Abstract
Background: The aim of this prospective study was to identify clinical factors associated with the development of shoulder pain in stroke patients with pronounced arm paresis. Methods: At stroke onset, 485 patients were initially assessed in 2007–2009. Sixty-three patients with pronounced arm paresis completed the study, and 21 of these developed shoulder pain. Clinical findings were recorded fortnightly by the attending physiotherapist during hospital stay. Results: Hand oedema on the paretic side was more common in patients developing shoulder pain compared with those who did not develop shoulder pain. The onset of shoulder pain was associated with concomitant hand oedema. High NIHSS score was associated with developing shoulder pain. Patients with a history of shoulder pain developed pain earlier than those without previous shoulder pain. Patients with haemorrhagic stroke were significantly more prone to developing shoulder pain. Conclusions: One-third of the stroke patients with pronounced arm paresis developed shoulder pain. Concomitant hand oedema seems to be an additional symptom of shoulder injury. Patients with low general status are more vulnerable to develop post-stroke shoulder pain.
Introduction
Shoulder pain is a common complication after stroke; reported incidence varies between 9% and 64% (Citation1–6). Several studies found that lost or impaired motor function of the arm is a predictor of shoulder pain after stroke (Citation1–3,Citation6–7). Other factors related to shoulder pain are low general status, sensory abnormalities (Citation1,Citation2) and limitations of external rotation in the glenohumeral joint (Citation6,Citation8). Two observation studies (one 6-month and one 16-month study post-stroke onset), reported a high percentage of shoulder pain onsets within the first 2 weeks – 55% and 32%, respectively (Citation1,Citation2). Similar findings were reported from a third study (Citation3). Among those with shoulder pain, the presence of central pain was found in 14% and 6%, respectively (Citation1,Citation2). The prevalence of central post-stroke pain may differ between 1% and 12% (Citation9).
One study describes the complexity in normal shoulder function and the vulnerability of the paretic stroke shoulder. The extended range of movement of the arm is controlled by a complex system of articulations that depend on muscle coordination and stabilization. The loss of muscle stabilization makes the glenohumeral joint highly susceptible to soft-tissue tears and impingement (Citation7). Therapeutic strapping of the hemiplegic shoulder may limit shoulder pain (Citation10). A sonography (ultrasound) study concluded that acute stroke patients with poor upper limb function are more prone to soft tissue injury of the shoulder during rehabilitation (Citation11). Another sonography study reported a higher prevalence of abnormal findings in the painful post-stroke shoulders than in the non-involved shoulders (Citation12). Studies using magnetic resonance imaging report adhesive capsulitis as a possible cause of shoulder pain after stroke (Citation13,Citation14). More prospective studies are needed to understand the circumstances or situations associated with injury in the post-stroke shoulder, especially in patients with pronounced arm paresis.
Aim
The aim of this prospective study was to identify clinical factors associated with the development of shoulder pain in stroke patients with pronounced arm paresis.
Methods
Subjects
A total of 485 patients with acute stroke were consecutively recruited (October 2007 to May 2009) mainly through the Stroke Unit or in a few cases from any of the other wards at the Department of Medicine at Skellefteå Hospital, Sweden. The main inclusion criterion was pronounced arm paresis, determined by the physiotherapists, at stroke onset. Arm motor function was measured with the Modified Motor Assessment Scale according to Uppsala University Hospital 1995 (M-MAS UAS-95) (scored 0–5, 5 = normal function), a validated and reliable Swedish version of the originally MAS. Arm motor function is one out of eight subscales in M-MAS (UAS-95) (Citation15,Citation16). Patients were included if their arm function score was low (M-MAS UAS-95, score 0–1), i.e. supine starting position, the affected arm extended towards the ceiling, not able to flex the elbow to touch the forehead and return to the starting position. This is closest comparable with not able to manage a score of 3 in the original MAS (Citation16).
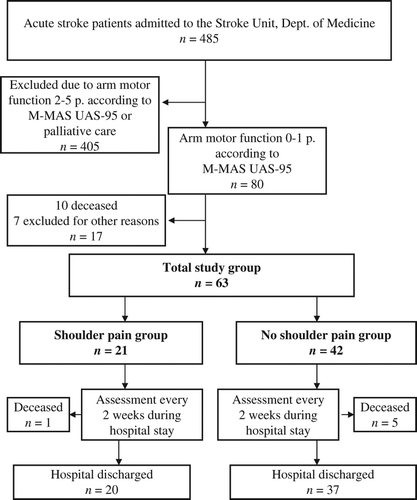
Exclusion criteria were patients in palliative care, patients denying participation and patients developing central pain. Out of 485 patients, 80 fulfilled the inclusion criteria. Seventeen patients were excluded due to the following reasons: difficulties in assessments (n = 2), earlier traumatic shoulder injury with severe shoulder pain (n = 1), earlier pronounced arm paresis with other diagnosis (n = 1), central pain (n = 1), denying to participate (n = 1), later observation of palliative care (n = 1) and deceased before first assessment (n = 10). The flow chart of recruited patients is further described in .
The remaining study group (n = 63) exhibited pronounced arm paresis. During the study period, 21 patients – the ‘shoulder pain group’ – developed shoulder pain and 42 did not develop shoulder pain – the ‘no shoulder pain group’ (). Members of the shoulder pain group reported new onset of shoulder pain or worsened shoulder pain compared with their status before stroke onset. Shoulder pain was defined as either pain at rest, pain during daily activities, or pain during treatment or exercise. Pain only detected in extreme positions of the arm was not recorded. Six patients from the total study group who deceased before hospital discharge were assessed and recorded during hospital stay and were therefore included in the study ().
Procedure
All assessments at hospital were performed by the clinical physiotherapists at the Stroke Unit with support from the supervising physiotherapist (MI). Before the study period started and twice during the study period, the physiotherapists met with the supervisor to calibrate the assessments. Patients were assessed by clinical physiotherapists continuously during hospital stay (weekdays), but assessments were recorded at admission, every second week and at hospital discharge.
Ethical considerations
Each participant provided informed consent; if this was not possible, a family member provided consent. As reporting only referred to the groups, individuals were not identified in the study. Ethical approval was obtained from the regional ethical review board in Umeå, Sweden.
Assessments
Baseline at admission
The following baseline variables were registered at admission: age, gender, date of stroke onset, affected hemisphere measured by computer tomography and/or clinical symptoms, incidence of falls at stroke onset, information regarding previous or current shoulder pain of affected shoulder, and arm motor function (M-MAS UAS-95) (Citation15,Citation16). Functional Ambulation Classification (FAC) was used to measure gait (scored 0–5, 5 = ambulation independent) and has been found to have excellent reliability, good validity and good responsiveness in patients with stroke (Citation17–19). Stroke severity at onset was measured using the National Institute of Health Stroke Scale (NIHSS), an instrument with high degree of reliability and validity (Citation20,Citation21). The NIHSS is a tool to objectively quantify the impairment caused by a stroke (scored 0–42, 0 = no stroke symptoms). The NIHSS is composed of 11 items: level of consciousness, horizontal eye movement, visual field test, facial palsy, motor arm, motor leg, limb ataxia, sensory, language, speech, and extinction & inattention. Data on stroke severity, type of stroke and history of previous stroke, were obtained from patients’ records.
Follow-up during hospital stay
During hospital stay, assessments were performed continuously and recorded every 2 weeks after stroke onset, and at hospital discharge. The following variables were registered: arm motor function (M-MAS UAS-95), gait (FAC), presence and onset of hand oedema, presence of inferior subluxation in the glenohumeral joint by palpation with the patient in the sitting position. Moreover, additional variables were registered: any occurrence of passive range of motion exercise of the affected arm, presence of neglect symptoms, sensory disturbances with light touch of the arm, proprioception of the arm, occurrence of resting position on the hemi paretic side, communication disorders such as dysphasia/aphasia, as well as other communication disorders such as dementia, confusion, deafness, fatigue and dysarthria. It was also registered whether the patients needed assistance when dressing the upper body, whether they were receiving assistance with personal hygiene in bed, and whether a lift was used to move from a bed to a wheelchair and from a wheelchair to a toilet. At shoulder pain onset, clinical physiotherapists were asked for date of onset and any known causative trauma. Onset of shoulder pain, onset of hand oedema, onset of passive range of motion exercise, and falls before onset of pain were double-checked by referring to medical records.
Statistics
Descriptive results are presented as median, interquartile ranges (IQR), numbers (n) and percentages (%). Differences in proportions between the two groups were analyzed using chi-squared test. For age, NIHSS, days at hospital and days until shoulder pain onset, the Mann–Whitney test was used. In the shoulder pain group, Spearman correlation and linear regression was used to identify associations between shoulder pain and concomitant hand oedema. Statistical significance was defined as p < 0.05.
Results
Characteristics at admission
The median age of the total study group was 79 and included 37 women (59%) and 26 men (41%) (). The median length of hospital stay was 39 days for patients in the shoulder pain group compared with 32 days for the patients in the no shoulder pain group. A significantly higher NIHSS score was assessed in the shoulder pain group (p = 0.04). The two groups also differed significantly (p = 0.02) with respect to type of stroke: haemorrhagic stroke was more common in the shoulder pain group (24%) compared with the no shoulder pain group (5%). No other statistical differences were found for any other characteristics at admission between the two groups.
Table I. Characteristics at admission of all stroke patients with pronounced arm paresis, data presented for those who did or did not develop shoulder pain.
Clinical findings during hospital care
Out of 63 patients with pronounced arm paresis, 21 (33%) developed shoulder pain during the study period and 42 (67%) did not. Fifteen patients developed shoulder pain during the first half of the study period, thus only six patients in the second half. The two groups differed significantly with respect to hand oedema on affected side, but for no other characteristics (). In the shoulder pain group, 13 patients (62%) developed hand oedema; in the no shoulder pain group, 11 patients (26%) developed hand oedema. Presence of neglect, exercise with passive range of motion, and sensory disturbance for light touch were somewhat more common among those patients who developed shoulder pain, although the differences were not significant.
Table II. Presence of clinical findings in stroke patients with and without shoulder pain at any occasion during hospital care.
Out of 63 participants in our study, 33 fell at stroke onset (), but only two developed shoulder pain within 3 days. These two also reported earlier shoulder pain. During hospital stay, another three participants in the shoulder pain group fell before pain onset. Of these three, one patient reported immediate shoulder pain. We found no associations between subluxation in the glenohumeral joint and shoulder pain among our study participants.
Clinical findings of the shoulder pain group
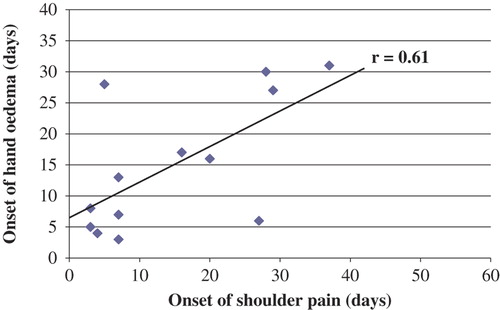
A linear regression analysis found a moderate association (p = 0.02) between the onset of shoulder pain and concomitant finding of hand oedema in the shoulder pain group (). Eleven (52%) of the patients in the shoulder pain group developed pain within 7 days. The median number of days to onset of shoulder pain among those with a history of shoulder pain (n = 10) was 3.5 days compared with 15 days for those with no history of shoulder pain (n = 11) (p = 0.02).
Discussion
Our main findings were that hand oedema on the paretic side was more common among patients with shoulder pain than among those without shoulder pain, and an association between the onset of shoulder pain and concomitant hand oedema was found. High NIHSS score was associated with developing shoulder pain. Patients with a history of shoulder pain developed pain earlier than those without previous shoulder pain. Patients with haemorrhagic stroke were significantly more prone to develop shoulder pain.
Methodological considerations
Impaired arm motor function is often reported to be a contributing factor to post-stroke shoulder pain (Citation1–3,Citation6,Citation7,Citation11). By defining the inclusion criterion as ‘pronounced arm paresis’ at admission, we were able to study a high-risk group and to identify additional associations with clinical factors. An advantage of this study was that all patients with pronounced arm paresis were consecutively recruited and measured at stroke onset and forward. However, the inclusion criterion was accompanied by low general status (measured using NIHSS), which led to limited patient participation, and dropout problems.
The lack of statistically significant differences in our clinical findings, for example presence of neglect, presence of passive range of motion exercise and sensory disturbances for light touch, may be due to the limited number of patients developing shoulder pain in our study. Ratnasabapathy et al. surmised that dysphasia might lead to under-reporting of shoulder pain after stroke (Citation3). This under-reporting may be the case in our no shoulder pain group that had high prevalence communicative problems accompanied by sensory loss, cognitive problems, hemi-neglect and hand oedema. Twenty-one patients developed shoulder pain during the total study period, but very few developed shoulder pain during the second half of the period (n = 6). Due to the focus of the study performed, the involved healthcare personnel might have increased their attention of the risk for shoulder pain onset among the stroke patients. A decrease of shoulder pain incidence during the later part of the study period might indicate this. A similar effect is also considered in a study that investigated therapeutic strapping of the hemiplegic shoulder (Citation10).
The main instrument used in the study, M-MAS (UAS-95), has been tested for reliability and validity in a Swedish context. In addition, the methodology of our study could be considered a strength as the calibration of assessments was performed three times during the study period. Furthermore, the clinical physiotherapist's assessments were performed continuously during weekdays but recorded once every second week during hospital stay. This strategy made it possible to observe clinical findings related to development of shoulder pain continuously, such as onset of hand oedema and onset of shoulder pain. On the other hand, we chose not to follow up after hospital discharge that probably some readers will miss.
The measurement of circumference to identify hand oedema by comparison with the non-paretic hand was found to be unreliable due to normal asymmetries. Instead, the clinical physiotherapist's subjective visual assessment of hand oedema was used. To our knowledge, the reliability of this is not studied.
Our findings in relation to previously published research
Turner-Stokes & Jackson (Citation7) thoroughly describe the complexity of the normal shoulder and the vulnerability of the paretic stroke shoulder. The scapula relation to the thoracic wall continuously seeks the optimal position for the glenoid fossa in order to provide the arm with an extended range of movement. Depending on the scapula coordination and position, the caput humerii has to stay centred in the glenoid fossa, mastered mainly by the rotator cuff, during arm movement. The loss of muscle activity and coordination after stroke onset makes the glenohumeral joint susceptible to impingement and tension trauma. Adding sensory loss and body image disturbance makes the post-stroke shoulder even more vulnerable to soft tissue injury.
Fall after stroke is common (Citation4,Citation5). Out of 63 participants in our study, 33 fell at stroke onset. Only two of these 33 patients developed shoulder pain within 3 days after stroke onset falling. These two also reported earlier shoulder pain, and combined with other symptoms the causes for pain onset were not entirely conclusive. During hospital stay, another three participants in the shoulder pain group fell before pain onset. Of these three, one patient reported immediate shoulder pain. Based on our study findings, falls may interact with other causes, but falls have not been shown to be a major single cause of developing shoulder pain early after stroke onset. We could not identify other single trauma causes. Thus, we hypothesize that repeated multiple low force traumas cause shoulder pain.
An ipsilateral hand oedema was more prevalent among those who developed shoulder pain compared with those who did not, and the time of onset of hand oedema was associated with the onset of shoulder pain. These results might indicate that both shoulder pain and concomitant ipsilateral hand oedema are symptoms of soft tissue injuries in the shoulder among stroke patients with pronounced arm paresis. To our knowledge, this is a new observation.
Lindgren et al. (Citation2) reported that low general status at baseline, measured as high NIHSS mean scores, is a predictor of shoulder pain. Our inclusion criterion pronounced arm paresis, as expected, was accompanied by a high median score for NIHSS at admission (). Although a low general status was observed in our total study group, a significantly higher NIHSS median score for those developing shoulder pain was found. The NIHSS assessment tool measures loss of functions that might serve as protective mechanisms for the shoulder joint such as arm motor function, sensory function, spatial awareness and body image. If we consider impaired arm motor function as one key premise for developing shoulder pain after stroke, the loss of other protective mechanisms probably increases the risk for developing shoulder injury and pain.
Out of the 21 stroke patients who developed shoulder pain, about 50% of the cases occurred within a week after stroke onset. Similar observations regarding early shoulder pain onset are reported by Gamble et al. (Citation1) and Lindgren et al. (Citation2), although there are differences in the selection of patients and study design between our study and these two studies as we only included patients with pronounced arm paresis during hospital stay. A difference was observed within our shoulder pain group: the patients with early onset of shoulder pain more often reported a history of shoulder pain. This seems to be logical, as loss of muscle stabilization in the shoulder probably revealed or provoked earlier injuries. Another reason for early onset of shoulder pain might be that the patients are in their lowest and most vulnerable status immediately after stroke onset. It would have been of interest to follow the study participants for more than 3 months to gain more information about long-term outcomes. Unfortunately, no follow-up was performed.
Haemorrhagic stroke was more frequent in the shoulder pain group in our study, but to our knowledge, no other studies have reported similar findings. As very few patients were diagnosed as haemorrhagic stroke, our study could not analyse differences within haemorrhagic subgroups. It is notable, however, that NIHSS admission median score for the haemorrhagic stroke patients was higher compared with the ischemic stroke patients (data not shown).
Different results have been reported about the association between subluxation in the glenohumeral joint and shoulder pain after stroke (Citation2,Citation7,Citation8,Citation22). Lindgren et al. (Citation2) reported an association, but we did not, even though we used their assessment method. This difference is probably due to differences in inclusion criteria, resulting in different comparison designs. Logically, a shoulder joint with a subluxation ought to be more vulnerable than a shoulder joint with caput humerii in its normal physiological position. Our study results, however, might indicate that subluxation of the shoulder joint has a stronger relation to pronounced arm paresis than developing shoulder pain after stroke onset.
Sensory disturbance from light touch has been reported to be a predictor of shoulder pain after stroke (Citation1,Citation2). As no association was found in our study, our study may lack statistical power. The same phenomenon might be applied for the association between neglect and shoulder pain. However, larger studies are needed to determine such associations.
Among stroke patients with pronounced arm paresis developing shoulder pain, concomitant hand oedema may be another symptom of shoulder injury. When investigating factors that cause post-stroke shoulder injury and pain, we must consider repetitive low force traumas in daily care, such as training, exercise, dressing, personal hygiene, transfers and positioning. These factors may warrant further investigation.
NOTICE OF CORRECTION
The version of this article published online ahead of print on 8 October 2013 contained errors. On pages 3 and 5 “shoulder group” should have read “no shoulder group” in four instances. These errors have been corrected for this version.
Conflict of interest: None to declare.
This study was supported by grants from the County Council of Västerbotten and the National Stroke Association in Sweden.
References
- Gamble GE, Barberan E, Laasch HU, Bowsher D, Tyrrell PJ, Jones AKP. Post stroke shoulder pain: A prospective study of the association and risk factors in 152 patients from a consecutive cohort of 205 patients presenting with stroke. Eur J Pain. 2002;6:467–74.
- Lindgren I, Jönsson AC, Norrving B, Lindgren A. Shoulder pain after stroke. A prospective population-based study. Stroke. 2007;38:343–8.
- Ratnasabapathy Y, Broad J, Baskett J, Pledger M, Marshall J, Bonita R. Shoulder pain in people with a stroke: A population-based study. Clin Rehabil. 2003;17:304–11.
- Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, et al. Medical complications after stroke: A multicenter study. Stroke. 2000;31:1223–9.
- McLean DE. Medical complications experienced by a cohort of stroke survivors during inpatient, tertiary-level stroke rehabilitation. Arch Phys Med Rehabil. 2004;85:466–9.
- Aras MD, Gokkaya NKO, Comert D, Kaya A, Cakci A. Shoulder pain in hemiplegia: Results from a national rehabilitation hospital in Turkey. Am J Phys Med Rehab. 2004; 83:713–9.
- Turner-Stokes L, Jackson D. Shoulder pain after stroke: A review of the evidence base to inform the development of an integrated care pathway. Clin Rehabil. 2002;16:276–98.
- Zorowitz RD, Hughes MB, Idank D, Ikai T, Johnston MV. Shoulder pain and subluxation after stroke: Correlation or coincidence?Am J Occup Ther. 1996;50:194–201.
- Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: Clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009;8:857–68.
- Griffin A, Bernhardt J. Strapping the hemiplegic shoulder prevents development of pain during rehabilitation: A randomised controlled trial. Clin Rehabil. 2006;20:287–95.
- Pong YP, Wang LY, Wang L, Leong CP, Huang YC, Chen YK. Sonography of the shoulder in hemiplegic patients undergoing rehabilitation after a recent stroke. J Clin Ultrasound. 2009;37:199–205.
- Lee IS, Shin YB, Moon TY, Jeong YJ, Song JW, Kim DH. Sonography of patients with hemiplegic shoulder pain after stroke: Correlation with motor recovery stage. Am J Roentgenol. 2009;192:W40–W44.
- Pompa A, Clemenzi A, Troisi E, DiMario M, Tonini A, Pace L, et al. Enhanced-MRI and ultrasound evaluation of painful shoulder in patients after stroke: A pilot study. Eur Neurol. 2011;66:175–81.
- Távora DGF, Gama RL, Bomfim RC, Nakayama M, Silva CEP. MRI findings in the painful hemiplegic shoulder. Clin Radiol. 2010;65:789–94.
- Barkelius K, Johansson A, Kõrm K, Lindmark B. Reliability and validity testing of modified motor assessment scale according to Uppsala University Hospital-95. Nordisk Fysioterapi. 1997;1:121–6 (in Swedish).
- Carr J, Shepherd R, Nordholm L, Lynne D. Investigation of a new Motor Assessment Scale for stroke patients. Phys Ther. 1985;65:175–80.
- Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl-Baker L. Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness. Phys Ther. 1984; 64:35–40.
- MacKnight C, Rockwood K. Assessing mobility in elderly people. A review of performance-based measures of balance, gait and mobility for bedside use. Clin Gerontol. 1995;5: 464–86.
- Mehrholz J, Wagner K, Rutte K, Meissner D, et al. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch Phys Med Rehabil. 2007;88:1314–9.
- Lyden P, Brott T, Tilley B, Welch KMA, Mascha EJ, Levine S, et al. Improved reliability of the NIH stroke scale using video training. Stroke. 1994;25:2220–6.
- Kasner SE, Chalela JA, Luciano JM, Cucchiara BL, et al. Reliability and validity of estimating the NIH stroke scale score from medical records. Stroke. 1999;30:1534–7.
- Paci M, Nannetti L, Rinaldi LA. Glenohumeral subluxation in hemiplegia: An overview. J Rehabil Res Dev. 2005;42: 557–68.