Abstract
The present study evaluates the effect of oxamate derivatives (N-ethyl, N-propyl, N-butyl oxamates) on functional murine sperm parameters, towards a new male non-hormonal contraceptive. These derivatives are selective inhibitors of lactate dehydrogenase-C4 (LDH-C4). LDH-C4 is a sperm-specific enzyme that plays an important role in ATP production for maintaining progressive motility as well as to induce capacitation and hyperactivation. The results demonstrate that all oxamate derivatives selectively inhibited LDH-C4 in mouse sperm extracts. The IC50 values for hexokinase and glyceraldehyde-3-phosphate dehydrogenase were at least an order of magnitude greater than LDH-C4 IC50 values. Prodrugs of oxamate derivatives assayed on sperm cells diminished normal sperm motility parameters, acrosome reaction, and cell viability in a concentration dependent manner. Also, we performed in vivo studies to determine the potential toxicity and possible contraceptive ability of these inhibitors. Mouse sperm were more sensitive to the N-butyl oxamate ethyl ester (NBOXet). Furthermore, results showed that NBOXet was of a low toxicity substance that diminished the total and progressive motility as well as the kinematic parameters of sperm cells. Data from in vitro and in vivo studies showed that N-butyl oxamate and its prodrug, are selective inhibitors of sperm LDH-C4, has low toxicity, and inhibits sperm progressive motility, offering some of the desirable characteristics of a male contraceptive: effect, low toxicity, and selectivity.
Introduction
The control of human fertility is an important global issue because overpopulation and unintended pregnancy have personal and social impact. Fortunately, the notion that contraception is only a woman's responsibility is changing. With this change of attitude, along with progress in understanding male reproductive physiology, there has begun a search for an effective, selective, safe, and reversible male contraceptive agent.
Research in this area includes studies on α-chlorohydrin and deoxysugars with highly unspecific effects [Bone et al. Citation2001; Kawaguchi et al. Citation2004]; different testosterone analogues with a series of negative effects [Nieschlag Citation2009], and immunologic contraception [Gupta Citation1999, Citation2012]. More recently, the inhibitory effect performed by an antagonist of the retinoic acid receptor on spermatogenesis was demonstrated [Chung et al. Citation2011]. However, the discovery of the ideal male contraceptive drug remains a challenge.
Lactate dehydrogenase-C4 (LDH-C4) (EC 1.1.1.27) is an isozyme found in mature testes and spermatozoa of many species with internal fertilization [Blanco et al. Citation1976]. Lactate dehydrogenase-C4 inhibitors and Ldhc null mouse have demonstrated that LDH-C4 performs an essential role in maintenance of glycolysis and ATP production in the flagellum, which are required for progressive and hyperactivated motility, sperm capacitation, and mouse fertility [Odet et al. Citation2008; Odet et al. Citation2011]. These unique functions, along with unusually high cell specificity, have made it a potential target for developing contraceptive methods regulating male fertility.
In previous studies, we reported that oxamate analogues have potential for use in contraception because N-isopropyl and propyl oxamates were selective inhibitors of LDH-C4 from testes [Rodríguez-Páez et al. Citation2011; Wong et al. Citation1997]. Furthermore, N-propyl oxamate (NPOX) significantly reduced ATP levels and capacitation of mouse sperm. N-isopropyl oxamate inhibited basal motility in a concentration- and time-dependent manner. The present study examined the effect of three N-substituted oxamates and their ethyl esters, N-ethyl, N-propyl, and N-butyl oxamates (NEOX, NPOX, and NBOX, respectively), on the enzymatic activity of key glycolytic enzymes besides LDH-C4, such as hexokinase (HK1S) (EC 2. 7. 1. 1) and glyceraldehydes-3-phosphate dehydrogenase (GAPDS) (EC 1. 2. 1. 12) from mouse spermatozoa. Functional sperm parameters were evaluated using produgs of these oxamate analogues.
Results
Determination of the glycolytic enzyme activities
We evaluated the inhibitory potencies of oxamate derivatives on mouse sperm sonicates containing LDH-C4, GAPDS, and HKS1. We used the concentration-response plot for comparing the extent of inhibition. shows the values of the half-maximal inhibitory concentrations (IC50) from NEOX, NPOX, and NBOX, and their respective ethyl esters (NEOXet, NPOXet, NBOXet). All compounds inhibited the three sperm isozymes in a concentration-dependent manner, however, and as expected, LDH-C4 was inhibited to the greatest extent by all compounds, i.e. the oxamate derivatives showed selectivity towards LDH-C4. N-ethyl oxamate was the compound that most effectively inhibited LDH-C4, whereas NPOX and NBOX poorly inhibited LDH-C4 with very similar IC50 values. The IC50 values for LDH-C4 were several times smaller than the IC50 values for the GAPDS, and 2 or 3 orders of magnitude smaller with respect to the HK1S values. N-ethyl oxamate was the most potent inhibitor for all three sperm isozymes, however, it was less selective towards LDH-C4. N-propyl oxamate ethyl ester was the less potent inhibitor towards the sperm isozymes. The most selective oxamate derivative towards LDH-C4was NBOXet. The prodrugs may have exerted their inhibitory effect as such or as the free salts, due to the hydrolytic action of the sperm nonspecific carboxylesterases [Yanagimachi, Citation1994]. The NEOXet and NBOXet prodrugs inhibited LDH-C4 more effectively. However, NEOXet was 2.6 times less potent than the free salt. N-butyl oxamate ethyl ester inhibited LDH-C4 to a similar extent as the acid form. In comparison NPOXet was 4-fold less effective inhibiting LDH-C4 than the acid from which it comes. The inhibitory response produced by the prodrugs on GAPDS was similar in all three cases. Only the acid form of NEOXet was significantly less effective. In contrast the two other prodrugs inhibited GAPDS to the same extent as the acid form. Hexoquinase was only inhibited by NEOXet and its corresponding acid.
Table 1. Inhibition of the oxamate derivatives on glycolytic enzymes from sperm mice extracts.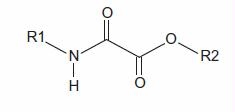 .
.
Evaluation of the motility, viability, and the acrosome reaction
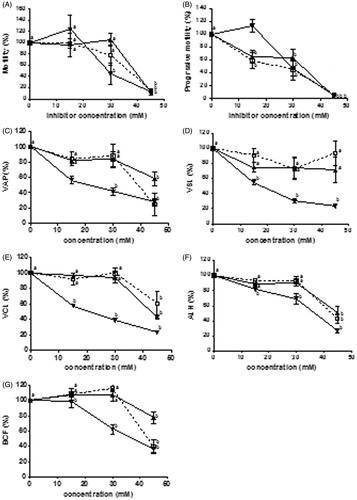
To determine if the inhibition of LDH-C4 provoked alterations in the sperm fertilizing capacity, we assayed the inhibitory effect of the oxamic acid analogues against three fertility parameters: motility, viability, and the acrosome reaction. The normal sperm motility parameters measured by the TOX-IVOS sperm analyzer system were recorded in the presence of the oxamate analogues after 30 min of incubation. Pyruvate and lactate were the energy source. shows the effect of the prodrugs on selected mouse sperm parameters. It was observed that the three compounds inhibited motility in a concentration dependent manner. Sperm motility is close to zero in the presence of any of the three prodrugs at 45 mM. The same effect is observed when progressive motility is measured. The three prodrugs inhibited in a concentration-dependent manner and at 45 mM, progressive motility is essentially zero. With the exception of NEOXet that did not inhibit straight line velocity (VSL) all other prodrugs inhibited all velocity parameters, average path velocity (VAP), VSL, and track speed (VCL), as well as lateral amplitude (ALH) and beat frequency (BCF). These inhibitory effects were concentration-dependent. The most effective prodrug was NBOXet where all kinematic parameters measured were diminished with greater potency than the other compounds (). At 30 mM ethyl N-butyl oxamate, VAP, VSL, VCL, and BCF diminished by more than 50% compared to the control, the only exception being ALH. Higher concentrations were required of NEOXet and NPOXet to achieve the 50% decrease in kinematic parameters.
Figure 1. Effect of N-alkyl oxamate prodrugs on the parameters of mouse sperm motility. Each graph represents percentage of motile sperm after 30 min of incubation in absence and presence of different concentrations of N-alkyl oxamate prodrugs. NEOXet: N-ethyl oxamate ethyl ester (□), NPOXet: N-propyl oxamate ethyl ester (▴), and NBOXet: N-butyl oxamate ethyl ester (▾). (A) total motility (100% means 45.3%), (B) progressive motility (100% means 13.4%), (C) average path velocity (VAP) (100% means 69.9 µm/s), (D) straight line velocity (VSL) (100% means 44.9 µm/s), (E) track speed (VCL) (100% means 141 µm/s), (F) lateral amplitude (ALH) (100% means 10.06 µm), and (G) beat frequency (BCF) (100% means 32.23 Hz). Values are the mean ± SEM, n = 3. (a) means significant difference and (b) means not significant difference. Data analysis was performed using Dunnett’s multiple comparison test, p < 0.05.
We observed that viability diminished in a concentration dependent manner after 30 minute exposure to these prodrugs. As we expected, 45 mM NBOXet decreased viability to the greatest extent, i.e, 57.6% compared to the control (). To determine if these compounds affected the acrosome reaction, mouse sperm were incubated under capacitating conditions and then treated with calcium ionophore A23187, to induce the acrosome reaction. The prodrugs were also added to the medium. The results showed that oxamate analogues inhibited the induction of the acrosome reaction in a concentration dependent manner (). At 45 mM, NBOXet blocked the inhibited the acrosome reactions by approximately 60% as compared to control.
Figure 2. Acrosome reaction in mouse sperm exposed to N-alkyl oxamates prodrugs after capacitating. Bars represent percentage of mouse sperm with induced acrosome reaction with 10 µM calcium ionophore A23187 after 30 min of incubation in absence and presence of N-alkyl oxamate prodrugs. ▪ control, ▪ N-ethyl oxamate ethyl ester (NEOXet), ▪ N-propyl oxamate ethyl ester (NPOXet), ▪ N-butyl oxamate ethyl ester (NBOXet). Values are expressed as mean ± SEM (n = 3). Data analysis was performed using Dunnett’s multiple comparison test, p < 0.05. *: means significant difference.
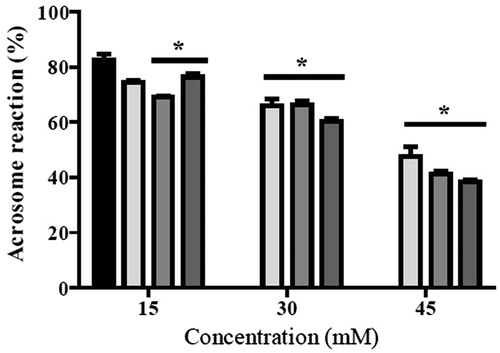
Table 2. Effect of N-alkyl oxamate prodrugs on the mice sperm viability after 30 min incubation.
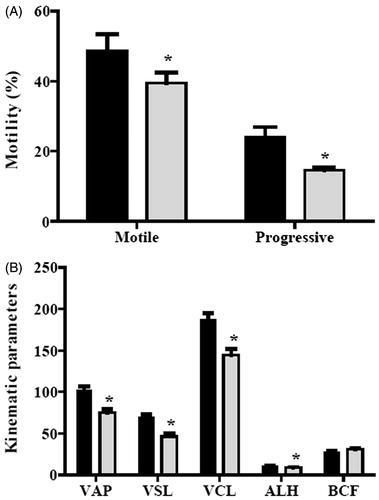
Given the above results, the in vivo effects of the NBOX prodrug were examined. Using the same mouse strain, the NBOXet LD50 was 693.30 mg/kg and thus using the classification of Loomis and Hayes [Loomis and Hayes Citation1996], deemed of low toxicity. Thereafter, a daily single dose of 200 mg/kg of NBOXet was administered intraperitoneally to a test group of 10 male mice, while the vehicle was administered to the same sized control group. Both groups were treated for seven days, taking into account that the epididimal transit time for mouse spermatozoa is five days [Dadoune and Alfonsi Citation1984]. The effect of this compound on spermatozoal motility is shown in . Both total motile sperm and progressively motile sperm decreased in the mice treated with NBOXet. There was a significant 19 % decrease in total motility and a 40 % decrease in progressive sperm from treated mice compared with control mice (). As expected, sperm kinematic parameters, including VAP, VSL, VCL, and ALH except BCF, significantly decreased in the treated mice (). There were no significant differences in the sperm concentration (106/mL) obtained from the vas deferens of both groups (26.8 ± 2.0 control vs. 30.2 ± 1.7 treated).
Figure 3. In vivo effect of N-butyl oxamate ethyl ester (NBOXet) on motility. A) Changes on mouse sperm motility after one week of administration with a single dose of NBOXet and (B) kinematic parameters of mice sperm from vas deferens. ▪ control, ▪ 200 mg/kg; average path velocity (VAP), straight line velocity (VSL), track speed (VCL) (µm/s), lateral amplitude (ALH) (µm), beat frequency (BCF) (Hz). Comparisons were performed using Student t test. *: means significant difference, p < 0.05.
Discussion
We tested the hypothesis that inhibition of LDH-C4 leads to a decrease in ATP production causing inhibition of sperm fertility functions requiring energy. Lactate dehydrogenase-C4 is a sperm-specific enzyme that plays an important role in ATP production for maintaining a progressive motility as well as to induce capacitation and hyperactivation [Goldberg et al. Citation2010; Odet et al. Citation2008; Odet et al. Citation2011]. In previous studies we showed that N-alkyl oxamate derivatives are competitive inhibitors of LDH isozymes. Mice testes LDH-C4 was more sensitive than LDH-A4 and LDH-B4 to inhibition by oxamate derivatives, and were selective inhibitors of LDH-C4, compared with other non-glycolytic mouse dehydrogenases [Rodríguez-Páez et al. Citation2011; Wong et al. Citation1997].
In this investigation, our first experiments were focused on determining the selectivity of NEOX, NPOX, and NBOX towards the mouse sperm LDH-C4, compared with other mouse sperm glycolytic enzymes (GAPDS and HK1S), that like LDH-C4, are exclusively expressed during spermatogenesis [Tanaka and Baba Citation2005]. The results showed that the mouse sperm LDH-C4 form was inhibited by three oxamate derivatives. N-ethyl oxamate inhibited LDH-C4 to the greatest extent in accord with the results previously from mice testes [Rodríguez-Páez et al. Citation2011]. Unexpectedly mouse sperm LDH-C4 was inhibited by NBOX to a greater extent than the mouse testes LDH-C4. It is possible that this result is influenced by the inhibition of the LDH-A4 isoenzyme present in mouse sperm since LDH-A4 was recently found in the principal piece of the flagellum [Odet et al. Citation2011]. Lactate dehydrogenase-A4 from mouse skeletal muscle was inhibited by NBOX although 12 times less than LDH-C4 [Rodríguez-Páez et al. Citation2011]. Hexoquinase and GAPDS sperm-specific glycolytic enzymes, were used as controls and were inhibited by the three oxamate derivatives. GAPDS may have been inhibited by the non-selective interaction of the compounds in the NAD binding site because it is a hydrophobic region where all tested derivatives might interact with the GAPDS [Rossmann et al. Citation1975]. However, the IC50 was at least an order of magnitude greater than that of the LDH-C4 IC50. Thus these three compounds were selective towards LDH-C4.
The inhibitory potential of the ethyl ester prodrugs (es) of the three oxamate derivatives against LDH-C4, HK1S, and GAPDS was examined. Inhibition was lessened and this possibly reflects the time required for the initial enzymatic hydrolysis of the esters to yield the active form. Thus to prodrugs were used for the cellular and in vivo studies to permit sufficient time to permeate the cell’s membranes for subsequent activation to the acid form by spermatozoon carboxyl esterases [Testa and Mayer Citation2003; Yanagimachi Citation1994].
Spermatozoa require high levels of ATP to perform their basic general functions. Adenosine triphosphate is also required for those important functions, i.e. progressive motility, protein tyrosine phosphorylation, hyperactivation/capacitation, the acrosome reaction, and others required to reach and fertilize the egg [De Jonge Citation2005; de Lamirande and O’Flaherty Citation2008; Visconti et al. Citation2002; Yanagimachi Citation1994]. Sperm cells exhibit a great versatility in their metabolism using different mechanisms for energy production using the wide array of substrates available to maintain sperm viability and fertilizing capacity [Goodson et al. Citation2012; Jones and Milmlow Citation1997; Marin et al. Citation2003; Rodriguez-Gil Citation2006; Storey Citation2008]. Mammalian sperm obtains its energy from glycolysable and/or non-glycolysable substrates through glycolysis and/or glycolysis combined with the Krebs cycle and/or the Krebs cycle alone. The equilibrium between both systems is controlled by the specific external conditions encompassing the sperm including medium composition, O2 pressure, extracellular pH, the metabolic phenotype linked to the species studied, and finally, the specific functional status of the sperm at the moment of analysis. Oxidative pathways are very active when sperm energy consumption is elevated. Under these conditions maintenance of the appropriate intracellular ATP level is difficult to control optimally to ensure sperm survival [Piomboni et al. Citation2012; Ruiz-Pesini et al. Citation2007]. Exogenous lactate becomes an essential substrate in the absence of other energy sources to maintain a highly regulated metabolic capacity for the survival of rat sperm [Yamashiro et al. Citation2010], boar sperm [Jones and Milmlow Citation1997], [Medrano et al. Citation2006], and mouse sperm [Mukai and Okuno Citation2004]. Lactate can be introduced inside mouse sperm via specific transporters MCT1 and MCT2 located mainly in the midpiece of the flagellum [Mannowetz et al. Citation2012], ensuring the use of lactate as an energy substrate. The joined operation of the lactate carrier and the cytosolic and mitochondrial isoforms of LDH-C4 allow the transport of lactate from the cytosol for mitochondrial ATP production to cell survival [Burgos et al. Citation1995; Mukai and Okuno Citation2004; Passarella et al. Citation2008].
In this study, we used lactate and pyruvate, non-glycolytic energy substrates to maintain normal motility (progressive) and survival of mouse sperm. Under these conditions the prodrugs of mouse sperm LDH inhibitors, NEOXet, NPOXet, and NBOXet were tested.
Sperm motility is one of the most important physiological parameters that requires high concentrations of ATP. Normal sperm (progressive) motility depends on ATP, principally for the activity of the dynein ATPases, which promote axonemal microtubule sliding. A reduction in ATP content would decrease dynein ATPase activity, which will ultimately immobilize spermatozoa [Inaba Citation2003, Citation2011]. The ATP for hyperactivated motility is generated through glycolysis, and LDH-C4 and GAPDS are enzymes that are essential to synthesize glycolytic ATP to maintain this type of motility [Ford Citation2004; Goldberg et al. Citation2010; Miki et al. Citation2004; Mukai and Okuno Citation2004; Odet et al. Citation2008; Odet et al. Citation2011]. However, glycolytic ATP is not essential to maintain normal motility. Several groups have shown that ATP synthesized by the mitochondria maintains normal sperm motility from different species [Buffone et al. Citation2012; Ford Citation2004; Ruiz-Pesini et al. Citation2007; Goodson et al. Citation2012; Hereng et al. Citation2011; Mukai and Okuno Citation2004; Odet et al. Citation2013].
When we assayed the prodrugs on mouse normal sperm motility using pyruvate and lactate as an energy source, we found that all prodrugs decreased the total and progressive motility as well as kinematic parameters of sperm motility. These included decreased progression (VAP and VSL) and vigorousness parameters (VCL, ALH, and BCF) [Mortimer Citation1997]. That is, the prodrugs impair sperm motility, causing antifertility effects. In previous reports by us and by others, we showed that inhibitors of LDH, diminished ATP levels and motility in mouse and human sperm [Hereng et al. Citation2011; Rodríguez-Páez et al. Citation2002; Rodríguez-Páez et al. Citation2011]. On one hand, the results of this study showed that NBOXet is better than sodium oxamate, a nonspecific inhibitor of LDH isozymes, because 40 mM sodium oxamate inhibited normal motility of human spermatozoa by 31% when incubated in capacitating conditions for 120 min [Hereng et al. Citation2011]. On the other hand, 100 mM sodium oxamate, had no effect on the motility of mouse spermatozoa incubated for two hours in the presence of glucose [Odet et al. Citation2011]. In comparison, others have reported that mouse spermatozoa incubated with >80 mM sodium oxamate in capacitating conditions for 90 min inhibited motility by 100% [Duan and Goldberg Citation2003]. In that article they reported that N-isopropyl oxamate, a selective inhibitor of LDH-C4 from mouse sperm [Wong et al. Citation1997] was a better inhibitor of motility than sodium oxamate, because it required 30 mM N-isopropyl oxamate to completely inhibit mouse sperm motility under the same incubation conditions as sodium oxamate. In a previous study, we found that 33 mM N-isopropyl oxamate ethyl ester inhibited 50% of mouse sperm motility when incubated for 30 min with pyruvate and lactate as the energy source [Itach et al. Citation2012; Medrano et al. Citation2005]. However, 67 mM 1-butanol inhibited total motility of mouse spermatozoa by 100% when incubated in capacitating conditions for 60 min. All these results confirm that (1) the LDH-C4 inhibitors decrease sperm motility, (2) N-alkyl oxamate derivatives are much better inhibitors of sperm motility than oxamate, (3) millimolar concentrations are needed to inhibit sperm motility; (4) one must take into account the different energetic conditions determining motility, and (5) the species-specific differences.
The prodrugs tested in this study diminished sperm viability after a 30 min incubation in a culture medium with pyruvate and lactate as energy substrates. This decrease in viability was concentration dependent on the prodrug, with NBOXet affecting cell viability the most. That viability decreased by inhibition of LDH-C4 demonstrates the importance of LDH-C4 in mitochondrial ATP production for cell survival. Several investigators have demonstrated the importance of lactate as an energy source and LDH-C4 to optimize the survival ability of spermatozoa [Medrano et al. Citation2005; Piomboni et al. Citation2012; Rodriguez-Gil Citation2006; Ruiz-Pesini et al. Citation2007] and the absence of energy substrates causes the death of sperm. Hence, one of the effects of inhibitors of LDH-C4 is the decreased viability of the sperm.
Ejaculated spermatozoa must undergo physiological priming as they traverse the female reproductive tract before they can bind to the egg’s extracellular coat, the zona pellucida, undergo the acrosome reaction, and fertilize the egg [Naz and Rajesh Citation2004]. The preparatory changes are the net result of a series of biochemical and functional modifications collectively referred to as capacitation. A central contributor of capacitation and the acrosome reaction is ATP, emphasizing that the energy supply plays a greater role in these two processes leading to fertilization [Miki Citation2007; Odet et al. Citation2008; Storey Citation2008; Urner and Sakkas Citation2003; Williams and Ford Citation2001]. Our results showed that, in a concentration dependent manner, all prodrugs added after inducing sperm capacitation inhibited the acrosome reaction induced by the calcium ionophore A23187. This effect was as expected since LDH-C4 is an essential enzyme for the synthesis of ATP necessary for fertilization, so these LDH-C4 inhibitors, blocked the acrosome reaction. These results are in agreement with those of O’Flaherty and colleagues, who demonstrated that inhibition of LDH-C4 by 1 mM sodium oxamate, prevents the bovine sperm acrosome reaction induced by heparin, lysophosphatidylcholine, or NADH [O’Flaherty et al. Citation2002; O’Flaherty et al. Citation2005]. It must be noted that capacitating spermatozoa produce controlled amounts of reactive oxygen species (ROS) that are key elements in the regulation of protein kinases associated with the acrosome reaction [de Lamirande and O’Flaherty Citation2008]. O'Flaherty did not focus on the participation of LDH-C4 in the energy supply of sperm, but rather suggested that LDH-C4 may participate in the regulation of the redox status required to achieve the acrosome reaction in bovine spermatozoa [O’Flaherty et al. Citation2005]. We determined the effect of NBOXet on ROS levels in non-capacitated mouse sperm and found that the amount of ROS decreased in a concentration-dependent manner, 45 mM of NBOXet inhibited 50% of ROS (results not shown). These results are consistent with the possible involvement of LDH-C4 in maintaining ROS levels required for the acrosome reaction.
It is noteworthy that sperm undergo the acrosome reaction after they are capacitated. One requirement for induction of capacitation is glucose [Goodson et al. Citation2012; Storey Citation2008; Urner and Sakkas Citation2003; Williams and Ford Citation2001]. N-alkyl oxamates could have exerted its inhibitory effect on the acrosome reaction by blocking the glycolytic production of ATP necessary for capacitation by inhibiting LDH-C4. In another study, we showed that 2.5 mM N-propyl oxamate ethyl ester, inhibited 90% of mouse sperm capacitation [Rodríguez-Páez et al. Citation2011]. Similarly, [Duan and Goldberg Citation2003] were able to block capacitation of mouse sperm by 82% with 50 mM sodium oxamate. According to the above, oxamate derivatives, inhibitors of LDH-C4, could inhibit the acrosome reaction prior to capacitation or directly at the acrosome reaction.
The greater inhibitory effect of NBOXet on the other prodrugs on motility, viability, and the acrosome reaction could be a mixture of various factors such as: (1) this prodrug inhibited sperm LDH with great effectiveness, (2) it is the most hydrophobic of the three prodrugs with enhanced cellular uptake via passive diffusion through the cellular membrane, (3) the differential ease of hydrolysis of the ethyl esters by sperm intracellular esterases, and (4) the toxicity thereof. However, we did not quantify participation for each of these processes.
After performing in vitro studies on some of the biological functions of mouse sperm, we conducted an in vivo study to determine the potential toxicity and possible contraceptive ability of these inhibitors of LDH-C4. The results showed that NBOXet was a low toxic substance (LD50 693.3 mg/kg) and diminished the total and progressive motility as well as the kinematic parameters from vas deferens sperm. N-butyl oxamate ethyl ester administered daily for seven days with 200 mg/kg was the more effective prodrug against mouse sperm LDH-C4 activity, viability, motility, and the acrosome reaction. We previously showed the dose-dependent antifertility effect, i.e., a reduction in the total number of pregnancies, of N-isopropyl oxamate and its prodrug, N-isopropyl oxamate ethyl ester, administered to male mice for 45 days [Rodríguez-Páez et al. Citation2002]. Other studies in humans have associated decreased seminal LDH-C4 activity with decreased sperm motility and infertility [Gavella and Cvitković Citation1985; Sawane et al. Citation2002]. This suggests that the decreased in vivo motility caused by NBOXet occurred because mouse sperm cannot generate sufficient glycolytic or mitochondrial energy when LDH-C4 is inhibited, thereby decreasing progressive motility from vas deferens sperm. This can compromise sperm functions and cause infertility. The fact that this selective LDH-C4 inhibitor is a compound of low toxicity and has an inhibitory effect on mouse sperm motility in vivo, confirms the important role of LDH-C4 in maintaining sperm motility needed to achieve fertility and the possible use of selective LDH-C4 inhibitors on male contraception.
The present study showed that NEOX, NPOX, or NBOX treatment of mice sperm induced a variety of responses. These cells showed different responses to the prodrugs with NBOXet being the most active. The data show that all the oxamate derivatives tested selectively inhibited mouse sperm LDH-C4. The ethylic ester prodrugs of oxamate derivatives assayed on sperm cells diminished normal sperm motility parameters, acrosome reaction, and cell viability. Mouse sperm were more sensitive to NBOXet. These effects were obtained in a concentration-dependent manner. The in vitro and in vivo studies in mice showed that NBOX or its prodrug NBOXet, are selective inhibitors of sperm LDH-C4, with low toxicity that inhibit sperm progressive motility. Both are necessary to attain a potential antifertility effect and are some of the characteristics of an ideal male contraceptive [Mathew and Bantwal Citation2012]. Nevertheless, further in vivo investigations are needed in order to achieve a more detailed analysis of the antifertility potential of these N-alkyl oxamate derivatives.
Materials and Methods
Materials and reagents
All materials and reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA) or Mallinckrodt Baker (Phillipsburg, NJ, USA). Other chemicals used were of the highest purity available.
Animal procedures and sperm preparation
All the experiments were carried out in male adult CD1 albino mice of 20–25 g weight. We obtained these rodents from the ENCB-IPN vivarium. The animals were housed in polypropylene cages at room temperature and under normal light/dark cycle. All animals were fed with a standard pellet diet and water ad libitum. The investigation and animal protocols were approved by The Institutional Ethics Committee of Escuela Nacional de Ciencias Biológicas of the IPN on January the 7th of 2014.
Mouse sperm were collected from vas deferens according to a published procedure [Mújica et al. Citation1991]. Sperm were allowed to disperse for 10 min at 37°C in Tyrode medium of pH 7.6 (120 mM NaCl, 2.8 mM KCl, 11.9 mM NaHCO3, 0.36 mM NaH2PO4, 0.49 mM MgCl2, 0.25 mM sodium pyruvate, 20 mM sodium lactate). The spermatozoa concentration was 8–10 × 106 spermatozoa/mL for each experiment. Parallel incubations with and without inhibitors were started by the additions of the pre-warmed (37°C) medium.
Preparation of the enzyme extracts
LDH-C4, GAPDS, and HK1S were obtained from adult CD1 albino mouse spermatozoa sonicates by the modified method reported by [Duan and Goldberg Citation2003]. This extract was the source of all the enzymes needed to test the selectivity of oxamate derivatives.
General procedure for the preparation of N-alkyl oxamates
All oxamate derivatives (N-ethyl, N-propyl, and N-butyl oxamates) used in this study were synthesized and characterized according to a previously published method [Rodríguez-Páez et al. Citation2011]. The ethyl esters of these oxamate derivatives were used as prodrugs in intact spermatozoa and in vivo studies.
Determination of activities of glycolitic enzymes
The in vitro dose-response effect was determined by using different concentrations of oxamate derivatives and their corresponding ethyl esters (3 to 100,000 µM) dissolved in 2.5% of DMSO. Half-maximal inhibitory concentrations (IC50) were calculated from the concentration-dependent inhibition curves.
LDH-C4
LDH-C4 activity was determined by recording the absorbance change at 340 nm produced by the oxidation of NADH. Assays were performed at 37°C. The reagent mixture contained 0.115 mM NADH, 50 mM sodium phosphate buffer, pH 7.4, 0.31 mM sodium pyruvate, and the enzyme preparation in Tyrode medium pH 7.4, to provide a ΔA340 of 0.06–0.07 per min in a 1 cm light path. The enzyme, the inhibitor (oxamate derivative), and the coenzyme were incubated with the buffer used in the assay for 10 min at 37°C before adding the substrate. IC50 was determined using various concentrations of the oxamate derivatives at a constant substrate concentration [Wong et al. Citation1997].
GAPDS
GAPDS activity was determined by recording the absorbance change at 340 nm produced by the reduction of NAD+. Assays were performed at 37°C. The reagent mixture contained 15 mM NAD+, 50 mM sodium pyrophosphate buffer, pH 8.5, 15 mM glyceraldehyde-3-phosphate, the enzyme preparation in Tyrode medium, pH 7.4, and 0.3 M sodium arsenate to provide a ΔA340 of 0.06–0.07 per min in a 1 cm light path. The enzyme, the substrate, the inhibitors (oxamate derivatives), and the coenzyme were incubated with the buffer used in the assay for 10 min at 37°C before adding the sodium arsenate. IC50 was determined using various concentrations of the oxamate derivatives at a constant substrate concentration [Allison Citation1964].
HK1S
HK1S activity was determined by recording the absorbance change at 340 nm produced by the reduction of NADPH formed by the coupled reaction catalyzed by glucose-6-phosphate dehydrogenase; this enzyme takes the glucose-6-phosphate produced by HK1S and oxidizes it to 6-phosphogluconolactone. The reagent mixture contained 25 mM glucose, 15 mM magnesium chloride, 7 mM ATP, 0.067 mM NADP, and 3 units of glucose-6-phosphate dehydrogenase, all in 1.5 mL 30 mM Tris-HCl, pH 7.5. The mixture was incubated for 5 min at 30°C before the enzyme preparation, in Tyrode medium pH 7.4, was added to provide a ΔA340 of 0.1 per min when the activity was assayed in a 1 cm light path. The enzyme, the substrate, the inhibitors (oxamate derivatives), and the coenzyme were incubated with the buffer used in the assay for 10 min at 37°C before adding the substrate. IC50 was determined using various concentrations of the oxamate derivatives at a constant substrate concentration [Easterby and Qadri Citation1982].
Sperm motility evaluation
The sperm suspensions were incubated in Tyrode medium pH 7.6 at 37°C under noncapacitating conditions (normal motility) during 30 min in presence and absence of different concentrations of the ethyl oxamate derivatives. After incubation, quantitative parameters of sperm motility were determined using a computer-assisted sperm analysis (CASA) instrument (TOX IVOS, software version 12.3; Hamilton Thorne Biosciences, Beverly, MA, USA) [Kato et al. Citation2001; Mortimer Citation2000] in a sample counting chamber pre-warmed to 37°C (MicroCell 20 Micron). Mice sperm motility were recorded at 60 frames/s for one s, analyzing velocities distribution and other kinematic parameters of mice spermatozoa motility: VAP, VSL, VCL, ALH, and BCF. Cells counted as progressive if VAP > 50 µm/s and STR > 50%. VAP cutoff was set at 10 µm/s and VSL cutoff at 0 µm/s. Sperms with VAP < VAP cutoff are excluded from the calculation of average cell velocity. At least 300 spermatozoa and three fields were assessed from each sample. Median values of each of the kinematic parameters were obtained for each sample. Experiments were performed in triplicate.
Sperm viability evaluation
The sperm suspensions were incubated in Tyrode medium pH 7.6 at 37°C during 30 min in presence and absence of different concentrations of the ethyl oxamate derivatives. Immediately after incubation, a double staining of FDA and PI [Jones and Senft Citation1985] was added to the sperm suspensions to evaluate the viability and the plasma membrane integrity. The effect of the oxamate derivatives on the sperm suspensions was measured by flow cytometry using a FASCcalibur flow cytometer (Becton Dickinson Towson, MD, USA), equipped with a laser beam of 488 nm. The results were analyzed with the Cellquest software (Becton Dickinson) [Díaz et al. Citation2004].
Sperm acrosome reaction in vitro
The sperm cells were maintained at 37°C in a modified Tyrode medium (120 mM NaCl, 2.8 mM KCl, 11.9 mM NaHCO3, 0.36 mM NaH2PO4, 0.49 mM MgCl2, 0.25 mM sodium pyruvate, 20 mM sodium lactate, 3 mg/mL BSA, 1 mg/mL glucose, 1.7 mM CaCl2). Sperm were incubated 2 h. Afterwards, 10 µM calcium ionophore A23187 was added to the sperm suspension [Pietrobon et al. Citation2001] and, at same time, different concentrations of the ethyl oxamate derivatives, were added. Then, the sperm suspensions were incubated at 37°C during 30 min. When this time ended, the cells were stained with fluorescein isotiociante-Pissum sativum aglutinina (FITC-PSA). The assay was performed by counting 100 cells per sample in high-magnification at 400x. The samples were counted according to the sperm status characterized by the fluorescence pattern as described by [Cross and Meizel Citation1989].
Evaluation of acute toxicity
The mean lethal dose (LD50) was determined in male CD1 mice weighing 22 ± 3 g. The compound was administered intraperitoneally using isotonic saline solution as a vehicle (1/100 of the animal weight). The number of deaths was registered at 24 h. LD50 was determined according the protocol described by [Klassen Citation2001].
In vivo effect on mouse sperm
To perform in vivo evaluations of the ethyl N-butyl oxamate, two groups were made, control and treated, with ten male CD1 mice weighing from 22 ± 3 g. A daily dose was administered intraperitoneally for 7 d. After the administration period, the animals were sacrificed and the sperm cells obtained from the vas deferens and gently mixed to evaluate the kinematic parameters of sperm motility using the TOX IVOS sperm analyzer system, software version 12.21. Experiments were performed in triplicate.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software). All data are shown as the mean ± SEM. Statistical significance of the results was determined using one-way ANOVA followed by Dunnett test. The t-test was used when comparing the mean differences between motility parameters in treated and untreated mice. Differences were considered significant at p < 0.05.
Declaration of interest
This work was partially supported by research grants from the Secretaría de Investigación y Posgrado del Instituto Politécnico Nacional (SIP-IPN), México. CW and LRP are fellows of the COFAA-IPN and of the SNI-CONACyT. JCM and ChAA were fellows of the CONACyT and PIFI-IPN. The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported in the present manuscript.
Author contributions
Performed the research: JCM; Coached JCM with the experiments and thoroughly revised the manuscript: LRP; Helped with the data analysis and thoroughly revised the manuscript: ChAA; Designed the experiments: CW.
| Abbreviations | ||
| LDH-C4 | = | lactate dehydrogenase-C4 |
| GAPDS | = | glyceraldehyde-3-phosphate dehydrogenase |
| HK1S | = | hexoquinase |
| NEOX | = | N-ethyl oxamate |
| NPOX | = | N-propyl oxamate |
| NBOX | = | N-butyl oxamate |
| NEOXet | = | N-ethyl oxamate ethyl ester |
| NPOXet | = | N-propyl oxamate ethyl ester |
| NBOXet | = | N-butyl oxamate ethyl ester |
| VAP | = | average path velocity |
| VSL | = | straight line velocity |
| VCL | = | track speed |
| ALH | = | lateral amplitude |
| BCF | = | beat frequency |
| LD50 | = | lethal dose 50% |
| IC50 | = | half maximal inhibitory concentration |
| ROS | = | reactive oxygen species |
References
- Allison, M. (1964) Carbohydrates Metabolism. In: Methods in enzymology. Vol. 9. Wood, W.A., ed. New York: Academic Press Inc, pp. 210--215
- Blanco, A., Burgos, C., Gerez de Burgos, N.M. and Montamat, E.E. (1976) Properties of testicular lactate dehydrogenase isoenzyme. Biochem J 153:165–72
- Bone, W., Jones, A., Morin, C., Nieschlag, E. and Cooper, T. (2001) Susceptibility of glycolytic enzyme activity and motility of spermatozoa from rat, mouse, and human to inhibition by proven and putative chlorinated antifertility compounds in vitro. J Androl 22:464–70
- Buffone, M.G., Ijiri, T.W., Cao, W., Merdiushev, T., Aghajanian, H.K. and Gerton, G.L. (2012) Heads or tails? Structural events and molecular mechanisms that promote mammalian sperm acrosomal exocytosis and motility. Mol Reprod Dev 79:4–18
- Burgos, C., Maldonado, C., Gerez de Burgos, N.M., Aoki, A. and Blanco, A. (1995) Intracellular localization of the testicular and sperm-specific lactate dehydrogenase isozyme C4 in mice. Biol Reprod 53:84–92
- Chung, SS., Wang, X., Roberts, S.S., Griffey, S.M. Reczek, P.R. and Wolgemuth, D.J. (2011) Oral administration of a retinoic acid receptor antagonist reversibly inhibits spermatogenesis in mice. Endocrinology 152:2492–502
- Cross, N.L. and Meizel S. (1989) Methods for evaluating the acrosomal reaction status for mammalian sperm. Biol Reprod 41:635–41
- Dadoune, J.P. and Alfonsi, M.F. (1984) Autoradiographic investigation of sperm transit through the male mouse genital tract after tritiated thymidine incorporation. Reprod Nutr Dev 24:927–35
- De Jonge, C. (2005) Biological basis for human capacitation. Hum Reprod Update 11:205–14
- de Lamirande, E. and O'Flaherty, C. (2008) Sperm activation: role of reactive oxygen species and kinases. Biochim Biophys Acta 1784:106–15
- Díaz, T.M., Moscoso, I., Centeno, A., López-Peláez, E., Ortega, D. and Doménech, N. (2004) Flow cytometry complement-mediated cytotoxicity assay detects baboon xenoantibodies directed to porcine epitopes undetected by hemolytic assay. Transpl Immunol 13:313–17
- Duan, C. and Goldberg, E. (2003) Inhibition of lactate dehydrogenase C4 (LDH-C4) blocks capacitation of mouse sperm in vitro. Cytogenet Genome Res 103:352–9
- Ford, W.C. (2004) Regulation of sperm function by reactive oxygen species. Hum Reprod 10:387–99
- Easterby, J.S. and Qadri, S.S. (1982) Carbohydrates Metabolism. In: Methods in enzymology. Vol. 90. Wood, W.A., ed. New York: Academic Press Inc, pp. 11--16
- Gavella, M. and Cvitković, P. (1985) Semen LDH-X deficiency and male infertility. Arch Androl 15:173–6
- Goldberg, E., Eddy, E.M., Duan, C. and Odet, F. (2010) LDHC: The ultimate testis specific gene. J Androl 31:86–94
- Goodson, S.G., Qiu, Y., Sutton, K.A., Xie, G., Jia, W. and O'Brien, D.A. (2012) Metabolic substrates exhibit differential effects on functional parameters of mouse sperm capacitation. Biol Reprod 87:75
- Gupta, G.S. (1999) LDH-C4: a unique target of mammalian spermatozoa. Crit Rev Biochem Mol Biol 34:361–85
- Gupta, G.S. (2012) LDH-C4: a target with therapeutic potential for cancer and contraception. Mol Cell Biochem 371:115–27
- Hereng, T.H., Elgstøen, K.B., Cederkvist, F.H., Eide, L., Jahnsen, T., Skålhegg, B.S., et al. (2011) Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa. Hum Reprod 26:3249–63
- Inaba, K. (2003) Molecular architecture of the sperm flagella: molecules for motility and signaling. Zoo Sci 20:1034–56
- Inaba, K. (2011) Sperm flagella: comparative and phylogenetic perspectives of protein components. Mol Hum Reprod 17:524–38
- Itach, S.B., Finklestein, M., Etkovitz, N. and Breitbart, H. (2012) Hyper-activated motility in sperm capacitation is mediated by phospholipase D-dependent actin polymerization. Dev Biol 362:154–61
- Jones, A.R. and Milmlow, D. (1997) Endogenous energy production by mature boar spermatozoa. J Reprod Fertil 111:285–90
- Jones, K.H. and Senft, J.A. (1985) An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem 33:77–9
- Kato, M., Fukunishi, K., Ikegawa, S., Higuchi, H., Sato, M., Horimoto, M., et al. (2001) Overview on rat sperm motion analysis using a Hamilton-thorn sperm analyzer collaborative working study. J Toxicol Sci 5:285–97
- Kawaguchi, T., Kawachi, M., Morikawa, M., Kazuta, H., Shibata, K., Ishida, M., et al. (2004) Key parameters of spem motion in relation to male fertility in rats given α-chlorhydrin or nitrobenzene. J Tox Sci 29:217–31
- Klassen, C.D. (2001) Toxicology. The Basic Science of Poisons. 6th ed. New York, USA: Mc Graw Hill
- Loomis, T. and Hayes, A. (1996) Loomis’ essentials of toxicology. 4th ed. San Diego, California, USA: Academic Press
- Mannowetz, N., Wandernoth, P. and Wennemuth, G. (2012) Basigin interacts with both MCT1 and MCT2 in murine spermatozoa. J Cell Physiol 227:2154–62
- Marin, S., Chiang, K., Bassilian, S., Lee, W.N., Boros, L.G., Fernández-Novell, J.M., et al. (2003) Metabolic strategy of boar spermatozoa revealed by a metabolomic characterization. FEBS Lett 554:342–6
- Mathew, V. and Bantwal, G. (2012) Male contraception. Indian J Endocrinol Metab 16:910–7
- Medrano, A., Fernández-Novell, J.M., Ramió, L., Alvarez, J., Goldberg, E., Montserrat-Rivera, M., et al. (2006) Utilization of citrate and lactate through a lactate dehydrogenase and ATP-regulated pathway in boar spermatozoa. Mol Reprod Dev 73:369–78
- Medrano, A., Peña, A., Rigau, T. and Rodrìguez-Gil, J.E. (2005) Variations in the proportion of glycolytic/non-glycolytic energy substrates modulate sperm membrane integrity and function in diluted boar samples stored at 15–17 degrees C. Reprod Domest Anim 40:448–53
- Miki, K., Qu, K., Goulding, E.H., Willis, W.D., Bunch, D.O., Strader, L.F., et al. (2004) Glyceraldehyde-3-phosphate dehydrogenase-S, a sperm specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Nat Acad Sci USA 101:16501–6
- Miki, K. (2007) Energy metabolism and sperm function. Soc Reprod Fertil 65:309–25
- Mortimer, S.T. (1997) A critical review of the physiological importance and analysis of sperm movement in mammals. Hum Reprod Update 3:403–39
- Mortimer, S.T. (2000) CASA–practical aspects. J Androl 4:515–24
- Mújica, A., Moreno-Rodríguez, R., Naciff, J., Neri, L. and Tash, J.S. (1991) Glucose regulation of guinea-pig sperm motility. J Reprod Fertil 92:75–87
- Mukai, C. and Okuno, M. (2004) Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod 71:540–7
- Naz, R.K. and Rajesh, P.B. (2004) Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod Biol Endocriol 2:75–98
- Nieschlag, E. (2009) Male hormonal contraception: Love’s labour’s lost? J Clin Endocrinol Metab 94:1890–2
- Odet, F., Duan, C., Willis, W.D., Goulding, E.H., Kung, A., Eddy, E.M., et al. (2008) Expression of the gene of mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol Reprod 79:26–34
- Odet, F., Gabel, S.A., Williams, J., London, R.E., Goldberg, E. and Eddy, E.M. (2011) Lactate dehydrogenase C and energy metabolism in mouse sperm. Biol Reprod 85:556–64
- Odet, F., Gabel, S., London, R.E., Goldberg, E. and Eddy, E.M. (2013) Glycolysis and mitochondrial respiration in mouse LDHC-null sperm. Biol Reprod 88:1--7
- O’Flaherty, C.M., Beorlegui, N.B. and Beconi, M.T. (2002) Lactate dehydrogenase-C4 is involved in heparin-and NADH-dependent bovine sperm capacitation. Androl 34:91–7
- O’Flaherty, C.M., Breininger, E., Beorlegui, N.B. and Beconi, M.T. (2005) Acrosome reaction in bovine spermatozoa: role of reactive oxygen species and lactate dehydrogenase C4. Biochim Biophys Acta 1726:96–101
- Passarella, S., de Bari, L., Valenti, D., Pizzuto, R., Paventi, G. and Atlante, A. (2008) Mitochondria and L-lactate metabolism. FEBS Lett 582:3569–76
- Pietrobon, E.O., Domínguez, L.A., Vicenti, E.A., Burgos, H.M. and Fornés, W.M. (2001) Detection of mouse acrosome reaction by acid phosphatase. Comparision with chlortetracycline and electron microscopy. J Androl 22:96–103
- Piomboni, P., Focarelli, R., Stendardi, A., Ferramosca, A. and Zara, V. (2012) The role of mitochondria in energy production for human sperm motility. Int J Androl 35:109–24
- Rodriguez-Gil, J.E. (2006) Mammalian sperm energy resources management and survival during conservation in refrigeration. Reprod Domest Anim 2:11–20
- Rodríguez-Páez, L., Guzmán-Ibarra, R., Acuña-González, C., Santillán-Báez, A., Moreno-Rodríguez, R. and Wong, C. (2002) The study of N-isopropyl oxamate on sperm motility and fertility, in mice. Proc West Pharmacol Soc 45:171–3
- Rodríguez-Páez, L., Chena-Taboada, M.A., Cabrera-Hernández, A., Cordero-Martínez, J. and Wong, C. (2011) Oxamic acid analogues as LDH-C4-specific competitive inhibitors. J Enzyme Inhib Med Chem 26:579–86
- Rossmann, M.G., Liljas, A., Branden, C.I. and Banaszak, L.J. (1975) Evolutionary and structural relationships among dehydrogenases. In: The Enzymes, Vol XI. Boyer, P.D., ed. New York: Academic Press, pp. 61–102
- Ruiz-Pesini, E., Díez-Sánchez, C., López-Pérez, M.J. and Enríquez, J.A. (2007) The role of the mitochondrion in sperm function: is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr Top Dev Biol 77:3–19
- Sawane, M.V., Kaore, S.B., Gaikwad, R.D., Patil, P.M., Patankar, S.S. and Deshkar, A.M. (2002) Seminal LDH-C4 isoenzyme and sperm mitochondrial activity: a study in male partners of infertile couples. Indian J Med Sci 56:560–6
- Storey, B.T. (2008) Mammalian sperm metabolism: oxygen and sugar, friend and foe. Int J Dev Biol 52:427–37
- Tanaka, H. and Baba, T. (2005) Gene expression in spermiogenesis. Cell Mol Life Sci 62:344–54
- Testa, B. and Mayer, J.M. (2003) The hydrolysis of carboxylic acid ester prodrugs. In: Hydrolysis in drug and prodrug metabolism. 1st ed. Testa, B. and Mayer, J.M., eds. Zürich, Switzerland: Verlag Helvetica Chimica Acta, pp. 419--534
- Urner, F. and Sakkas, D. (2003) Protein phosphorylation in mammalian spermatozoa. Biol Reprod 125:17–26
- Visconti, P.E., Westbrook, V.A., Chertihin, O., Demarco, I., Sleight, S. and Diekman, A.B. (2002) Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol 53:133–50
- Williams, K.M. and Ford, W.C. (2001) The motility of demembranated human spermatozoa is inhibited by free calcium ion activities of 500 nmol/L or more. Int J Androl 24:216–24
- Wong, R.C., Rodríguez-Páez, L., Nogueda, B., Pérez, O.A. and Baeza, R.I. (1997) Selective inhibition of the sperm-specific lactate dehydrogenase isozyme-C4 by N-isopropyl oxamate. Biochem Biophys Acta 1343:16–22
- Yamashiro, H., Toyomizu, M., Kikusato, M., Toyama, N., Sugimura, S., Hoshino, Y., et al. (2010) Lactate and adenosine triphosphate in the extender enhance the cryosurvival of rat epididymal sperm. J Am Assoc Lab Anim Sci 49:160–6
- Yanagimachi, R. (1994) Mammalian fertilization. In: The Physiology of Reproduction. Knobil, E. and Neill, J., eds. FL, USA: Raven Press, pp. 189–317
