Abstract
Context: The development of a reliable, eco-friendly process for synthesis of gold nanoparticles (AuNPs) has gained impetus in recent years to counter the drawbacks of chemical and physical methods. Objective: This study illustrates simple, green synthesis of AuNPs in vitro using cell lysate supernatant (CLS) of non-pathogenic bacteria and to investigate its potential antimicrobial activity. Materials and methods: Gold nanoparticles were synthesized by the reduction of precursor AuCl4− ions using the CLS of Bacillus licheniformis at 37°C upon 24 h of incubation. The nanoparticles were characterized for their morphology, particle size, optical absorption, zeta potential, and stability. Further the antimicrobial activity was assayed using cup-plate method. Results: The process of biosynthesis was extracellular and the gold ions were reduced to stable nanogold of average size 38 nm. However, upon storage of AuNPs for longer duration at room temperature stability was influenced in terms of increase in particle size and decrease in zeta potential with respect to as synthesized nanoparticles. SEM micrographs revealed the spherical shape of AuNPs and EDX analysis confirmed the presence of gold in the sample. Also clear zone of inhibition was observed against Bacilllus subtilis MTCC 8364, Pseudomonas aeruginosa MTCC 7925, and Escherichia coli MTCC 1698 confirming the antimicrobial activity of AuNPs. Discussion: The bioprocess under study was simple and less time consuming as compared to other methods as the need for harvesting AuNPs from within the microbial cells via downstream process will be eliminated. Nanoparticles exhibited good stability even in absence of external stabilizing agents. AuNPs showed good antimicrobial activity against several Gram-negative and Gram-positive pathogenic bacteria. Conclusion: The extracellular biosynthesis from CLS may serve as a suitable alternative for large scale synthesis of gold nanoparticles in vitro. The synthesis from lysed bacterial cell strongly suggests that exposure of microbial whole cells to the gold solution for nanoparticle formation is not necessary and that microorganism even in lysed state retained its bioreduction potential. Further the potential of biologically synthesized AuNPs as antimicrobial agents will be of great commercial importance.
Introduction
The recent surge of research on nanoparticles synthesis from natural living sources has attracted considerable attention in the field of nanobiotechnology and application research. The shape, morphology, and size of nanoparticles play an important role in controlling physical, chemical, optical, and electrical properties of these nanostructured materials (Kamat Citation2002, EI-Sayed Citation2001). A variety of “chemical” and “physical” methods are used for synthesis of metallic nanoparticle, however, they are associated with many drawbacks including use of toxic chemicals, imperfection of surface structures, and environmental hazards. Accordingly, there is an essential need to develop simple, economic, size controlled, high-yield, and environmentally benign procedures for synthesis of metallic nanoparticles (Thakkar et al. Citation2010).
Biosynthesis of gold nanoparticles (AuNPs) has been carried out by using different bacteria, fungi, actinomycetes, algae, and plant species (He et al. Citation2007, Wen et al. Citation2009, Bhambure et al. Citation2009, Ahmad et al. Citation2003, Ghodake et al. Citation2010, Du et al. Citation2011). Among various reported species, prokaryotes offer advantage over eukaryotes in terms of easy handling and genetic manipulation as a means for over-expressing specific enzymes involved in nanomaterial synthesis and is usually a single-step process without additional requirement of toxic chemicals and stringent conditions. Considering these advantages, a bacterial system could prove to be an excellent alternative for the synthesis of gold nanoparticles (Mandal et al. Citation2006, Sweeney et al. Citation2006).
Microorganisms are emerging as potential nanofactories for biosynthesis of nanoparticles owing to their ability to survive and grow in high metal ion concentration (Beveridge et al. Citation1997, Bruins et al. Citation2000). Also cellular enzymes like NADH and NADPH-dependant reductases are important factors in metal nanoparticles synthesis (Ahmad et al. Citation2003). Intracellular biosynthesis makes the job of downstream processing difficult and also beats the purpose of developing a simple and economical bioprocess as the surface-trapped gold nanoparticles or those which are formed inside the biomass would require an additional step of processing like ultrasonication (Ganesh and Gunasekaran Citation2009), cell disruption techniques by using detergents (Nangia et al. Citation2009) or lysis buffer (Samadi et al. Citation2009), for their release into the surrounding medium. The recovery process also results in considerable loss in the quantity of nanoparticles. Many microorganisms reported for biosynthesis of AuNPs are pathogenic to plants and/or humans and require longer incubation (> 120 h) for generating nanoparticles (Bai et al. Citation2011). This would make the handling and disposal of biomass a major threat to environment and also unsuitable for commercialization of the bioprocess.
Selection of suitable microorganism and bioprocess design plays an important role in efficient biosynthesis of gold nanoparticles. We have selected Bacillus licheniformis, as a potential “nanofactory” for synthesis of AuNPs as it is neither a human pathogen nor a toxigenic microorganism (Rey et al. Citation2004). Strategies involving release of specific enzymes from microbial cell into external medium to be used for extracellular bioreduction of AuNPs can do away with the necessity of downstream processing for releasing of nanoparticles from within the cell as in case of intracellular synthesis. Hence, the present study describes a novel method for the use of CLS of Bacillus licheniformis as a source for extracellular biosynthesis of AuNPs, its subsequent characterization, stability, and assessing its potential role as antimicrobial agent which would be a great advantage for commercial application.
Materials and methods
Bacterial strain and growth condition
Bacillus licheniformis MTCC 429 (Gram positive, thermophilic bacteria) was procured from Institute of Microbial Technology (IMTECH), Chandigarh, India. The culture was maintained at 4°C in nutrient agar slants and subcultured at regular intervals.
Preparation of cell lysate supernatant
Bacillus licheniformis was grown in modified nitrate broth supplemented with glucose as carbon source. The cultures were incubated at 100 rpm at 37°C for 24 h. The cultures were then centrifuged at 5000 rpm for 20 min. The obtained pellet was resuspended in 50 ml of 0.05 M phosphate buffer solution. Further ultrasonic disruptions of cells were carried out with an ultrasonic processor (Sonics Vibra Cell VC 130, Newton, USA) for 5 min (30sec on/off cycle) to obtain the CLS. The sonicated sample was then centrifuged at 8000 rpm for 10 min. The resultant supernatant was used for the synthesis of gold nanoparticles.
In vitro biosynthesis of gold nanoparticles
The obtained CLS supernatant was incubated with aqueous HAuCl4 solution of 1mM concentration for 24 h at 200 rpm at 37°C. Simultaneously, a control of the CLS of B. licheniformis (without HAuCl4) was also maintained at same condition. Aliquots of the reaction solution were removed periodically and characteristic absorption of synthesized AuNPs was measured.
Characterization of gold nanoparticles
The excitation spectra of the samples containing AuNPs were measured by UV-visible spectrophotometer. While the particle size distribution of gold nanoparticles was measured by dynamic light scattering technique using Zetasizer (NanoZS; Malvern Instruments, Worcestershire, UK). The measurement conditions were as follows: He-Ne Red laser, 4.0 MW, 633 nm, temperature 25°C, refractive index 1.333, or with adjustment if needed. All measurements were done in triplicate using disposable polystyrene cuvettes (Malvern Instruments, Worcestershire, UK). The zeta potential of the sample was measured by photon correlation spectroscopy using Zetasizer (NanoZS, Malvern Instruments, worcestershire, UK) equipped with 4.0 mW He-Ne Red laser (633 nm). The preparations were diluted with double distilled water for measurement of zeta potential. All measurements were done at 25°C in triplicate. Further, the air-dried sample containing AuNPs were further subjected to field emission scanning electron microscope (SEM) (JEOL JSM-6390 LV) and simultaneously the EDX spectrum was also recorded.
Antimicrobial activity of the gold nanoparticles
The antimicrobial activity for the synthesised gold nanoparticles was evaluated by cup plate method (Rose and Miller Citation1939). The three different bacterial strains Bacilllus subtilis MTCC 8364, Pseudomonas aeruginosa MTCC 7925, and Escherichia coli MTCC 1698 were selected for antimicrobial study. A definite bacterial density (equivalent to 0.5 Mcfarland standards) was mixed in molten Mueller Hinton agar (MHA) media. The AuNPs were dispersed in deionized water and the solution was further added to the cup prepared in solidified media. Further the zone of inhibition was observed after incubating the plate at 37°C for 12 h.
Results
Characterization of gold nanoparticles
In vitro biosynthesis of gold nanoparticles
The study on extracellular biosynthesis of gold nanoparticles by CLS of B. licheniformis has been demonstrated. The aqueous chloroaurate ions (Au3+) were reduced to metallic gold (Auo) upon exposure to the bacterial CLS. The color of the reaction mixture turned from pale yellow to pink upon 24 h of incubation at 37°C, indicating the formation of gold nanoparticles while the CLS control retained its original color as shown in .
Surface plasmon resonance of gold nanoparticle by UV-visible spectroscopy
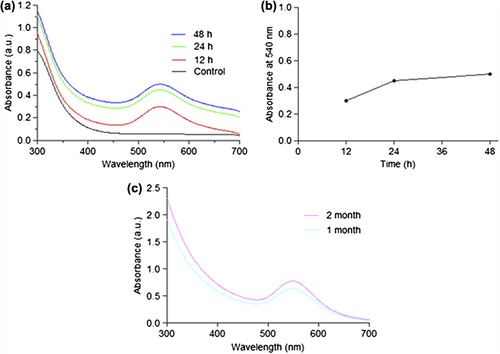
The AuNPs synthesized were primarily characterized by measuring the absorption and formation of surface plasmon resonance band by UV-Vis spectroscopy. UV- visible spectra of gold nanoparticle obtained from Bacillus licheniformis CLS showed an intense peak at 540 nm which is a characteristic absorbance for gold nanoparticles whereas the control showed no evidence of absorption in the range of 300–700 nm as depicted in a.
Figure 2. The optical absorption spectra of gold nanoparticle at different time intervals (a) Absorbance of gold nanoparticles against wavelength (control, 12h, 24h, and 48h); (b) The graph shows relationship between optical absorbance of gold nanoparticles with incubation time; (c) Stability of gold nanoparticles at room temperature (1 month and 2 month).
Stability study of biosynthesized gold nanoparticles
In order to assess the nanoparticle stability, absorbance spectra were measured periodically to ascertain the intensity of bioreduction as a function of time. It was observed that the absorbance increased progressively until 24 h of incubation after which there was not much increase as observed till 48 h, indicating AuNPs in solution were quite stable a, b as a matter of fact that the absorption peak at 24 h and 48 h remained in close proximity at 540 nm indicates that the AuNPs were well dispersed in the solution and there was not much aggregation. However, when aged for 2 months there was change in optical properties, size, and zeta potential of the AuNPs in suspension c owing to degradation of suitable capping agents, active biomolecules, enzymes, and proteins leading to aggregation of particles. As particle size increased, the zeta potential was found to decrease with time as shown in .
Table I. Optical properties, size, and zeta potential of synthesized gold nanoparticles from cell lysate supernatant of B. licheniformis at different time interval.
Particle size measurement and zeta potential of gold nanoparticles
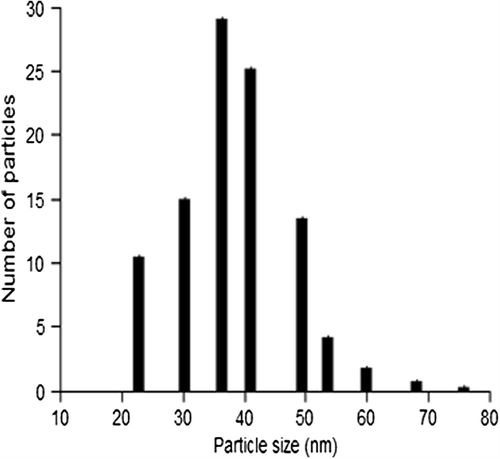
The particle size range of the biosynthesized gold nanoparticles was determined using dynamic light scattering method and the corresponding size histogram has been depicted in . The AuNPs synthesized were in the size range of 20–75 nm and the average particle size was 38 ± 2.57. The zeta potential for the gold nanoparticle in suspension was found to be − 24.6 ± 1.76 indicating the nanoparticles were relatively stable.
SEM and EDX study of gold nanoparticles
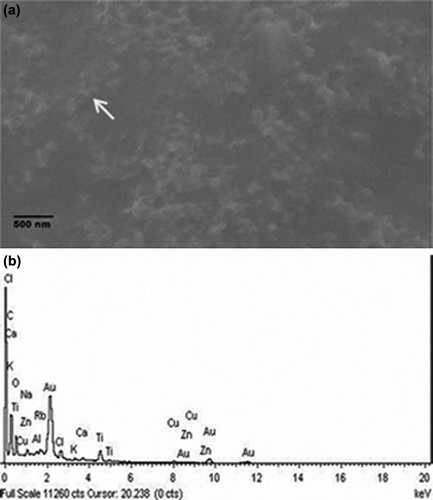
A representative SEM micrograph of the sample containing AuNPs showed the formation of spherical nanoparticles and elemental analysis using EDX has also confirmed the presence of gold in the sample. It is evident from the SEM images that AuNPs were well dispersed and spherical in size a. A spot EDX profile derived from focussing one of the nanoparticles generated a sharp peak corresponding to Au as depicted in b.
Antimicrobial activity of the gold nanoparticles
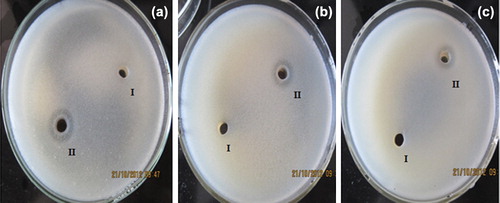
The antimicrobial activity of AuNPs was clearly demonstrated by the appearance of clear zone of inhibition in both Gram positive and Gram negative bacteria. We observed zone of inhibition around the cup (II) in the plate () containing dispersed AuNPs, indicating the antimicrobial activity of AuNPs against Bacilllus subtilis MTCC 8364, Pseudomonas aeruginosa MTCC 7925. and Escherichia coli MTCC 1698; the control experiment with only deionized water in the cup (I) exhibited no zone of inhibition.
Discussion
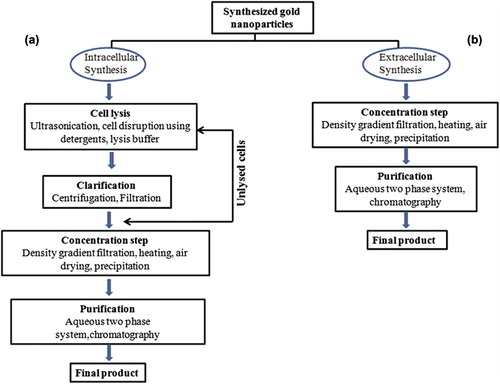
Gold nanoparticles are well known to exhibit vivid colors (Mulvaney Citation1996) depending upon the surface plasmon absorption. Moreover, it would be useful if the nanoparticles could form extracellularly, as it will eliminate the need to harvest the nanoparticles formed within the cells. Also the time required in downstream processing to recover and purify gold nanoparticles from cell lysate supernatant is reduced in the present studies as the two steps namely repeated cell lysis and subsequent centrifugation required in intracellular synthesis are not applicable in this case as illustrated in . It is speculated that cellular biomolecules, enzymes, and proteins present in the CLS of the organisms aided in the reduction of chloroaurate ions into gold atoms and further, formation of gold nanoparticles. The frequency and width of surface plasmon is dependent on size and shape of metallic nanoparticles as well as the dielectric constant of the surrounding medium and the metal itself (Burda Citation2005). It is also hypothesized that the CLS obtained from bacterial biomass harvested at stationary growth phase had optimal quantity of cellular enzymes and membrane proteins specially those involved in ion transport that could have also lead to increased and size-controlled biosynthesis of gold nanoparticles in in vitro conditions. Preferably the role of cellular enzymes NADPH-dependant reductases and nitrate reductases is anticipated in bioreduction of chloroaurate ions to elemental AuNPs. Also B. licheniformis is known to be a good source for these enzymes that are present at the cell membrane as respiratory enzymes (Rey et al. Citation2004). The role of phosphate capping via NADPH- reductases (Nangia et al. Citation2009) present in the CLS is possibly the important factor in stabilization of the biosynthesized gold nanoparticles which is also evident from a, b. Also zeta potential of the suspension is one of the key parameters in assessing the stability of the synthesized nanoparticle as it gives the net electrostatic potential of any particle in suspension. Larger particle size exhibit low zeta potential and ultimately the nanoparticles are unstable. Whereas higher zeta potential corresponds to stability by creating repulsive force between the nanoparticle and keeping them away from each other, thereby limiting the aggregation of AuNPs in suspension (Bac et al. Citation2011). To compete with the chemical process of nanoparticles synthesis, biological process has to gain a very strict control over average particle size in a specific size range and uniform particle morphology (Gericke and Pinches Citation2006). Physical parameters play a major role in achieving size-controlled biosynthesis. From stability reports, it is evident that the reaction time is an important factor in controlling the morphology of the AuNPs and hence by controlling the reaction time and modulating the physical parameters small size, spherical, monodispersed AuNPs could be obtained from the “novel green nanofactories”. Further it has been also observed in our studies that gold nanoparticles synthesised from Bacillus licheniformis has shown potent antimicrobial activity against the Gram positive (Bacillus subtilis) and Gram negative (Pseudomonas aeruginosa and Escherichia coli) microorganisms. Further this potential of AuNPs is of great advantage as it may be useful in water purification (Das et al. Citation2009) and also in overcoming the problems of bacterial resistance to antibiotics. Extracellular biosynthesis of nanoparticles is now attracting a considerable interest as it is more economical and an efficient approach. Extracellular biosynthesis of AuNPs using cell filtrate and cell supernatant have been reported recently (He et al. Citation2007, Hussieny et al. 2007, Wen et al. Citation2009, Du et al. Citation2011). However, our study of B. licheniformis CLS-mediated extracellular synthesis of AuNPs serves as a novel approach as there are no published reports on the use of bacterial CLS as source for AuNPs biosynthesis to the best of our knowledge.
Conclusions
We report here a novel green synthesis of extracellular gold nanoparticles using Bacillus licheniformis CLS at 37°C without using any chemical reducing agents. This single-step greener approach is low cost, nontoxic, simple, and environmentally benign procedure for synthesis of gold nanoparticles. Further, the extracellular bioreduction of gold nanoparticles would have advantage from process point of view as it will minimise the steps of downstream processing of the nanoparticles. The results also imply that exposure of whole cells to the gold solution during nanoparticle formation is not necessary and that microorganism even in lysed state retains its bioreduction potential. Identification of the active reducing proteins or enzymes involved in biosynthesis could potentially allow for a process in a cell-free environment, where the size and shape of the particles can be precisely controlled. Further by modulating the physical parameters involved, and using suitable capping agents to passivate the surface of gold nanoparticles, size- and shape-controlled biosynthesis could be achieved. The potential of synthesized AuNPs as antimicrobial agent will open new avenues in medical and environmental research.
Acknowledgement
Authors thankfully acknowledge the financial supports from Department of Science and Technology, Government of Jharkhand to carry out this research and technical assistance provided by Central Instrumentation Facility, Birla Institute of Technology, Mesra, Ranchi, Jharkhand, India.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the contents and writing of the paper.
References
- Ahmad A, Senapati S, Khan MI, Kumar R, Sastry M. 2003. Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, Thermomonospora sp. Langmuir. 19:3550–3553.
- Bac LH, Kim JS, Kim JC. 2011. Size, optical and stability properties of gold nanoparticles synthesized by electrical explosion of wire in different aqueous media. Rev Adv Mater Sci. 28:117–121.
- Bai HJ, Yang BS, Chai CJ, Yang GE, Jia WL, Yi ZB. 2011. Green synthesis of silver nanoparticles using Rhodobacter Sphaeroides. World J Microbiol Biotechnol. 27:2723–2728.
- Beveridge TJ, Hughes MN, Lee H, Leung KT, Poole RK, Savvaidis I, et al. 1997. Metal-microbe interactions: contemporary approaches. Adv Microb Physiol. 38:177–243.
- Bruins RM, Kapil S, Oehme FW. 2000. Microbial resistance to metals in the environment. Ecotoxicol Environ Saf. 45:198–207.
- Burda C, Chen X, Narayanan R, El-Sayed MA. 2005. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 105:1025–102.
- Bhambure R, Bule M, Shaligram N, Kamat M, Singhal R. 2009. Extracellular biosynthesis of gold nanoparticles using Aspergillus niger – its characterization and stability. Chem Eng Technol. 32:1036–1041.
- Das SK, Das AK, Guha AK. 2009. Gold nanoparticles: microbial synthesis and application in water hygiene management.Langmuir. 25:8192–8199.
- Du L, Xian L, Feng JX. 2011. Rapid extra-/intracellular biosynthesis of gold nanoparticles by the fungus Penicillium sp. J Nanopart Res. 13:921–930.
- EI-Sayed MA. 2001. Facile synthesis of micrometer-sized gold nanoplates through an aniline-assisted route in ethylene glycol solution. Acc Chem Res. 34:257–264.
- Ganesh BMM, Gunasekaran P. 2009. Production and structural characterization of crystalline silver nanoparticles from Bacillus cereus isolate. Colloids Surf B Biointerfaces. 74:191–195.
- Ghodake GS, Deshpande NG, Lee YP, Jin ES. 2010. Pear fruit extract assisted room temperature biosynthesis of gold nanoplates. Colloids Surf B Biointerfaces. 75:584–589.
- Gericke M, Pinches A. 2006. Biological synthesis of metal nanoparticles. Hydrometallurgy. 83:132–140.
- He S, Guo Z, Zhang Y, Zhang S, Wang J, Gu N. 2007. Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulate. Mater Lett. 61:3984–3987.
- Husseiny MI, Abd El-Aziz M, Badr Y, Mahmoud MA. 2007. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim Acta A. 67:1003–1006.
- Kamat PV. 2002. Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. J Phys Chem B. 106:7729–7744.
- Mandal D, Bolander ME, Mukhopadhyay D, Sarkar G, Mukherjee P. 2006. The use of microorganisms for the formation of metal nanoparticles and their application. Appl Microbiol Biotechnol. 69:485–492.
- Mulvaney P. 1996. Surface plasmon spectroscopy of nanosized metal particles. Langmuir. 12:788–800.
- Nangia Y, Wangoo N, Goyal N, Sharma S, Wu JS, Dravid V, Shekhawat GS, Suri CR. 2009. Facile biosynthesis of phosphate capped gold nanoparticles by a bacterial isolate Stenotrophomonas maltophilia. Appl Phys Lett. 94:233901–233903.
- Rose SB, Miller RE. 1939. Studies with the agar cup-plate method I. A standardized agar cup-plate technique. J Bacteriol. 38:525–537.
- Rey MW, Ramaiya P, Nelson BA, Brody-Karpin SD, Zaretsky EJ, Tang M, et al. 2004. Complete genome sequence of the industrial bacterium Bacillus licheniformis and comparisons with closely related Bacillus species. Genome Biol. 5:R77.
- Samadi N, Golkaran D, Eslamifar A, Jamalifar H, Fazeli MR, Mohseni FA. 2009. Intra/extracellular biosynthesis of silver nanoparticles by an autochthonous strain of Proteus mirabilis isolated from photographic waste. J Biomed Nanotechnol. 5:247–253.
- Sweeney SF, Woehrle GH, Hutchison JE. 2006. Rapid purification and size separation of gold nanoparticles via diafiltration. J Am Chem Soc. 128:3190–3197.
- Thakkar KN, Mhatre SS, Parikh RY. 2010. Biological synthesis of metallic nanoparticles. Nanomedicine. 6:257–262.
- Wen Li, Lin Z, Gu P, Zhou J, Yao B, Chen G, Fu J. 2009. Extracellular biosynthesis of monodispersed gold nanoparticles by a SAM capping route. J Nanopart Res. 11:279–288.