C.S. (2011) 17beta-estradiol signaling and regulation of Sertoli cell function. Spermatogenesis 1:318–24
- Lucio, R.A., Tlachi-Lopez, J.L., Eguibar, J.R., and Agmo, A. (2013) Sperm count and sperm motility decrease in old rats. Physiol Behav 110–111:73–9
- Luo, L., Chen, H., and Zirkin, B.R. (2005) Temporal relationships among testosterone production, steroidogenic acute regulatory protein (StAR), and P450 side-chain cleavage enzyme (P450scc) during Leydig cell aging. J Androl 26:25–31
- Maffei, L., Rochira, V., Zirilli, L., Antunez, P., Aranda, C., Fabre, B., et al. (2007) A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin Endocrinol (Oxf) 67:218–24
- Meachem, S.J., Robertson, D.M., Wreford, N.G., McLachlan, R.I., and Stanton, P.G. (2005) Oestrogen does not affect the restoration of spermatogenesis in the gonadotrophin-releasing hormone-immunised adult rat. J Endocrinol 185:529–38
- Muyan, M., Ryzmkiewicz, D.M., and Boime, I. (1994) Secretion of lutropin and follitropin from transfected GH3 cells: evidence for separate secretory pathways. Mol Endocrinol 8:1789–97
- Neaves, W.B., Johnson, L., Porter, J.C., Parker, C.R., Jr., and Petty, C.S. (1984) Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab 59:756–63
- O'Donnell, L., Robertson, K.M., Jones, M.E., and Simpson, E.R. (2001) Estrogen and spermatogenesis. Endocr Rev 22:289–318
- Oliveira, C.A., Carnes, K., Franca, L.R., and Hess, R.A. (2001) Infertility and testicular atrophy in the antiestrogen-treated adult male rat. Biol Reprod 65:913–20
- Paniagua, R., Martin, A., Nistal, M., and Amat, P. (1987a) Testicular involution in elderly men: comparison of histologic quantitative studies with hormone patterns. Fertil Steril 47:671–9
- Paniagua, R., Nistal, M., Amat, P., Rodriguez, M.C., and Martin, A. (1987b) Seminiferous tubule involution in elderly men. Biol Reprod 36:939–47
- Pearl, C.A., Berger, T., and Roser, J.F. (2007) Estrogen and androgen receptor expression in relation to steroid concentrations in the adult boar epididymis. Domest Anim Endocrinol 33:451–9
- Pearl, C.A., Jablonka-Shariff, A., and Boime, I. (2010) Rerouting of a follicle-stimulating hormone analog to the regulated secretory pathway. Endocrinology 151:388–93
- Pentikainen, V., Erkkila, K., Suomalainen, L., Parvinen, M., and Dunkel, L. (2000) Estradiol acts as a germ cell survival factor in the human testis in vitro. J Clin Endocrinol Metab 85:2057–67
- Pereira-Simon, S., Xia, X., Catanuto, P., and Elliot, S. (2012) Oxidant stress and mitochondrial signaling regulate reversible changes of ERalpha expression and apoptosis in aging mouse glomeruli and mesangial cells. Endocrinology 153:5491–9
- Perheentupa, A., and Huhtaniemi, I. (2009) Aging of the human ovary and testis. Mol Cell Endocrinol 299:2–13
- Petersen, P.M., and Pakkenberg, B. (2000) Stereological Quantification of Leydig and Sertoli Cells in the Testis from Young and Old Men. Image Analysis and Stereology 19:215–9
- Pitteloud, N., Dwyer, A.A., DeCruz, S., Lee, H., Boepple, P.A., Crowley, W.F.Jr., et al. (2008) The relative role of gonadal sex steroids and gonadotropin-releasing hormone pulse frequency in the regulation of follicle-stimulating hormone secretion in men. J Clin Endocrinol Metab 93:2686–92
- Quinn, R. (2005) Comparing rat's to human's age: how old is my rat in people years? Nutrition 21:775–7
- Robb, G.W., Amann, R.P., and Killian, G.J. (1978) Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J Reprod Fertil 54:103–7
- Robertson, K.M., O'Donnell, L., Jones, M.E., Meachem, S.J., Boon, W.C., Fisher, C.R., et al. (1999) Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proc Natl Acad Sci USA 96:7986–91
- Royer, C., Lucas, T.F., Lazari, M.F., and Porto, C.S. (2012) 17Beta-estradiol signaling and regulation of proliferation and apoptosis of rat Sertoli cells. Biol Reprod 86:1–13
- Saunders, P.T., Millar, M.R., Macpherson, S., Irvine, D.S., Groome, N.P., Evans, L.R., et al. (2002) ERbeta1 and the ERbeta2 splice variant (ERbetacx/beta2) are expressed in distinct cell populations in the adult human testis. J Clin Endocrinol Metab 87:2706–15
- Setchell, B.P., and Cox, J.E. (1982) Secretion of free and conjugated steroids by the horse testis into lymph and venous blood. J Reprod Fertil Suppl 32:123–7
- Setchell, B.P., Laurie, M.S., Flint, A.P., and Heap, R.B. (1983) Transport of free and conjugated steroids from the boar testis in lymph, venous blood and rete testis fluid. J Endocrinol 96:127–36
- Sharma, P.K., and Thakur, M.K. (2004) Estrogen receptor alpha expression in mice kidney shows sex differences during aging. Biogerontology 5:375–81
- Sinkevicius, K.W., Laine, M., Lotan, T.L., Woloszyn, K., Richburg, J.H., and Greene, G.L. (2009) Estrogen-dependent and -independent estrogen receptor-alpha signaling separately regulate male fertility. Endocrinology 150:2898–905
- Tenover, J.S. (2003) Declining testicular function in aging men. Int J Impot Res 15 Suppl 4:S3–8
- Waites, G.M., and Einer-Jensen, N. (1974) Collection and analysis of rete testis fluid from macaque monkeys. J Reprod Fertil 41:505–8
- Wang, C., Leung, A., and Sinha-Hikim, A.P. (1993) Reproductive aging in the male brown-Norway rat: a model for the human. Endocrinology 133:2773–81
- Wang, C., Sinha Hikim, A.P., Lue, Y.H., Leung, A., Baravarian, S., and Swerdloff, R.S. (1999) Reproductive aging in the Brown Norway rat is characterized by accelerated germ cell apoptosis and is not altered by luteinizing hormone replacement. J Androl 20:509–18
- Wicks, N., Crouch, S., and Pearl, C.A. (2013) Effects of Improvac and Bopriva on the testicular function of boars ten weeks after immunization. Anim Reprod Sci 142:149–59
- Zaya, R., Hennick, C., and Pearl, C.A. (2012) In vitro expression of androgen and estrogen receptors in prepubertal and adult rat epididymis. Gen Comp Endocrinol 178:573–86
- Zhao, C., Dahlman-Wright, K., and Gustafsson, J.A. (2008) Estrogen receptor beta: an overview and update. Nucl Recept Signal 6:e003
- Zirkin, B.R., and Chen, H. (2000) Regulation of Leydig cell steroidogenic function during aging. Biol Reprod 63:977–81
- Zirkin, B.R., Chen, H., and Luo, L. (1997) Leydig cell steroidogenesis in aging rats. Exp Gerontol 32:529–37
- Zirkin, B.R., Santulli, R., Strandberg, J.D., Wright, W.W., and Ewing, L.L. (1993) Testicular steroidogenesis in the aging brown Norway rat. J Androl 14:118–23
- Zirkin, B.R., and Tenover, J.L. (2012) Aging and declining testosterone: past, present, and hopes for the future. J Androl 33:1111–8