Abstract
Our aim was to test the influence of cold ischaemia on replanted limbs, focusing on muscular atrophy and neurological recovery. Inbred wild-type and green fluorescent protein (GFP) transgenic (Tg) Lewis rats aged 8–10 weeks were used. The amputated limbs were transplanted at several cold ischaemic times (0, 1, 8, 12, 24, 48, and 72 hours). An arterial ischaemic model and a denervation model were used as controls. To study nerve regeneration, a GFP limb was transplanted on to the syngenic wild Lewis rat. These animals were evaluated histologically, electrophysiologically, and immunohistochemically. The longer the ischaemic time, the more evident was atrophy of the muscles. Electrophysiological investigation showed that the latency at 3 weeks was longer in the transplantation models than in the normal controls, particularly in the longer ischaemia group. Larger numbers of migrating Schwann cells were seen in the group with no delay than in the group that had been preserved for 12 hours. Ischaemia after amputation of a limb causes muscle cells to necrose and atrophy, and these changes worsen in proportion to the ischaemic preservation time. A delay in nerve regeneration and incomplete paralysis caused by malregeneration also affect muscular atrophy.
Introduction
With the advances in vascular surgical techniques, replantation after major amputation of a limb has become relatively common in microsurgical centres. Amputated tissues have been thought to be tolerant of ischaemia for 4–6 hours of normothermia and 12–24 hours of hypothermia [Citation1–4]. However, poor functional results are still seen as a result of insufficient recovery of motor neurons, joint contractures, and muscular atrophy [Citation3].
Skeletal muscle is most sensitive to ischaemia, so the success of its replantation greatly depends on minimisation of ischaemia. Muscular atrophy in replants can be either vascular or neurogenic [Citation5]. The degree of function in the replanted limb is dependent on preserved nerve function and range of movement of the joints in addition to the quantity of surviving muscle cells. The purpose of this study was to test the influence of the cold ischaemic time of hind-limb grafts and study the underlying mechanisms of regaining function of the limbs. Muscular atrophy of the grafted limb and neurological recovery were examined both histologically and electrophysiologically.
Materials and methods
Animals
Male wild-type rats (an inbred strain of Lewis rats) aged 8–10 weeks and weighing 250–320 g were used throughout the study. To assess nerve regeneration, green fluorescent protein (GFP) transgenic (Tg) Lewis rats [Citation6,7] aged 8 weeks were used as recipients in Group D, described below.
Experimental groups
The animals operated on were divided into four groups.
Group A: Syngenic transplantation in a Lewis to Lewis combination; the right hind limb was amputated at the mid-thigh level. The amputated limbs were preserved at 4°C for 0, 1, 8, 12, 24, 48, and 72 hours (n = 3 each), then transplanted to other Lewis rats using microsurgical techniques [Citation8]. Group B: An arterial ischaemic model (n = 3) used as a control. By ligation of collateral and re-entry vessels, we reduced the flow to 37% of the control value [Citation9]. Group C: A denervation model (n = 3) was used as a second control in which the sciatic nerve was resected 10 mm at the mid-thigh. Group D: To study the influence of ischaemia on nerve regeneration, the right hind limb was transplanted using wild-type Lewis rats as donors and GFP transgenic rats as recipients. The amputated limbs were transplanted after preservation for 0 and 12 hours (n = 3 each).
Operations
Transplantation models: Transplantation of the rat hind limb was as previously shown [Citation8]. Briefly, the rats were anaesthetised with sodium pentobarbital 50 mg/kg intraperitoneally. In the transplantation model a skin incision was made around the mid-thigh where the femoral vessels and sciatic nerve were prepared and divided. The amputated limbs were wrapped in wet gauze and immediately preserved in a refrigerator (at 4°C). We did not use a preservative solution. The recipient limbs were amputated in the same manner as the donors. Intramedullary fixation of the femur was first achieved with an 18-G needle; the muscles were approximated; the sciatic nerve, femoral artery, and vein were anastomosed by microsurgical techniques; and finally the skin was closed.
Ischaemic models: Procedures followed the protocol of Seifert et al. [Citation9].
A midline laparotomy was made, and within the left retroperitoneum, the testicular artery and all lumbar arteries below the renal artery were ligated and divided. The inferior mesenteric artery, caudal artery, and left iliac artery were ligated and divided. The viscera were then returned to the abdominal cavity and the incision closed. Five days after the first operation the left femoral artery was ligated through an oblique inguinal incision.
In denervation models [Citation10] the left sciatic nerve was exposed by splitting the gluteal muscle, a 10 mm segment of the nerve was resected proximal to the bifurcation, and the skin was closed.
Evaluation
Animals in Groups A and B were evaluated both electrophysiologically and histologically to assess the influence of ischaemia. Animals in Groups C and D were evaluated histologically alone.
Histological examination
Biopsy specimens from the gastrocnemius muscle were harvested 8 weeks after the operation. Specimens were fixed in 4% paraformaldehyde for 24 hours, and stored in 20% sucrose for 24 hours and 30% sucrose for a further 24 hours. Transverse 10-µm serial sections were cut on a Cryostat for routine haematoxylin and eosin staining.
One hundred randomly selected cross-sectional diameters of muscle cells were measured in 10 fields (×400).
Electrophysiological measurements
Electrophysiological recordings were made 2, 3, 4, and 8 weeks after the operation. An evoked electromyography was recorded with needle electrodes. The sciatic nerve was stimulated with the cathode placed 10 mm proximal to the sciatic nerve suture (20–30 µA monophasic pulses at 1 Hz; pulse duration, 20 ms). The recording electrode was attached to the belly of the gastrocnemius. The distance between the stimulating and recording sites was standardised at 20 mm to compare distal motor latencies. Electrical activity recorded from the gastrocnemius muscle was amplified (filter setting, 20 Hz to 20 kHz) and stored.
Assessment of nerve regeneration
Sutured sciatic nerves were removed from the animals in Group D 2 weeks after the operation. The specimens were fixed in 4% paraformaldehyde for 24 hours and stored in 20% sucrose for 24 hours, and 30% sucrose for a further 24 hours. Cross sections were cut at the point 5 mm distal to the suture. Expression of the GFP gene was detected using a fluorescence microscope under a 489-nm wavelength excitation light. Immunohistochemical analysis was used to characterise the migrating GFP cells. After blocking non-specific reactions, the specimens were incubated in phosphate buffered saline (PBS) containing polyclonal rabbit anti-S100 protein antibody (1:500, Dako, Osaka, Japan) for 1 hour. After they had been washed with PBS, they were incubated with anti-rabbit IgG (1:500, Vector Laboratories, Burlingame, Calif, USA) for 1 hour. After washing with PBS, they were incubated with secondary antibodies labelled with horse-radish peroxidase (HRP) (1:200, Vector Laboratories, Burlingame, Calif, USA) for 1 hour, and incubated in DAB-H202 (30% H2O2 3.3 µl/ DAB 5 mg/ PBS 10 ml) for 3 minutes. They were then counterstained with haematoxylin for 1 minute, dehydrated, clarified with xylene, and covered with a glass. Numbers of immunohistochemical and fluorescence-stained cells were counted.
Statistics
The data were analysed with the help of a paired Student's t test and multiple comparison analysis to assess the significance of differences between groups. Probabilities of less than 0.05 were accepted as significant.
Results
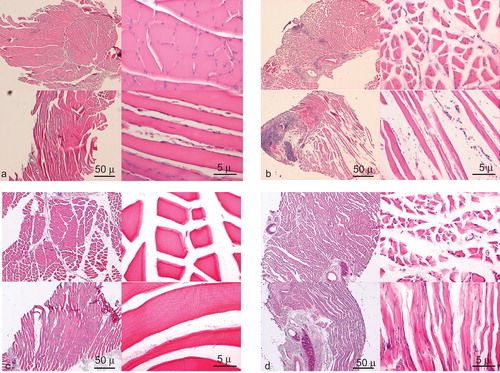
Histological studies obtained from Group A showed no distinguishable changes from normal muscle at shorter ischaemic times (0–1 hour). Slightly atrophied fibres and widening of intramuscular spaces were seen in the 8-hour ischaemic group. Centrally-positioned nuclei and fibroses were seen after 12–24 hours of ischaemia (). Focal necrosis and reactive fibroblast proliferation were seen and degenerative muscle fibres were ubiquitous at 72 hours of ischaemia. The longer the ischaemic time, the more evident were the changes. On the other hand, there were no definite changes from normal muscle and the intramuscular space was slightly widened in Group B, the arterial ischaemic model (). Uniform muscle atrophy as a result of denervation was obvious in Group C, the denervation model, compared with other groups ().
Figure 1. Gastrocnemius muscle specimens of rat hind limb. Haematoxylin and eosin stain; left, above: cross-section (original magnification ×40) left, below: longitudinal section (original magnification ×40), right above; cross section (original magnification ×400), right below: longitudinal section (original magnification ×400). Group A: transplantation model, preservation time (a) 1 hour, no distinguishable change, (b) at 72 hours, focal necrosis and degenerative muscle were seen. (c) Group B: arterial ischaemic model, no definite changes. (d) Group C: GFP transplantation model, uniform muscle atrophy was obvious. Scale bars, 50 μm (original magnification ×40), 5 μm (original magnification ×400).
The summarised results of measurements of muscle cell diameter for Groups A, B, and C are shown in . The longer the ischaemic time, the more evident were the atrophic muscles in Group A. There were no significant differences between the normal, 0-hour ischaemia, and 1-hour ischaemia groups. There were significant differences, however ( p < 0.05), between the <1-hour ischaemia groups and the >8-hour ischaemia groups. There was no significant difference seen among the >8-hour ischaemia groups.
Figure 2. The results of measurement of the diameters of muscle cells in groups A, B, and C. In group A, the longer the ischaemic time, the more evident was muscle atrophy. In group B muscle atrophy was not pronounced. Group C showed severe atrophy. *p < 0.05.
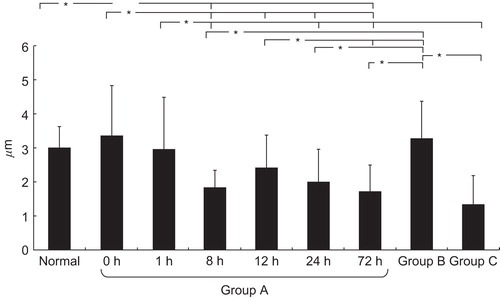
Muscle atrophy was not seen in the arterial ischaemia model; these muscle fibres retained their diameters. Reductions up to 37% in hind limb flow did not result in significant atrophy. In contrast, severe atrophy was seen in the denervation model.
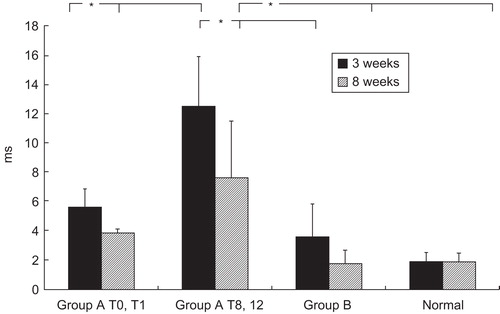
To assess the physiological importance of various ischaemic conditions, we examined the percentage of the nerves that responded to electrical stimulation. According to the histological results, we divided Group A into two subgroups: shorter ischaemia (0–1 hour) and longer ischaemia (8–12 hours). At 3 weeks postoperatively, 5 of 9 of the nerves in the longer ischaemia group responded to stimulation compared with all 6 of those in the shorter ischaemia group. This electrophysiological investigation showed that the latency at 3 weeks was longer in the transplantation models than in the controls, especially in the longer ischaemia group (). Velocity and amplitude showed no significant differences between the groups (data not shown). Latency persisted at 3 weeks in the shorter ischaemia group, but there was no significant difference between it and the normal control group at 8 weeks. The longer ischaemia groups had more evident delays at 3 weeks and did not recover by 8 weeks, and both were significantly different from the normal controls. The arterial ischaemia group did not differ significantly from the normal controls, which indicates that nerve tissue can tolerate ischaemia to some degree.
Figure 3. Distal motor latencies of the sciatic nerve. Comparison of shorter ischaemia group (0, 1 hours ischaemia), longer ischaemia group (8, 12 hours ischaemia), arterial ischaemic group, and normal controls. *p < 0.05.
Histological and electrophysiological studies obtained from Groups A, B, and C (prolongation of latency) suggested that the number, diameter, and myelination of axons were insufficient [Citation2,11–13] and reinnervation of muscle is poor after longer ischaemic times. A limb with a longer ischaemic time might have delayed, insufficient, reinnervation.
We used a new green fluorescent protein (GFP) rat strain [Citation7] to examine nerve regeneration. We previously developed GFP Tg Wistar rats that expressed increased GFP in various organs, including the peripheral nerves [Citation6,7,14]. We used the new GFP Tg Lewis rat to see how peripheral nerves regenerated distally beyond the anastomosis. Visualisation and numerical expression of regenerative nerve cells could be done by using GFP Tg rats. In this new GFP Tg rat transplantation model, the GFP-positive cell extended into the distal nerve fibres from the point of anastomosis. The typical pattern is shown in . GFP–S100 immunohistochemical and fluorescence-stained cells are newly extended Schwann cells accompanied by regenerating axons. We can check the number of regenerating axons 2 weeks after transplantation, which is the most important time in the recovery of muscular function. The sciatic nerve controls contained 196 Schwann cells. Total numbers of GFP–S100 immunohistochemical and fluorescence-stained cells 5 mm distal from the anastomosis in each subgroup are shown in . Definite differences were apparent between the 0-hour and 12-hour ischaemia groups. Prolonged ischaemia severely reduces the number of regenerated peripheral nerves in transplanted limbs.
Figure 4. The GFP stained cells of the sciatic nerve in group D, the GFP transplantation model, at the point of 5 mm distal from suture. Cold preservation time 0 hour, at two weeks after transplantation. 489-nm wave length excitation light (original magnification ×40).
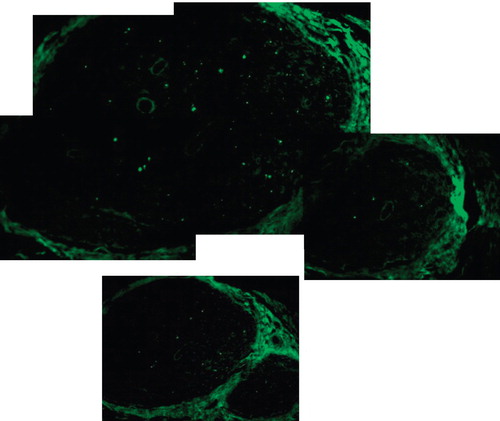
Table I. Numbers of GFP-S100 doubly-stained cells 5 mm distal from the anastomosis of the sciatic nerve. The difference between 0 hour and 12 hours cold preservation groups was significant. *p = 0.0002.
Discussion
Replantation after major limb amputation is now common at many microsurgical centres. In major limb amputation, where large quantities of muscle are lost, functional restoration is often poor as a result of muscle atrophy, joint contracture, and insufficient recovery of motor neurons. Skeletal muscle is the most sensitive to ischaemia [Citation15–17], so the success of replantation depends greatly on minimisation of ischaemia. Many techniques have been used to try and minimise ischaemic injury to traumatically amputated tissues. Hypothermia [Citation3,4,16] and tissue perfusion [Citation18,19] are used as preservative measures. Hypothermia is unarguably the most commonly regarded technique used to minimise ischaemic damage to devascularised tissues. Several experiments suggested that replantation of major extremities should not exceed 4–6 hours in warm ischaemia and 12–24 hours in cold ischaemia [Citation3,4,15–17].
In this study longer ischaemic times resulted in more severe morphological changes to the muscle cells; that is, muscle cell atrophy, necrosis, and fibrosis were proportionate to ischaemic time. Various histological changes in the muscle of transplanted limbs have been reported, but we know of no reports that have compared ischaemic muscle cells by preservation time and ischaemic muscle cell diameters by preservation time. In this study, we showed the objective numerical value correlated muscular atrophy and ischaemic time. It is encouraged that cold ischaemic periods should be <8 hours in order to help restore good function of major muscular extremities.
The arterial ischaemic model showed slight atrophy; some cases even showed no change from the control muscle. We suspect that rat arterial flow might be easily recovered even with ligation of major artery, as the degrees of reduction in hind limb flow were not severe enough to cause chronic muscular atrophy 8 weeks after ligation.
On the other hand, muscular atrophy by denervation was obvious. Clinically, recovery from paralysis after 3 weeks results in disuse atrophy. No response at three weeks in this electrophysiological examination can lead to the after effects of paralysis. If a longer ischaemic time also damages nerves and nerve regeneration, functional replantation results would be poor.
In Group D, axonal regeneration delayed by ischaemia also occurred simultaneously in longer ischaemic models. This histological result can lead to the conclusion that muscular atrophy is caused not only by direct damage to the muscle cells as a result of a longer ischaemic time but also by delayed regeneration of motor nerves and incomplete recovery. Nerve fibres were also affected by ischaemia in the transplantation model. We also showed that the speed of regeneration and the numbers of regenerating nerve fibres decrease with prolonged ischaemia. As a result, reinnervated muscle replanted after a long ischaemic time is likely to have insufficient functional recovery.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Muramatsu I, Yakahata N, Masamichi U, Metabolic and histologic changes in the ischemia muscles of replanted dog legs. Clin Orthop Relat Res 1985;196:292–9.
- Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome. Cardiovasc Surg 2002;10:620–30.
- van Alphen WA, Smith AR, ten Kate FJ. Maximum hypothermic ischemia in replants containing muscular tissue. J Hand Surg 1988;13A:415–22.
- Eckert P, Schnackerz K. Ischemic tolerance of human skeletal muscle. Ann Plast Surg 1991;26:77–84.
- Furuno K, Goodman MN, Goldberg AL. Role of different proteolytic systems in the degradation of muscle proteins during denervation atrophy. J Biol Chem 1990;265:8550–7.
- Hakamata Y, Tahara K, Kobayashi et al. Green fluorescent protein-transgenic rat: a tool for organ transplantation research. Biochem Biophys Res Commun 2001;286:779–85.
- Inoue H, Ohsawa I, Murakami T, Development of new inbred transgenic strains of rats with LacZ or GFP. Biochem Biophys Res Commun 2005;329:288–95.
- Kihira M, Miura T, Ishiguro N. Preservation of skeletal muscle in tissue transfers using rat hindlimbs. Plast Reconstr Surg 1991;88:275–84.
- Seifert FC, Banker M, Lane B, An evaluation of resting arterial ischemia models in the rat hind limb. J Cardiovasc Surg (Torino) 1985;26:502–8.
- Beehler BC, Sleph PG, Benmassaoud L, Reduction of skeletal muscle atrophy by a proteasome inhibitor in a rat model of denervation. Exp Biol Med (Maywood) 2006;231:335–41.
- Ide C. Peripheral nerve regeneration. Neurosci Res 1996;25:101–21.
- Donoso RS, Ballantyne JP, Hansen S. Regeneration of sutured human peripheral nerves: an electrophysiological study. J Neurol Neurosurg Psychiatry 1979;42:97–106.
- Ide C. Peripheral nerve regeneration. Neurosci Res 1996;25:101–21.
- Kimura A, Ajiki K, Hakamata Y, Transmigration of donor cells involved in the sciatic nerve graft. Transplant Proc 2005;37:205–7.
- Brunelli GA, Brunelli GR. Tissue changes at different periods of ischemia. Int Angiol 1995;14:253–63.
- Ren PH, Koshima I, Soeda S. Electron microscopic findings of muscles after warm or cold ischemia in amputated legs. J Jpn PRS 1991;11:20–7.
- Petrasek PF, Homer-Vanniasinkam S, Walker PM. Determinants of ischemic injury to skeletal muscle. J Vasc Surg 1994;19:623–31.
- Norden MA, Rao VK, Southard JH. Improved preservation of rat hindlimbs with the University of Wisconsin solution and butanedione monoxime. Plast Reconstr Surg 1997;100:957–65.
- Tsai TM, Jupiter JB, Serratoni F, The effect of hypothermia and tissue perfusion on extended myocutaneous flap viability. Plast Reconstr Surg 1982;70:444–54.