Abstract
Complex interactions between pain, depression, and anxiety impact quality of life in patients with ALS. Psychological approaches to pain control may be useful. This study explored the role of self-efficacy in mitigating pain. Individuals registered with the Agency for Toxic Substances and Disease Registry National ALS Registry and who experienced pain were invited to participate in an online survey. Subjects completed the Brief Pain Inventory-Short Form, Hospital Anxiety and Depression Scale, and Chronic Pain Self-Efficacy Scale. Correlations between variables were determined. Multiple linear regression models assessed relationships between depression, anxiety and self-efficacy predictions, and pain severity, interference, and relief. Results recorded that there were 197 participants (58% males, mean age 59 ± 10 years). Cases or borderline cases of depression or anxiety were common. Mean levels of pain were moderate. Higher pain self-efficacy scores predicted lower pain severity, lower pain interference, and higher pain relief with treatment. As depression scores increased, pain interference with daily life was higher. In conclusion, anxiety and depression are common in patients with ALS and pain. Self-efficacy appears to mitigate pain. A multifactorial approach to pain management should be considered in these patients, addressing mental health and self-efficacy to augment pharmacologic pain treatments.
Introduction
Pain is a common symptom in amyotrophic lateral sclerosis (ALS). It is experienced in all stages of the disease, and has a moderately severe impact on patients’ daily activities (Citation1–6). Many factors contribute to pain in these patients, including muscle atrophy, damage to connective tissue, increased strain on joints, joint contractures and discomfort from pressure due to limited mobility, and muscle cramps and spasms (Citation4). Pain has been found to directly affect quality of life (QoL). Patients with ALS with untreated or inadequately treated pain report a significantly lower QoL, as well as a more dramatic decline in QoL during disease progression, compared to patients who report that their pain has been adequately treated (Citation7). ALS patients have a relatively high prevalence of psychological morbidity, demonstrating significantly higher levels of depression, hopelessness and anxiety than the general population. As a group, ALS patients have been found to be as distressed as 68% of the psychiatric outpatient population (Citation8). Research on the prevalence of depression in patients with ALS has shown broad ranges spanning from 2% to 75% (Citation9), with the wide variability likely due to the use of differences in depression assessment instruments (Citation10). The prevalence of anxiety in ALS is less studied but has been reported to range between 8% and 26% (Citation9). There appears to be a link between pain, depression, and anxiety (Citation11,Citation12). Thus, pain has the potential to be a major contributor to mood disturbances in patients with ALS. A more complete knowledge of the factors contributing to both physical pain and the psychological experience of pain and suffering in ALS patients would be an important advance in the management and treatment of the disease.
Little is known of psychological factors that may be used to mitigate the complex interactions of pain, depression, and anxiety in the ALS patient population. One avenue by which to approach this would be through a better understanding of self-efficacy in these patients. Self-efficacy is the expectation that one can control one’s outcomes by executing a behavior (Citation13). The strength of self-efficacy is dependent on behavioral effort in changing an outcome and persistence in attempts (Citation14). Stronger self-efficacy results in better coping under stress, and has been shown to contribute to the chronic pain patient’s coping, including pain coping behaviors and compliance with pain treatment programs (Citation14). The aim of the current study is to explore the relationship between depression, anxiety, self-efficacy, and the pain experience in patients with ALS.
Subjects and methods
Patients included individuals registered with the Agency for Toxic Substances and Disease Registry (ATSDR) National ALS Registry who indicated that they would like to be informed about research studies. The registry is the largest dataset on Persons with ALS (PALS) in the United States and has over 5000 registrants. The website permits PALS to select whether they wish to be told about research trials. Researchers who want to contact PALS in the Registry must send ATSDR information about their study, including Institutional Review Board approval. If ATSDR approves the request, ATSDR will email eligible and interested PALS information about the study. PALS who wish to do so may then contact the researcher about participating in the study. This study recruited participants with ALS from the ATSDR ALS Registry who experienced pain of any kind.
Methods
This study utilized a cross-sectional observational design using a public online and anonymous survey. The survey was hosted on the internet using REDCAP™ and was posted from 1 January to 15 April 2015. Written notification posted on the survey website provided participants with information regarding the research objectives, risks and benefits, directions for how to complete the survey and contact information for the study coordinator for questions or concerns. Informed consent was obtained on the website before beginning the study survey. Institutional Review Board exempt approval was obtained (IRB Study #1918).
Instruments
ALS Functional Rating Scale-Revised (ALSFRS-R) (Citation15). This is a 12-item questionnaire measuring speech, swallowing, fine and gross motor skills and respiratory function. Total scores vary from 0 (worst possible function) to 48 (normal function). The self-administered version used for this study has been found to be valid compared to the standard evaluator-administered version (Citation16).
Brief Pain Inventory-Short Form (BPI) (Citation17). The BPI assesses pain at its ‘worst’ and ‘least’ in the last 24 h, and also assesses pain ‘on the average’ and ‘right now’. Scores range from zero (no pain) to 10 (pain as bad as you can imagine). The BPI measures the extent to which pain has interfered with the following over the past 24 h: general activity, walking, work, mood, enjoyment of life, relations with others, and sleep. BPI pain interference is scored as the mean of the seven interference items, each of which is scored from zero (does not interfere) to 10 (completely interferes). The BPI also asks patients to assess the amount of relief of pain that treatments have provided in the past 24 h, ranging from 0% (no relief) to 100% (complete relief). BPI is a reliable and valid instrument (Citation17–19). High test-retest reliability and alternate-form reliability is demonstrated when pain is stable or when pain changes in a predictable way (Citation17).
Hospital Anxiety and Depression Scale (HADS) (Citation20). The HADS comprises 14 items rated on a 4-point Likert scale (range 0–3). It is designed to screen for the presence and severity of depression and anxiety over the past week in medical patients. It possesses a seven-item depression subscale (HADS-D) and a seven-item anxiety subscale (HADS-A). Each subscale score is the sum of the respective seven items (ranging from 0 to 21). Scores in each of the domains of 0–7 represent non-cases, scores of 8–10 are borderline cases and scores of 11–21 represent clinical cases of depression and anxiety (Citation20). Subsequent analyses have shown greatest sensitivity and specificity in detecting depression and anxiety when each of these conditions is defined as a score of 8 or higher (Citation21). The scale comprises primarily items unrelated to somatic symptoms of depression and anxiety, and has been used to assess patients with ALS (Citation22).
Chronic Pain Self-Efficacy Scale (CPSS) (Citation14). The CPSS is a validated, 22-item self-report questionnaire that is designed to measure chronic pain patients’ perceived self-efficacy to cope with the consequences of pain. The scale comprises three factors: (1) self-efficacy for pain management; (2) self-efficacy for coping with symptoms; (3) self-efficacy for physical function. Each item is posed as a question, and the subject is asked to rate on a 10-point Likert scale from 10 to 100 how certain they are that they can accomplish a specific task (e.g. that they can decrease their pain by methods other than taking extra medication, or that they can keep their pain from interfering with their sleep). The physical function factor contains items which are inappropriate for an ALS sample (e.g. how certain are you that you can walk a half mile, how certain are you that you can lift a 10 pound box?). Therefore, only items from the two factors (self-efficacy for pain management and for coping) were used in the current study, knowing that they are only a portion of the validated measure. Normative data have not been published for this instrument.
Statistics
All variables were summarized initially with frequencies and percentages or means, medians, and standard deviations. Several variables were calculated for analysis: the ALSFRS-R score by summing the individual components, a measure of interference in daily activity by taking the average of the Brief Pain Inventory (BPI) activity questions, a HADS depression and HADS anxiety score, and pain self-efficacy and coping self-efficacy scores. Several of these variables were either somewhat skewed or more ordinal in nature, so correlations between variables were determined using a Spearman correlation rather than the usual Pearson correlation. Three multiple linear regression models were used to look for relationships of depression, anxiety and self-efficacy predictions on pain severity, pain interference, and pain relief. Disease duration, gender, age, and function as measured by the ALSFRS-R were included as predictors in each of the regression models. p <0.05 was used to test for significance. Model assumptions were checked with variance inflation factor statistics and residual plots. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patient demographics
One hundred and ninety-seven participants responded to the survey. The mean age was about 60 years, nearly 60% were males, and most demonstrated limb onset. Participant demographics are reported in .
Table 1. Patient characteristics.
Pain presentation and pain management
Pain data from the BPI are presented in . Mean levels of pain on average, pain interference with daily activities, and pain relief were reported as moderate. Pain relief data were not normally distributed, with the most common response to the pain relief question being 0% (no relief of pain) in 23% of the sample (). The clinicians most commonly managing pain were neurologists, followed by primary care physicians. Close to 30% of the sample were not receiving any pain management.
Figure 1. Pain relief. Patients were asked to rate the amount of relief that pain medications or treatments have provided in the past 24 h, ranging from 0% (no relief) to 100% (complete relief). Nearly a quarter obtained no relief from their pain.
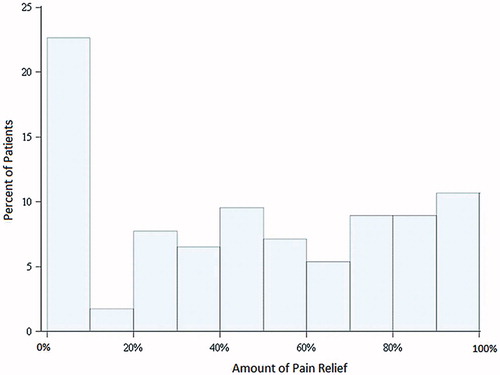
Table II. Pain features and management.
Depression, anxiety and self-efficacy
Depression and anxiety were relatively common in the sample, with more than half the subjects screening as cases or borderline cases of depression (). Anxiety was less common, but nearly one-fifth screened as clinical cases of anxiety and more than one-fifth were borderline cases. Pain self-efficacy and coping self-efficacy scores were, on average, moderate (). Correlations among depression, anxiety, self-efficacy and the pain variables of severity, interference and relief are provided in . While many of the correlations were significant, some of the strongest correlations were observed between the pain and coping self-efficacy scores and the scores for pain severity and interference.
Table III. Descriptive statistics of depression, anxiety and self-efficacy.
Table IV. Correlations of depression, anxiety, self-efficacy, and pain.
Multiple linear regression was used to model the influence of depression, anxiety, and self-efficacy along with disease characteristics of age, gender, disease duration and function as predictors of pain severity, interference, and relief (). Pain self-efficacy but not coping self-efficacy was a significant predictor of pain severity, pain interference, and relief from pain with treatment. Higher pain self-efficacy scores predicted lower pain severity, lower pain interference, and higher pain relief with treatment. Gender was a significant predictor of pain severity and pain relief as well. On average, females reported greater pain severity but also higher pain relief from treatments than males. Pain interference scores decreased as the age of the patients increased. The correlations between depression and pain interference indicated that as depression scores increased, pain interference with daily life was higher. Anxiety, disease duration and function were not significant predictors of any of the pain outcomes in the study.
Table V. Pain severity, interference, and relief: multiple linear regression.
Discussion
There appears to be a need for more effective pain management in patients with ALS. Pain in our series was, on average, moderate in intensity, but was severe in some patients, and a barrier to daily life activities. Similar findings have been observed by others (Citation2,Citation3,Citation6) and by us in a previous series of patients recruited by a different method (Citation23). Importantly, relief as a result of pain treatments was not impressive, averaging less than 50% (100% = complete relief). Nearly a quarter of the sample experienced no pain relief, and this is a major concern. Others have found that the use of opioids is common, yet those same series demonstrate significant average pain (Citation2,Citation3,Citation6). It is unknown whether the poor pain control is due to lack of adequate assessment of pain by the health care team, ineffective treatments, or other causes, but it certainly is meaningful that almost 30% of our study patients were not receiving any treatment for their pain. Combined with the modest level of pain control and the presence of pain interference, this points to a clinical management gap in ALS care.
The impact of age and of gender that were found in our study parallel those in the literature and point to the need to consider these factors when assessing and treating those with ALS and pain. The elderly generally rate the intensity and unpleasantness of painful stimuli lower than do younger individuals (Citation24), and they perceive some type of physiological, disease-associated pain to be less severe than do those who are younger (Citation25). On a biological level, this may be due to age-dependent reductions in inflammation, in primary afferent fibers, or in pain receptors (Citation25). Psychosocial factors may play a role, as the elderly may expect pain with aging, and regard it as less bothersome than do younger individuals (Citation26). With regard to gender, females in epidemiological studies are more likely than males to report pain. Females also have reduced pain thresholds and reduced pain tolerance to noxious stimuli. Several studies have demonstrated greater responses to opioids in females than males, which may explain the greater pain relief in females in our sample (Citation27).
Depression and anxiety, as assessed in this series and measured by others, are common also in ALS patients (Citation8–10,Citation22). The current study does not provide information about the treatments or therapies used for depression and anxiety or how effective they were. However, it is clear that depression and anxiety are significant concerns for this disease population.
Correlations demonstrated significant relationships between both pain self-efficacy and coping self-efficacy, and the pain experience. However, a multilevel regression model demonstrated that pain self-efficacy and not coping self-efficacy was a significant predictor of pain and pain relief. The complex relationships between pain, QoL, anxiety, and depression argue for a multi-modal approach to pain management. It has been noted that psychological approaches to symptom management in ALS are under-explored and under-utilized. Mindfulness has been shown to impact QoL and psychological well-being but other psychologically-based studies are rare (Citation28,Citation29). The results of our study point to the importance of acknowledging the role of self-efficacy in the pain experience. Assessment of self-efficacy appears to be clinically important in the understanding of an individual’s pain experience and their potential adjustment to chronic pain. Interventions that focus on utilizing self-efficacy theory (Citation13) may aid in reducing perceptions of pain or help in the relief of pain. Cognitive-behavioral interventions can help to develop self-efficacy using activities such as coping skills and self-management training with rehearsal and practice in the individual’s daily environment (Citation30–32). Successful experiences in pain control can produce the greatest changes in self-efficacy beliefs (Citation14).
This study demonstrates the potential value of the ATSDR ALS Registry for research, permitting recruitment of a relatively large sample in a short period of time, and demonstrating the utility of using the ALS registry for public online surveys to understand the disease. However, the limitations of such a method must be considered. The patient population was limited to those motivated enough to register, to indicate they wished to be notified about research, and to respond to the invitation for this particular research study. However, the sample demographics are similar in age, gender distribution, and prevalence of familial cases to those of the ALS patient population as a whole. The study group appears to have more slowly progressive disease than average, in view of the relatively well-preserved ALSFRS-R despite a mean disease duration of nearly four years. It is likely that severely depressed patients would lack the motivation to participate in the study. The socioeconomic status of our patients was not assessed, so it is possible that access to care as a result of socioeconomic status may have influenced the availability and efficacy of pain treatments for some.
In conclusion, pain, anxiety, and depression are all common in ALS, and the relationships between them are complex. Self-efficacy appears to be a mechanism that warrants further exploration as a means to mitigate pain. In view of the relationship of pain to QoL in ALS, and to anxiety and depression more generally, approaches that help patients gain self-efficacy may be effective, non-pharmacologic mechanisms for improving the lives of patients with ALS. Pain management protocols in this disease population should be multifactorial, addressing significant mental health and self-efficacy contributions to the pain experience as a way of augmenting standard symptomatic pain management treatments.
Acknowledgements
This work was supported by the Paul and Harriet Campbell Fund for ALS Research, the ALS Association Greater Philadelphia Chapter, and many private donations.
Declaration of interest
Z. Simmons has received reimbursement from Neuralstem, Inc., for serving on a Data Safety Monitoring Board for an ALS therapeutic trial. Other authors have no conflicts of interest to declare.
References
- Ganzini L, Johnston WS, Hoffman WF. Correlates of suffering in amyotrophic lateral sclerosis. Neurology. 1999;52:1434–1440.
- Wallace VCJ, Ellis CM, Burman R, Knights C, Shaw CE, Al-Chalabi A. The evaluation of pain in amyotrophic lateral sclerosis: a case-controlled observational study. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:520–7
- Hanisch F, Skudlarek A, Berndt J, Kornhuber ME. Characteristics of pain in amyotrophic lateral sclerosis. Brain Behav. 2015;e00296.
- Handy CR, Krudy C, Boulis N, Federici T. Pain in amyotrophic lateral sclerosis: a neglected aspect of disease. Neurol Res Int. 2011;2011:403808.
- Rivera I, Ajroud-Driss S, Casey P, Heller S, Allen J, Siddique T, et al. Prevalence and characteristics of pain in early and late stages of ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:369–72.
- Chiò A, Canosa A, Gallo S, Moglia C, Ilardi A, Cammarosano S, et al. Pain in amyotrophic lateral sclerosis: a population based controlled study. Eur J Neurol. 2012;19:551–5.
- Pagnini F, Lunetta C, Banfi P, Rossi G, Fossati F, Marconi A, et al. Pain in amyotrophic lateral sclerosis: a psychological perspective. Neurol Sci. 2012;33:1193–6.
- Felgoise SH, Chakraborty BH, Bond E, Rodriguez J, Bremer BA, Walsh SM, et al. Psychological morbidity in ALS: the importance of psychological assessment beyond depression alone. Amyotroph Lateral Scler. 2010;11:351–8.
- Simmons, Z. Rehabilitation of Motor Neuron Disease. In: Barnes M, Good D, eds. Neurorehabilitation. Handbook of Clinical Neurology (Michael Aminoff, series ed). Amsterdam: Elsevier; 2013. pp 483–98.
- Wicks P, Abrahams S, Masi D, Hejda-Forde S, Leigh PN, Goldstein LH. Prevalence of depression in a 12-month consecutive sample of patients with ALS. Eur J Neurol. 2007;14:993–1001.
- Lerman SF, Rudich Z, Brill S, Shalev H, Shahar G. Longitudinal associations between depression, anxiety, pain, and pain-related disability in chronic pain patients. Psychosom Med. 2015;77:333–41.
- Rzewuska M, Mallen CD, Strauss VY, Belcher J, Peat B. One-year trajectories of depression and anxiety symptoms in older patients presenting in general practice with musculoskeletal pain: a latent class growth analysis. J Psychsom Res. 2015;79:195–201.
- Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215.
- Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain.1995;63:77–84.
- Cedarbaum JM, Stambler N, Fuller C, Hilt D, Thurmond B, Nakanishi A, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169:13–21.
- Kasarskis EJ, Dempsey-Hall L, Thompson MM, Luu LC, Mendiondo M, Kryscio R. Rating the severity of ALS by caregivers over the telephone using the ALSFRS-R. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:50–4.
- Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with non-cancer pain. Clin J Pain. 2004;20:309–18.
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapor. 1994;23:129–38.
- Atkinson TM, Rosenfeld BD, Sit L, Mendoza TR, Fruscione M, Lavene D, et al. Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI). J Pain Symptom Manage. 2011;41:558–65.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361–70.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77.
- Taylor L, Wicks P, Leigh PN, Goldstein LH. Prevalence of depression in amyotrophic lateral sclerosis and other motor disorders. Eur J Neurol. 2010;17:1047–53.
- Stephens HE, Lehman E, Raheja D, Yang C, Walsh S, McArthur, et al. Pain in amyotrophic lateral sclerosis: patient and physician perspectives and practices. Amyotroph Lateral Scler Frontotemporal Degener. (in press). DOI: 10.3109/21678421.2015.1074701.
- Petrini L, Matthiesen ST, Arendt-Nielsen L. The effect of age and gender on pressure pain thresholds and suprathreshold stimuli. Perception. 2015;44:587–96.
- Daoust R, Paquet J, Piette E, Sanogo K, Bailey B, Chauny J-M. Impact of age on pain perception for typical painful diagnoses in the emergency department. J Emerg Med. 2016;50:14–20. doi: 10.1016/j.jemermed.2015.06.074.
- Yong HH, Gibson SJ, Horne DJ, Helme RD. Development of a pain attitude questionnaire to assess stoicism and cautiousness for possible age differences. J Gerontol B Psychol Sci Soc Sci.2001;56:279–84.
- Paller CJ, Campbell CM, Edwards RR, Dobs AS. Gender-based differences in pain perception and treatment. Pain Med. 2009;10:289–99.
- Pagnini F. Psychological well-being and quality of life in amyotrophic lateral sclerosis: a review. Int J Psychol. 2013;48:194–205.
- Pagnini F, Phillips D, Bosma CM, Bosma MC, Reece A, Langer E. Mindfulness, physical impairment and psychological well-being in people with amyotrophic lateral sclerosis. Psychol Health. 2015;30: 503–17.
- O’Sullivan K, Dankaerts W, O’Sullivan L, O’Sullivan PB. Cognitive Functional Therapy for Disabling, Non-specific Chronic Low Back Pain: Multiple Case-Cohort Study. Phys Ther. 2015;95:1478–1488.
- Wilson M, Roll JM, Corbett C, Barbosa-Leiker C. Empowering Patients with Persistent Pain Using an Internet-based Self-Management Program. Pain Manag Nurs. 2015;16:503–14.
- Rini C, Porter LS, Somers TJ, McKee DC, DeVellis RF, Smith M, et al. Automated internet-based pain coping skills training to manage osteoarthritis pain: a randomized controlled trial. Pain. 2015;156:837–48.
