Abstract
Objective: This study aims to provide a comprehensive review of the role of Collagenase Clostridium histolyticum (CCH). Methods: This review is based on a literature review and practical experience. Results: This review provides practical management strategies for using collagenase by sharing clinical experiences over the past few years; logistical aspects of in-clinic treatment, lessons learned, and novel approaches to correct traditionally hard-to-treat contractures are discussed. In addition a brief, yet comprehensive overview is provided on the pathophysiology of the disease, the mechanism of collagenase action and results of clinical studies. Conclusion: CCH has an evolving role as one of the tools available for treating Dupuytren's disease.
Background
Dupuytren’s disease
Dupuytren’s disease (DD) is a progressive fibroproliferative disorder that affects the palmar fascia, whereby early nodular tissue develops into a collagen cord. As the cord thickens and shortens over time, flexion deformity of the affected joints and web spaces ensues [Citation1–3]. Impaired hand function is the usual presenting complaint, as affected individuals have difficulty performing daily activities secondary to the joint contracture [Citation4–6]. Many are also embarrassed by the visible deformity [Citation5].
DD is relatively common across Europe, in particular in people of Northern European origin [Citation7,Citation8]. It is more common in men [Citation9], and the incidence increases with advancing age [Citation10,Citation11]. Additional risk factors include ethnicity [Citation12], diabetes [Citation13–15], exposure to anti-epileptics [Citation16,Citation17], alcohol consumption and cigarette smoking [Citation18,Citation19], and history of hand trauma and vibratory work [Citation20–23]. Among the genetic predispositions, a mitochondrial rRNA mutation [Citation24], changes in human leukocyte antigen (HLA) class II alleles [Citation25], and alterations in the Wnt signalling pathway [Citation26] are included. There is no cure for DD, and corrective surgery has been the mainstay of treatment for Dupuytren’s contracture (DC) since the clinical features of the disease were first described more than 200 years ago.
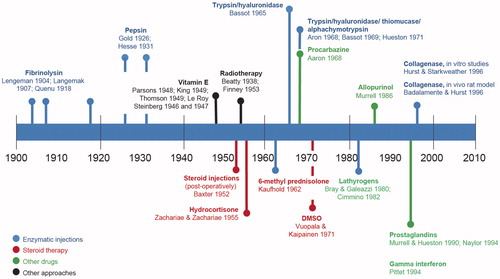
Collagenase Clostridium histolyticum (CCH) is the first non-surgical, pharmacologic treatment for DC with a palpable cord approved for use in the US and Europe. Clinical trials [Citation27–29] and post-marketing surveillance [Citation30] have demonstrated the efficacy and safety of CCH treatment for correcting DC. However, treating DC enzymatically is not a new concept. Early investigations began already in the 1960s, and in 1971 the use of a combination of digestive enzymes during open surgery was referred to as an “enzymic fasciotomy” [Citation31] ().
Figure 1. Historic highlights in the development of non-surgical approaches for Dupuytren’s contracture.
In this review, we aim to briefly describe the pathophysiology of DD and provide practical management strategies for the use of CCH in patients with DC by summarising the clinical experiences over the past few years [Citation29].
Pathophysiology of Dupuytren’s disease
During the past 30 years, great advances have been made in our understanding of the pathophysiology of DD. The important role of myofibroblasts was first described in 1972 [Citation32]. Later the presence of non-muscle myosin, actin, and fibronectin in DD tissue was identified [Citation33] and the importance of myofibroblasts and their regulation by calcium adenosinetriphosphatase in the contractile process was described [Citation34,Citation35]. The importance of integrin and fibronectin in the contractile process was also described [Citation36]. In more recent years profiling of haematopoietic and mesenchymal stem cells in tissues biopsied from DD patients and from those undergoing surgery for carpal tunnel syndrome (controls) was performed to identify the potential source of DD cells. RNA expression of stem cell markers was higher in DD patients and distinct stem cell populations were identified in DD tissues including cords, nodules, perinodular fat, and skin, supporting the hypothesis that DD may result from mesenchymal progenitor cell expansion [Citation37].
Three histologic stages characterise the disease process [Citation38]:
Proliferative stage: there is an increased production of fibroblasts that cluster to form nodules.
Involutional stage: fibroblasts differentiate into myofibroblasts, and growth factors, particularly transforming growth factor-β, facilitate their rapid and extensive proliferation [Citation39]. A combination of chemical and tensile forces confers contractile properties onto the tissue and results in the deposition of extracellular matrix (ECM) proteins, especially collagen [Citation40].
Residual stage: cellular activity ceases, leaving the inelastic collagen cord to span joints of the hand, shorten, and cause fixed flexion contracture (FFC) of the affected joint [Citation41].
Of the ECM proteins assembled and deposited by myofibroblasts, collagen types I, III, IV, and V are the most prevalent [Citation42]. In normal palmar fascia, type I collagen predominates, whereas in DD affected tissue the predominant form is type III collagen [Citation43–46], which is also a prominent contributor to the pathogenesis of DD [Citation41]. Compared with normal fascia, there is a 10%–20% increase in type III collagen in nodules and a 30%–40% increase in cords.
Due to its triple helix structure, cross-linking, and glycosylation, collagen is a stable protein. Matrix metalloproteinases (MMPs) and cathepsins (L and H) are the only mammalian enzymes that possess collagenase activity. Relevant for DD is the collagenase activity of MMP, which is controlled through synthesis, activation of latent MMPs, inactivation of other active enzymes, and an acidic pH environment [Citation47,Citation48]. Collagen accumulation in DD is the net result of deposition and degradation, the balance of which is determined by the local environment.
Collagenase Clostridium histolyticum (CCH)
The discovery of collagenase started in the early 1930s when the digestive activity of filtrates from cultured Clostridium histolyticum on small fragments of equine Achilles tendon was described [Citation49,Citation50]. The enzyme responsible for the effect was later named collagenase, as its digestive activity turned out to be exclusive to collagen [Citation51]. In 1996, CCH, which is composed of two distinct collagenases isolated from Clostridium histolyticum, was introduced as a potential candidate for pharmacotherapy for patients with DD for the first time [Citation52].
As a new molecular entity, CCH comprises a fixed-ratio mixture of two purified collagenolytic enzymes, clostridial type I collagenase (AUX-I) and clostridial type II collagenase (AUX-II) [Citation53]. Clostridial type I and type II collagenases are calcium- and zinc-dependent MMPs. The enzymes differ in terms of structure, affinity, cleavage site, and catalytic efficiency (). By virtue of these differences in attributes, when the two collagenases are combined, the enzymatic activity is improved over that observed with either class acting alone [Citation53].
Table 1. Characteristics of the type I and type II Clostridium histolyticum collagenases.
In the first study exploring the therapeutic potential of CCH experiments were performed on DD cords excised from patients undergoing fasciectomy. The goals were three-fold: (1) to determine the range of CCH doses required for clinical utility; (2) to define the minimal effective dose needed to cause complete cord rupture; and (3) to quantify the force required during the finger extension procedure to facilitate cord rupture. The study demonstrated that a minimal effective dose of 300 units was required to rupture a cord using normal extension force, and that the effect increased relative to dose [Citation52].
Later, the effect of CCH on primary fibroblasts obtained from different anatomic sites in tissue biopsied from patients with DD or carpal tunnel syndrome was investigated [Citation54]. At the cellular level, CCH inhibited the spreading, attachment, and proliferation of fibroblasts in a dose- and time-dependent manner. At the transcriptional level, CCH also showed dose-dependent inhibition of several ECM components, cytokines, and growth factors [Citation54]. When CCH was removed, the cellular processes of DD tissues recovered in nodules, cords, and skin, but not fat. This recovery may be due to the presence of myofibroblasts [Citation32] or stem-like cells associated with DD [Citation37].
Clinical efficacy of CCH
The clinical efficacy of CCH has been evaluated in at least 13 studies, including one phase I, three Phase II, and nine Phase III clinical trials conducted in the US, Europe, and Australia.
CORD I and CORD II
In the pre-licensing Phase III clinical trials, CORD I (Collagenase Option for Reduction of Dupuytren) and CORD II, as well as in a single centre study of similar design, CCH, was efficacious compared to placebo in reducing contractures to ≤5° of normal finger extension [Citation28,Citation29,Citation55].
JOINT I and JOINT II
JOINT I and II were identical open-label studies designed to follow clinical practice [Citation27]. Eligible patients could receive up to five CCH injections into cords that were prioritised by the extent of contracture. After the first, patients could opt to receive up to two more injections into the same cord or receive injections into other affected cords, regardless of the outcome for the first joint. The conclusion of the studies was that CCH was effective in treatment of DCs of ranging severity. Treatment of most cords required one single injection and earlier treatment in the course of the disease, resulted in improved outcome.
Efficacy and safety in patient sub-groups
In a retrospective study of data from three randomised placebo-controlled trials [Citation28,Citation29,Citation55], the efficacy and safety of CCH were evaluated in sub-groups of patients previously shown to be at increased risk for surgical complications [Citation56]. Gender, age, or diabetes did not affect the complication rate. The frequency or nature of AEs was also similar to other studies.
Efficacy after previous surgery
Recurrence after surgery for DC is frequent and may require retreatment. Retrospective data from 12 clinical trials were pooled to evaluate the relative efficacy of CCH in patients who had undergone previous surgery for DC [Citation57]. Overall, these findings suggest that previous surgery for DC does not affect its efficacy or safety, and that CCH is a viable option among patients with recurring DC.
Recurrence
CORDLESS (CORD Long-Term Evaluation of Safety Study) is a follow-up study of patients from the CORD and JOINT studies [Citation58]. Recurrence in a joint that had achieved clinical success with CCH was defined as either a ≥20° worsening in contracture with a palpable cord, or a medical/surgical intervention to correct a new or worsening contracture. At 3 years, of the 1080 joints that had been treated, 35% recurred (27% MP joint, 56% PIP joints) and, of these, an intervention was performed in 8%. By 5 years, 40% of metacarpophalangeal cords and 66% of proximal interphalangeal cords recurred.
Clinical practice
US chart review
In a retrospective review of charts from patients treated with CCH in 2010, an assessment of the effectiveness of CCH in clinical practice, rather than a research protocol, was performed. Overall, the effectiveness of CCH was comparable to the efficacy in clinical trials; in the “real-world” study, fewer injections were needed to gain an improvement than in the research studies. This is likely due to the widespread use of local anaesthetic during the extension procedure, which may have allowed the use of greater and more prolonged force to facilitate cord disruption compared to the clinical trials where local anaesthetic was not allowed [Citation30].
One-year US post-marketing safety study
During the first 3 years after the FDA approved CCH for DC (February 2010–February 2013), ∼49 000 injections were given, and 1732 AEs were reported for 864 patients [Citation59]. The most commonly reported AEs were skin tears (13%), peripheral oedema (9.5%), and contusion (9.7%). There were 26 tendon ruptures (0.05%). The nature and frequency of AEs during this period were comparable to those observed in the clinical trials; no new safety signals were detected in this analysis and no safety-related changes were made to the product label.
Practical guidance from experienced surgeons
Establishing a CCH practice
Candidates for CCH treatment are patients with a palpable cord and an identifiable functional problem rather than an indication strictly based on the degree of the contracture.
Symptoms are, however, most likely to manifest after a contracture of ≥ 30°. There is no indication for prophylactic treatment with CCH, or for nodules alone. Nor is the use of CCH advised for patients with deficient or severely damaged skin.
Educating patients regarding alternatives, treatment procedure, reasonable expectations, and potential risks is important.
Injection procedure
The instructions in the package insert and other educational material for dosing, reconstitution, and administration should be followed strictly. After CCH is reconstituted, the solution should preferably be drawn into a 0.5 ml syringe (with 0.01 ml intervals), which allows accurate divided administration. A fixed needle, rather than a removable needle, is advised to avoid expensive wastage in the event of detachment. In theory, a local anaesthetic before the injection may interfere with proper placement of the injection and mask inadvertent penetration of a digital nerve. Spray analgesia (e.g. ethyl chloride) reduces needle pain. Cold spray should not be sprayed onto the needle as this can constrict flow. Palpation is of utmost importance to create a mental image of the underlying pathoanatomy before an injection. Also, be aware that not all flexed fingers have Dupuytren’s cords – other pathologies such as a neurological contracture, post-traumatic PIP contracture, or locked trigger finger may co-exist.
Great care should be taken not to advance the needle with the plunger, such that the injection is too deep and is deposited dorsal to the cord. Using both hands to control the needle during the injection is often helpful. Digital cords can be injected parallel to the palm rather than in the perpendicular plane to reduce the chance of tendon penetration. Injections may be given by inserting the needle through the skin only once; however, placement of the needle in separate spots along the cord allows for more accurate yet widespread deposition of aliquots and aids in the management of complex cords. If only one joint is to be treated, but both MP and PIP joints are contracted, then CCH should be administered across the MP cord first. In many patients, a PIP contracture will correct due to the dynamism of the cord [Citation60].
Data from a recently published trial suggest that two affected joints can be effectively and safely treated with concurrent CCH injections into the same hand in patients with multiple contractures [Citation61]. Another study suggests that a larger dose can be used, off label, and with due consent and caution, for multiple cords [Citation62].
Finger extension procedure
According to the initial studies, the finger extension procedure should be performed the day after the injection, but recent studies show that a delay of up to 7 days after injection gives equivalent results [Citation63,Citation64]. Both the EMA and FDA have approved manipulation up to 72 hours later.
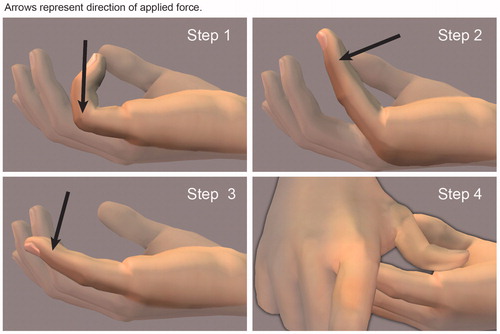
A four-step finger extension procedure is recommended to maximise disruption of the cord and minimise skin tears (). A local anaesthetic is administered as a metacarpal or wrist block before the extension procedure. Cord disruption is almost always successful after a single manipulation; however, some surgeons recommend repeating the extension procedure two or three times, even if the first procedure breaks the cord. If there are adjacent cords such as natatory connections to the adjacent finger, these should be manipulated as well; rupture is common. Secondary ruptures can occur, contributing to a better outcome. Massaging the cord, eliciting the sensation of “bubble wrap”, can very effectively improve the contracture.
Figure 2. Hurst’s four-step finger extension procedure after CCH injection of a central cord contracting both the MP and PIP joints of the little finger. The hand is placed on the examination table with the wrist oriented for palmar flexion with the forearm facing upward. Step 1: the MP joint is extended with the PIP joint flexed. Step 2: the PIP joint is extended with the MP joint flexed. Step 3: both the MP and PIP joints are extended. Step 4: with the finger extended, pressure is applied to the residual cord. Photos courtesy of LC Hurst.
Immediately after the manipulation, a soft compressive bandage can be applied, allowing patients to move the hand while protecting the skin from accidental snagging. The skin can be vulnerable for a few days after the injection, especially if there is a blood blister or skin tear. Some surgeons forgo a bandage, splinting the finger immediately after the extension procedure.
Splinting
There is no evidence about whether splinting confers benefit, and the extent to which type of splint may be best has not been studied in detail. Moreover, it has been shown that patient adherence with their use is low [Citation65,Citation66]. Use of splints will depend upon the surgeon’s and hand therapist’s opinion. Some recommend static night extension splinting for 3 months after CCH treatment. Starting the night splinting with a little flexion and gradually straightening it over several nights is generally less painful. Splints that can be adjusted by the patient are commercially available. For PIP joints that are still contracted after CCH lyses the cord, daytime use of a PIP reverse knuckle bender splint is appropriate. Occasionally, surgical addition of a Digit Widget™ might be considered for severely stiffened PIP joints, although we are not aware of any reports.
Finger flexion and extension exercises
Patients should be advised to use the hand as normally as possible immediately after the finger extension procedure, but avoid lifting heavy objects for 1–2 weeks. Follow-up by a qualified hand therapist may be necessary for some patients. Detailed instructions for daily hand and finger exercises can be found at: http://www.dupuytrens.stonybrook.edu/patient_exercise.cfm.
Frequency and purpose of follow-up visits
The frequency and nature of follow-up varies depending on the treating surgeon and the patient’s outcome, progress, and complications. If a skin tear occurs, the patient must be reassured that this is within the normal response after CCH injection. It will heal uneventfully in 2–4 weeks. At the 1-month follow-up visit, the results are checked and evaluated for the need for a second injection. If the first injection does not fully correct the contracture, or if there is more than one cord, then a further injection can be considered.
Patient satisfaction
Patient satisfaction depends on how successful the treatment is and on the patient’s expectations before the treatment. The patients may still be satisfied, even if there is some residual contracture [Citation67]. It is important to inform the patient about the likely risk of recurrence in due course. A well informed patient with realistic expectations is more likely to be satisfied.
Recurrence
There is no broadly accepted definition of recurrence. Surgery in a field previously treated with CCH is generally not more challenging than primary surgery [Citation68].
The use of CCH on patients with recurrence after surgery has been studied with good results and high patient satisfaction [Citation57]. CCH works well in cords where the contracture is secondary to a recurrent visible and palpable cord, the joint is not stiff and there is healed skin over the cord. If there is insufficient skin between the proximal flexion crease and PIP flexion crease of the finger or diffuse involvement of the skin, surgical excision of the cord and skin grafting is probably more appropriate.
Safety concerns
Allergic reactions
Allergic reactions are extremely rare after treatment with CCH. One anaphylactic reaction was reported in a post-marketing clinical study of CCH for Dupuytren’s contracture in a patient who had previous exposure to CCH for the treatment of Dupuytren’s contracture, demonstrating that severe reactions including anaphylaxis can occur, and an anaphylactic reaction set must be available and the doctor must be familiar with its use. An immune response to CCH can manifest as axillary pain and/or lymphadenopathy, and the patient should be informed about the risk of such reactions. After treatment the patient should be observed for 30 minutes before leaving the clinic in order to monitor for any appearance of allergic reactions.
Oedema
Local swelling and some ecchymosis is common and to be expected when a bioactive drug is injected into the hand. The swelling typically resolves within 24–48 hours after the injection. Patients should be advised to elevate their hand and to take prophylactic analgesics. Ecchymosis is usually mild; in more severe cases, the condition resolves within 10–14 days. Also blood blisters are relatively common after CCH injection and should be attended to and monitored, but are of little cause for concern.
Skin tears
In clinical practice, small skin tears occur in ∼13% of cases [Citation59] and are treated with standard wound care. The tears close rapidly and heal in 10–21 days and the quality of the regenerated skin is usually excellent. In rare cases, application of a graft has been necessary. It is thought that CCH spreading into the dermal-epidermal interval weakens the skin, and the tear develops during the extension procedure.
Artery and nerve injury
We are not aware of any instances of artery or nerve injury after CCH injection. No instances of artery or nerve injury occurred during CCH clinical trials [Citation28,Citation29,Citation55], and there were no reports of artery or nerve injury in the 3-year post-marketing safety study [Citation59].
Tendon rupture
In the CORD I study, two patients experienced tendon ruptures [Citation28] and in the CORD II study one patient had a flexion pulley rupture after treatment with CCH [Citation29]. During the first 36 months after FDA approval, there were 26 reports of tendon rupture after ∼ 49 000 injections of CCH were given to patients with DC in the US (0.05%) [Citation59]. During that time and since, several tactics have been developed to avoid or minimise the risk for tendon rupture. First, CCH should not be injected near the PIP flexion crease of the little finger; however, if this is unavoidable, the needle should be oriented so that it is parallel to the palm and facing outward rather than toward the tendon. Second, occasionally in some patients who have had previous surgery, the flexor sheaths rupture; consequently the flexor tendons are “bowstrung”, such that the displaced tendons can cause flexion contractures, and the tendon itself can look like a Dupuytren’s cord. Third, it is important to remember that the distance from the skin to the flexor sheath in the palm is ∼ 7 mm and at the PIP joint, this distance is only 4 mm. When assessing how deep to insert the needle, remember that the needle bevel measures 1¼ mm. Fourth, a tendon is more likely to rupture only if an entire dose of CCH has been injected into it.
Other serious injuries
Two cases of finger fractures have been reported. These events occurred as complications of the finger extension procedure.
Treatment advances in previously challenging cases
Efficacy of CCH in patients with comorbid conditions
Diabetes
Diabetes patients generally have increased surgical risk and CCH might for this reason be the preferred treatment option in this patient group. However, in clinical trials the efficacy and safety of CCH treatment was comparable between patients with or without diabetes [Citation56]. All patient medications and special diets should continue without modification.
Patients taking anticoagulants
The use of anticoagulants is restricted in those taking anticoagulants. The CCH label advises that the drug should be used with caution with the exception of up to 150 mg daily of acetylsalicylic acid. One possibility for patients taking anticoagulants is to transition them to heparin 4–7 days before the injection. Clotting rates can be checked on the injection day, and the transition back to their anticoagulant medication can begin the day after, if the finger extension procedure does not result in a skin tear.
The use of CCH in patients with a clotting disorder or anticoagulant drug should be individualised and fully discussed with the patient. There are significant risks in stopping anticoagulants in some patients. Some doctors may be content to use CCH with the same practice as they would with surgery which might allow its use if the INR is below 2.5 [Citation69].
Efficacy of CCH in hard-to-treat cords
PIP joints
PIP contractures are more difficult than MP contractures to treat using corrective surgery or percutaneous needle fasciotomy (PNF) [Citation70–72], and rates of recurrence are generally high [Citation73]. Anatomic differences leave the PIP joint more susceptible to blood blisters, tendon rupture, and higher recurrence rates with CCH. When both the MP and PIP joints on the same finger are contracted by the same cord, CCH treatment of the MP joint sometimes corrects both contractures. The portion of the PIP contracture caused by the cord is predictably corrected by the finger extension procedure. In some patients with severe PIP contractures (>40°), there may be several cords, some of which may not be palpable. In these cases, the use of ultrasound to guide the injection(s) may be useful. PIP contractures can also be caused by non-DD-related factors such as a contracted volar plate, contracted collateral ligaments of the PIP joint, arthritic changes in the joint, and attenuation of the extensor mechanism of the PIP joint. Thus, the likelihood of complete correction of a PIP contracture is not as good as that for complete correction of an MP contracture.
Central cord
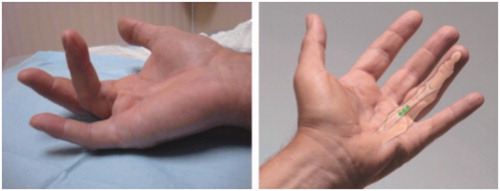
A central cord develops from the pretendinous band, which is a retention ligament extending from the palm into the fibrofatty tissues of the finger. This cord can cause an MP contracture or a combined MP and PIP contracture (). The typical result after CCH treatment of a combined MP and PIP contracture is full correction of the MP and partial but sufficient correction of the PIP cord.
Figure 3. Example of central cord contracture. (left) The structure of a pathologic combined MP/PIP contracture cord is easily visualised and palpated. (right) The surgeon imagines the underlying pathology and plans the placement of the aliquots of a single dose of CCH. Each aliquot is placed centrally in the portion of the cord that is most separated from the underlying flexor sheath. The typical result is full correction of the MP contracture and almost full correction of the PIP contracture. Photos courtesy of LC Hurst.
Crow-foot cord
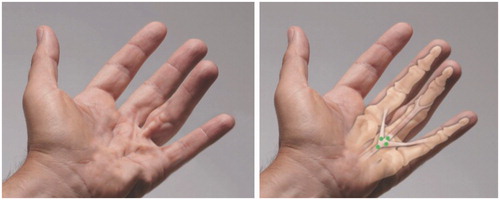
A crow-foot cord is a combination of a central cord and two natatory cords extending into the adjacent fingers on either side, causing MP contractures in three fingers (). Usually, all three contractures can be corrected with a single dose.
Figure 4. Example of a crow-foot cord. In a crow-foot cord the central cord influences two natatory cords causing MP contractures in three fingers. A single dose of CCH is divided into aliquots and injected as indicated by dots in the figure. Usually, all three contractures can be corrected with a single dose. Photos courtesy of LC Hurst.
“Y” cord and Super “Y” cord
The “Y” cord is a combination of a central cord and a natatory cord and adjacent finger, and the Super “Y” cord causes MP contractures in two fingers separated by an uninvolved finger with no contracture (). Usually, these contractures can be corrected with a divided, single dose treatment.
Figure 5. Examples of “Y” and Super “Y” cords. (top, left) The central cord extends into the ring finger, and the natatory cord contracts the little finger Frequently, this cord combination causes MP contractures in the adjacent fingers. (top, right) Visual examination and palpation allow the mental imagine of the underlying pathology. The single dose is injected in aliquots at the fork of the “Y” as indicated by dots in the figure (bottom, left). The Super “Y” cord is a combination of a central cord involving the palm only and two natatory cords extending into the adjacent fingers on either side of the central cord. This causes MP contractures in two fingers separated by an uninvolved finger with no contracture. (bottom, right). A single dose of CCH is divided into aliquots and injected as illustrated. Usually, these contractures can be successfully corrected with a single dose. Photos courtesy of LC Hurst.
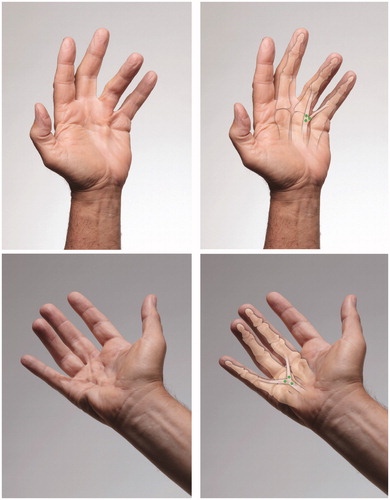
Abductor digiti minimi cords
The abductor digiti minimi cord starts at the insertion of the site of the abductor digiti minimi into the ulnar base of the fifth finger proximal phalanx. The cord extends distally to the ulnar base of the middle phalanx. This cord typically causes PIP contractures and does not involve the MP joint unless there is an associated central cord. An isolated abductor digiti minimi cord that has not been previously treated usually responds well to CCH treatment. Aliquots should be injected into the proximal half of the cord, as this area has the most separation from the underlying flexor tendon sheath.
Commissural and radial thumb cord
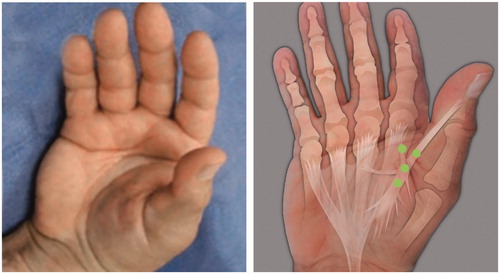
Thumb contractures () are caused by commissural cords and radial thumb cords, often occurring together. They typically close the first web space and adduct the thumb. In a small percentage of patients, these contractures can be severe and disabling, limiting the use of the thumb by making normal pinch and grip nearly impossible [Citation74,Citation75]. CCH is successfully used for treating thumb cords [Citation76]. Occasionally, thumb cords can be thick enough to require a second injection of CCH 30 days later.
Figure 6. Example of thumb cords (commissural and radial). (left) In this example the tip of the thumb cannot be moved away from the base of the index finger, and it cannot be abducted so that the thumb and palm can be placed flat on a table top. (right) The cords can be visualised and palpated to create a mental image, and the aliquots of a single dose can be injected around the junction site of the two cords as indicated by dots. Occasionally, thumb cords can be thick enough to require a second injection of CCH 30 days later. Photo and schematic diagram courtesy of LC Hurst.
Other complex cords
Various complex cord combinations exist that require careful analysis to determine junction points of the component cords and the areas that are most separated from the underlying flexor tendons. Such complex cords can also be successfully treated with a single dose of CCH.
Technical advances
Delayed finger extension procedure
The finger extension procedure is typically performed the day after the CCH injection. In the recent MULTICORD study the extension procedure was delayed until 48 or 72 hours without any difference in the efficacy or safety profile [Citation63,Citation64].
Multiple injections
In case additional treatment is required the next injections are given after 30 days. However, recent studies revealed that treatment of multiple joints using two concurrent injections resulted in comparable improvement to those seen in previous studies [Citation61,Citation62]. Multiple concurrent injections for effective treatment of patients with multiple affected joints would benefit the patient and enhance the cost-effectiveness of the drug, which is otherwise currently a challenge. For these reasons, the FDA in the US and the EMA in Europe have approved the use of two concurrent injections.
Dividing a single dose of CCH into aliquots is an alternative successful treatment of complex combined cords like the “Y” cord. A single dose can also be divided to treat two separate cords that are not connected. However, success will be determined by the thickness of the cords, since the volume splitting will only work if they are very thin with a small cross-sectional diameter.
Conclusion
Overall, the skills required to perform CCH injection and finger manipulation procedures are not difficult to acquire. A detailed knowledge of hand anatomy and the means to manage any complications is essential. CCH injection is a simple, efficacious, and well tolerated alternative to surgery. Patient satisfaction is generally high, especially when patients are well informed about the technique and have reasonable expectations regarding outcomes and are informed about the risk of recurrence. Recurrence rates after CCH are higher than surgery. Nevertheless, recurrence may occur in suitable cases with a second injection. If surgery is required after treatment with CCH, it is not more complex than a primary procedure.
Recent research suggests that an increased dose, multiple injections, and delayed manipulation appear to be safe and effective. These advances will increase the utility and improve the cost effectiveness of CCH.
Acknowledgements
This work was sponsored by Pfizer Inc. and Swedish Orphan Biovitrum AB. We thank Linda A. Goldstein, PhD, of UBC Scientific Solutions, who provided medical writing services funded by Pfizer Inc. and Kristina Lindsten, PhD, medical writer at Swedish Orphan Biovitrum AB for participation in manuscript revision. The authors acknowledge the support of Robert Gerber and Piotr Szczypa of Pfizer Inc., Jim Tursi of Auxilium Pharmaceuticals, and Hans Olivecrona of Swedish Orphan Biovitrum. Funding for Open Access reproduction of this paper has been provided by Swedish Orphan Biovitrum. The figures are provided by Dr L. Hurst who maintains copyright.
Disclosure statement
DW has received honoraria and travel expenses for advisory board work with Pfizer Ltd. and Swedish Orphan Biovitrum. JMAR, and GP have received honoraria and travel expenses for advisory board work with Pfizer Ltd. JW has served as a speaker and participated in advisory boards for Pfizer Ltd. LCH has received research support from Auxilium Pharmaceuticals, Biospecific Technologies Corp (BTC), the US Food and Drug Administration, and National Institutes of Health; LCH also serves as a consultant to Auxilium Pharmaceuticals and the State University of New York at Stony Brook and receives royalties from BTC. DW, GP, JW, and LCH were study investigators as part of the CCH clinical trial programme.
References
- Black EM, Blazar PE. Dupuytren disease: an evolving understanding of an age-old disease. J Am Acad Orthop Surg 2011;19:746–57.
- Rayan GM. Dupuytren disease: anatomy, pathology, presentation, and treatment. J Bone Joint Surg Am 2007;89:189–98.
- Shaw RB Jr., Chong AK, Zhang A, et al. Dupuytren's disease: history, diagnosis, and treatment. Plast Reconstr Surg 2007;120:44e–54e.
- Bayat A, McGrouther DA. Management of Dupuytren's disease-clear advice for an elusive condition. Ann R Coll Surg Engl 2006;88:3–8.
- Hobby JL, Dias JJ. A review of hand surgery provision in England. J Hand Surg Br 2006;31:230–5.
- Pratt AL, Byrne G. The lived experience of Dupuytren's disease of the hand. J Clin Nurs 2009;18:1793–802.
- Hindocha S, McGrouther DA, Bayat A. Epidemiological evaluation of Dupuytren's disease incidence and prevalence rates in relation to etiology. Hand (NY) 2009;4:256–69.
- Hart MG, Hooper G. Clinical associations of Dupuytren's disease. Postgrad Med J 2005;81:425–8.
- Wilbrand S, Ekbom A, Gerdin B. The sex ratio and rate of reoperation for Dupuytren's contracture in men and women. J Hand Surg Br 1999;24:456–9.
- Gudmundsson KG, Arngrimsson R, Sigfusson N, et al. Epidemiology of Dupuytren's disease: clinical, serological, and social assessment. The Reykjavik study. J Clin Epidemiol 2000;53:291–6.
- Hoèt F, Boxho J, Decoster E, et al. [Dupuytren's disease. Review of 326 surgically treated patients]. Ann Chir Main 1988;7:251–5.
- Saboeiro AP, Porkorny JJ, Shehadi SI, et al. Racial distribution of Dupuytren's disease in Department of Veterans Affairs patients. Plast Reconstr Surg 2000;106:71–5.
- Arkkila PE, Kantola IM, Viikari JS. Dupuytren's disease: association with chronic diabetic complications. J Rheumatol 1997;24:153–9.
- Aydeniz A, Gursoy S, Guney E. Which musculoskeletal complications are most frequently seen in type 2 diabetes mellitus? J Int Med Res 2008;36:505–11.
- Noble J, Heathcote JG, Cohen H. Diabetes mellitus in the aetiology of Dupuytren's disease. J Bone Joint Surg Br 1984;66:322–5.
- Arafa M, Noble J, Royle SG, et al. Dupuytren's and epilepsy revisited. J Hand Surg Br 1992;17:221–4.
- Critchley EM, Vakil SD, Hayward HW, Owen VM. Dupuytren's disease in epilepsy: result of prolonged administration of anticonvulsants. J Neurol Neurosurg Psychiatry 1976;39:498–503.
- Burge P, Hoy G, Regan P, Milne R. Smoking, alcohol and the risk of Dupuytren's contracture. J Bone Joint Surg Br 1997;79:206–10.
- Godtfredsen NS, Lucht H, Prescott E, et al. A prospective study linked both alcohol and tobacco to Dupuytren's disease. J Clin Epidemiol 2004;57:858–63.
- Bovenzi M. Hand-arm vibration syndrome: diagnostic aspects, dose-response relationship and exposure limits. Med Lav 1994;85:463–73.
- Cocco PL, Frau P, Rapallo M, Casula D. Occupational exposure to vibration and Dupuytren's disease: a case-controlled study. Med Lav 1987;78:386–92.
- Liss GM, Stock SR. Can Dupuytren's contracture be work-related?: review of the evidence. Am J Ind Med 1996;29:521–32.
- Thomas PR, Clarke D. Vibration white finger and Dupuytren's contracture: are they related? Occup Med (Lond) 1992;42:155–8.
- Bayat A, Walter J, Lambe H, et al. Identification of a novel mitochondrial mutation in Dupuytren's disease using multiplex DHPLC. Plast Reconstr Surg 2005;115:134–41.
- McCarty S, Syed F, Bayat A. Role of the HLA system in the pathogenesis of Dupuytren's disease. Hand (NY) 2010;5:241–50.
- Dolmans GH, Werker PM, Hennies HC, et al. Wnt signaling and Dupuytren's disease. N Engl J Med 2011;365:307–17.
- Witthaut J, Jones G, Skrepnik N, et al. Efficacy and safety of collagenase clostridium histolyticum injection for Dupuytren contracture: short-term results from 2 open-label studies. J Hand Surg Am 2013;38:2–11.
- Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren's contracture. N Engl J Med 2009;361:968–79.
- Gilpin D, Coleman S, Hall S, et al. Injectable collagenase Clostridium histolyticum: a new nonsurgical treatment for Dupuytren's disease. J Hand Surg Am 2010;35:2027–38.
- Peimer CA, Skodny P, Mackowiak JI. Collagenase clostridium histolyticum for dupuytren contracture: patterns of use and effectiveness in clinical practice. J Hand Surg Am 2013;38:2370–6.
- Hueston JT. Enzymic fasciotomy. Hand 1971;3:38–40.
- Gabbiani G, Majno G. Dupuytren's contracture: fibroblast contraction? An ultrastructural study. Am J Pathol 1972;66:131–46.
- Tomasek JJ, Schultz RJ, Episalla CW, Newman SA. The cytoskeleton and extracellular matrix of the Dupuytren's disease “ myofibroblast”: an immunofluorescence study of a nonmuscle cell type. J Hand Surg Am 1986;11:365–71.
- Tomasek JJ, Schultz RJ, Haaksma CJ. Extracellular matrix-cytoskeletal connections at the surface of the specialized contractile fibroblast (myofibroblast) in Dupuytren disease. J Bone Joint Surg Am 1987;69:1400–7.
- Badalamente MA, Stern L, Hurst LC. The pathogenesis of Dupuytren's contracture: contractile mechanisms of the myofibroblasts. J Hand Surg Am 1983;8:235–43.
- Magro G, Lanzafame S, Micali G. Co-ordinate expression of alpha 5 beta 1 integrin and fibronectin in Dupuytren's disease. Acta Histochem 1995;97:229–33.
- Hindocha S, Iqbal SA, Farhatullah S, et al. Characterization of stem cells in Dupuytren's disease. Br J Surg 2011;98:308–15.
- Luck JV. Dupuytren's contracture; a new concept of the pathogenesis correlated with surgical management. J Bone Joint Surg Am 1959;41:635–64.
- Badalamente MA, Sampson SP, Hurst LC, et al. The role of transforming growth factor beta in Dupuytren's disease. J Hand Surg Am 1996;21:210–15.
- Brickley-Parsons D, Glimcher MJ, Smith RJ, et al. Biochemical changes in the collagen of the palmar fascia in patients with Dupuytren's disease. J Bone Joint Surg Am 1981;63:787–97.
- Meyerding H, Black J, Broders A. The etiology and pathology of Dupuytren's contracture. Surg Gynecol Obstetr 1941;72:582–90.
- Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol 2007;127:526–37.
- Bailey AJ, Sims TJ, Gabbiani G, et al. Collagen of Dupuytren's disease. Clin Sci Mol Med 1977;53:499–502.
- Bazin S, Le Lous M, Duance VC, et al. Biochemistry and histology of the connective tissue of Dupuytren's disease lesions. Eur J Clin Invest 1980;10:9–16.
- Gelberman RH, Amiel D, Rudolph RM, Vance RM. Dupuytren's contracture. An electron microscopic, biochemical, and clinical correlative study. J Bone Joint Surg Am 1980;62:425–32.
- Menzel EJ, Piza H, Zielinski C, et al. Collagen types and anticollagen-antibodies in Dupuytren's disease. Hand 1979;11:243–8.
- Bode W. A helping hand for collagenases: the haemopexin-like domain. Structure 1995;3:527–30.
- Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011;41:271–90.
- Weinberg M, Randin A. Ferment fibrolytique d'origine microbienne. Compt Rend Soc Biol 1931;107:27–8.
- Weinberg M, Randin A. Propriétés physioco-chimiques du ferment fibrolytique d'origine microbienne. Compt Rend Soc Biol 1932;110:352–3.
- Ssadikow W. Über ein neues Kollagen-lösendes Ferment (Kollagenase). Biochem Z 1927;181:267–83.
- Starkweather KD, Lattuga S, Hurst LC, et al. Collagenase in the treatment of Dupuytren's disease: an in vitro study. J Hand Surg Am 1996;21:490–5.
- Briefing document for collagenase clostridium histolyticum (AA4500) in the treatment of advanced Dupuytren's disease [http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/ucm182012.html], 2009.
- Syed F, Thomas AN, Singh S, et al. In vitro study of novel collagenase (XIAFLEX®) on Dupuytren's disease fibroblasts displays unique drug related properties. PLoS One 2012;7:e31430.
- Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren's contracture. J Hand Surg Am 2007;32:767–74.
- Raven RB, 3rd, Kushner H, Nguyen D, et al. Analysis of efficacy and safety of treatment with Collagenase Clostridium histolyticum among subgroups of patients with Dupuytren contracture. Ann Plast Surg 2014;73:286–90.
- Bainbridge C, Gerber RA, Szczypa PP, et al. Efficacy of collagenase in patients who did and did not have previous hand surgery for Dupuytren's contracture. J Plast Surg Hand Surg 2012;46:177–83.
- Peimer C, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with Collagenase Clostridium histolyticum (CORDLESS Collagenase Option for Reduction of Dupuytren Long-Term Evaluation of Safety Study): 5-Year data. J Hand Surg (Am) 2015;40:1597–605.
- Peimer CA, McGoldrick CA, Greg Kaufman G. Nonsurgical treatment of Dupuytren contracture: 3-year safety results using Collagenase Clostridium histolyticum. J Hand Surg 2013;38:e52.
- Rodriguez JR, Zhang W, Scammell BE, Davis TRC. Dynamism in Dupuytren’s contractures. J Hand Surg 2015;40E:166–70.
- Coleman S, Gilpin D, Kaplan FT, et al. Efficacy and safety of concurrent collagenase clostridium histolyticum injections for multiple Dupuytren contractures. J Hand Surg Am 2014;39:57–64.
- Verhayden JR. Early outcomes of a sequential series of 144 patient with Dupuytren's disease treated by collagenase injection using an increased dose, multicord technique. J Hand Surg 2015;40:133–40.
- Kaplan TD, Badalamente M, Hurst L, et al. Delayed manipulation following Clostridial Collagenase histolyticum injection for Dupuytren contracture. J Hand Surg 2013;38:52–3.
- Mickelson DT, Noland SS, Watt AJ, et al. Prospective RCT comparing 1-versus 7-day manipulation following collagenase injection for Dupuytren contracture. J Hand Surg(Am) 2014;39:1933–41.
- Kemler MA, Houpt P, van der Horst CM. A pilot study assessing the effectiveness of postoperative splinting after limited fasciectomy for Dupuytren's disease. J Hand Surg Eur Vol 2012;37:733–7.
- Larson D, Jerosch-Herold C. Clinical effectiveness of post-operative splinting after surgical release of Dupuytren's contracture: a systematic review. BMC Musculoskelet Disord 2008;9:104.
- Warwick D, Arner M, Pajardi G, et al. Collagenase Clostridium histolyticum in patients with Dupuytren's contracture: results from POINT X, an open-label study of clinical and patient-reported outcomes. J Hand Surg 2015;40E:142–32.
- Hay DC, Louie DL, Earp BE, et al. Surgical findings in the treatment of Dupuytren’s disease after initial treatment with clostridial collagenase. J Hand Surg 2014;39E:463–5.
- Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg 2010;15:109–13.
- Au-Yong IT, Wildin CJ, Dias JJ, Page RE. A review of common practice in Dupuytren surgery. Tech Hand up Extrem Surg 2005;9:178–87.
- Engstrand C, Boren L, Liedberg GM. Evaluation of activity limitation and digital extension in Dupuytren's contracture three months after fasciectomy and hand therapy interventions. J Hand Ther 2009;22:221–6.
- Draviaraj KP, Chakrabarti I. Functional outcome after surgery for Dupuytren's contracture: a prospective study. J Hand Surg Am 2004;29:804–8.
- Crean SM, Gerber RA, Le Graverand MP, et al. The efficacy and safety of fasciectomy and fasciotomy for Dupuytren's contracture in European patients: a structured review of published studies. J Hand Surg Eur 2011;36:396–407.
- Figus A, Britto JA, Ragoowansi RH, Elliot D. A clinical analysis of Dupuytren's disease of the thumb. J Hand Surg Eur 2008;33:272–9.
- Milner RH. Dupuytren's disease affecting the thumb and first web of the hand. J Hand Surg Br 2003;28:33–6.
- Bendon CL, Giele HP. Collagenase for Dupuytren's disease of the thumb. J Bone Joint Surg Br 2012;94:1390–2.
