Abstract
In the area of water purification, nanotechnology provides efficient removal of pollutants and germs. Electrospun nanofibers membrane has a potential for water purification due to its high large surface area, and good mechanical strength. In the present study, PAMAM dendrimers composite nylon-6 nanofibers membrane was prepared by crosslinking method using glutaraldehyde. Modified membrane has drastically improved water purification efficiency. Further, the efficacy of the modified membrane can be renewed by mere exposure of the saturated membrane with the solution having acidic pH. The modified membrane can be used as an effective tool for water purification.
Introduction
Clean water free of toxic chemicals and pathogens is essential for human health. In developing countries like India majority of the diseases are due to contaminated drinking water. In the past few years polymeric nanofibres have attracted a lot of attention for water filtration because of their large surface area to volume ratio and their unique nanometre-scale architecture. Due to availability of very fine pore size, huge surface area and high charge density they possess high adsorption capacities as well as high microbial and ionic retention capacity. Moreover, nanofiber sheets offer higher permeability to water than conventional materials in current use due to very high porosities and interconnected pore structures (Thavasi et al. Citation2008). Bejorge et al. conducted a very effective experiment using electrospun nylon nanofiber sheet and showed that these membranes can be used for water filtration applications, but that further improvements are necessary before these membranes can be practically employed (Bjorge et al. Citation2010). Here, we employed one of the very successful tools, i.e. PAMAM dendrimer (generation 5), in collaboration with the nanofibers sheet to improve its efficacy for the removal of ionic impurities and microbial contamination in the local water. In vitro studies were conducted to evaluate the percent retention of ionic and microbial impurity. Dendrimers provided excellent opportunities to develop effective ultrafiltration (UF) processes for the purification of water contaminants such as toxic metal ions, radionuclide, organic and inorganic solutes, bacteria and viruses. Dendrimers are symmetrical and spherical macromolecules consisting of a relatively dense shell which includes a core, branching sites and terminal groups. The charge on the terminal groups and nonporous structure seems to be responsible for the retention of the ionic as well as microbial contaminations. Their interior may be similar or very different from the surface of the molecule. Moreover, their physicochemical properties such as reactivity, complex or salt formation, hydrophilicity and so forth can be varied and optimized by selecting an optimum polymer and processing conditions. Diallo et al. utilized poly (amidoamine) (PAMAM) dendrimers with ethylene diamine (EDA) core and terminal NH2 groups to recover Cu (II) ions from aqueous solutions and found that the Cu (II) binding capacities of the PAMAM dendrimers are much higher at a pH of aqueous solution than those of linear polymers with amine groups (Diallo et al. Citation2005). Amine- terminated PAMAM dendrimers possess high binding affinity for metal ions to their surface via coordination to the amine or acid functional groups. As the complexation of metal ions with the dendrimer is pH-dependent, the release of metal ions from dendrimers can be readily achieved by the protonation of amine functional groups by exposing them to low pH (Crooks et al. Citation2001). The adsorbed ions can therefore be recovered and the dendrimers based water filter can be recycled easily and this feature makes PAMAM dendrimers a suitable choice for metal ion separation.
Materials and methods
Materials
Polyamide-6 (Nylon-6, Mn = 10,000g/mol) PAMAM dendrimer (5th generation), formic acid were purchased from Sigma-Aldrich Pvt. Ltd., USA. All materials and solvents were of analytical grade and were used as received without further purification.
Methods
Modification of the polymer
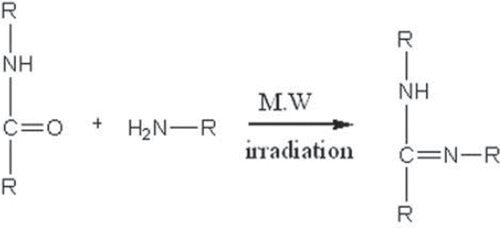
Nylon-6 was crosslinked with the dendrimer having a primary imine group through a microwave-assisted reaction (). For the crosslinking, Nylon -6 and dendrimer were dissolved in formic acid and adsorbed on silica 100–200 mesh. The dried adsorbed reaction mixture was placed in a domestic microwave oven and irradiated for 5 min. After irradiation, the reaction mixture was extracted by adding methanol to the silica-adsorbed reaction mixture. The procedure was repeated thrice to ensure complete extraction. The extract was concentrated on a rotary evaporator. The semisolid residue was further characterized by IR spectroscopy.
Preparation of nylon membrane
Nylon-6 membrane was prepared using both plain and modified polymer. Nanofibers membrane was prepared by electrospinning using the method optimized by Chowdhury and Stylios. The polymer was dissolved in Formic acid (96.7%) at a temperature of 20°C and stored at the same temperature. A fixed polymeric concentration of 20% was selected for the experiments. Electrospinning was carried out at room temperature in air. Nylon-6 fibers were electrospun from syringe (24 gauge needle) and collected by a stainless steel mandrel rotating at 350 rpm. The syringe was set up at a tip-to-collector distance of 8 cm and the feeding rate was fixed to 0.20 ml/hr and the electrospinning voltage was 15 kV (Chowdhury and Stylios 2012).
Removal of pathogens
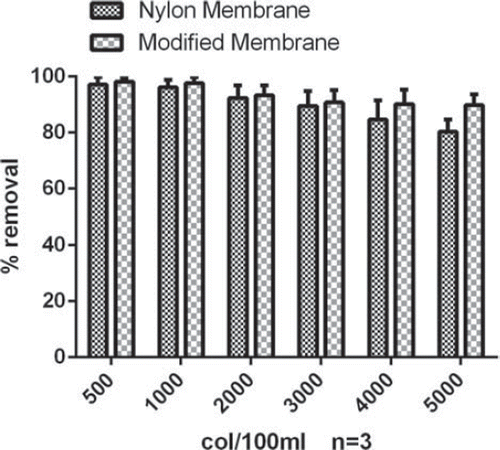
The samples were filtered through the filter membrane fitted in a syringe column with pressure. Water samples with different microbial concentrations (E. coli, colony/ 100 ml) were used to evaluate the efficiency of the membranes. The removal of pathogens was expressed as percent removal of the initial concentration used ().
Removal of ions
The membranes were fitted in a syringe column and a water sample (100 ml) containing fixed Li+ concentration 500ppm was passed through the membrane. The Li+ concentration was analyzed by flame photometry (microprocessor flame photometer, 1381 from environmental and scientific instrument co., India). The amount of Li+ in the filtrate was calculated by using standard curve. The final results are expressed as percentage of the total metal ions added initially. Subsequent batches were passed through the same membrane to evaluate the saturation point of the membrane.
Renewal of membrane activity
After the adsorption reached equilibrium, the membrane was removed followed by the desorption of the ions by incubating with 10 mL of the HCl solution (0.1 M) for 30 min. After the Li+ desorption, the regenerated adsorbent was washed thoroughly with deionized water for adsorption in the succeeding cycle and the results were compared with the results from the first batch.
Results
Characterization of the modified membrane
The imine formation was confirmed by observing the infra red spectrum of the residue. The appearance of the band at 1640 cm21 confirmed the presence of the imine group and the desired crosslinking of dendrimer with Nylon 6. An intense band was also observed at 1670 cm21 due to the amide carbonyl stretch indicating the presence of free amide groups also along with C = N stretch. The extent of crosslinking was not investigated as the aim of the study was just to link the dendrimer with nylon 6. Other bands observed correspond to C-H stretches (2920 cm21) and C-N stretch (1376 cm21).
Characterization of the electrospun nylon membrane
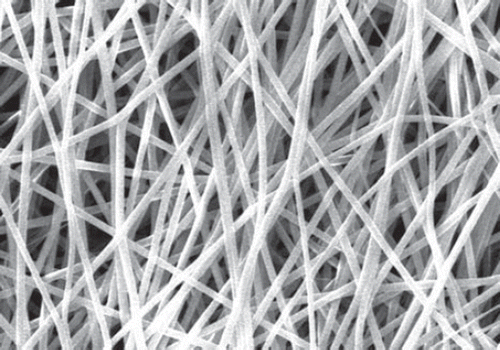
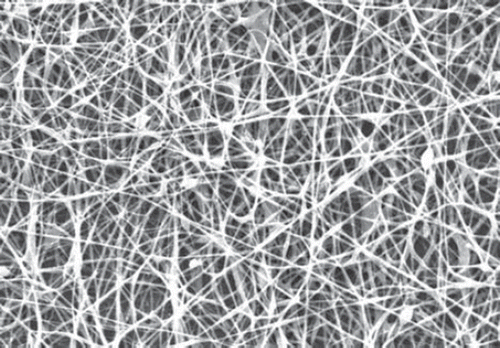
The morphology of the prepared nanofiber sheet was examined using scanning electron microscopy (SEM), Jeol JSM- 6100, JEOL BV, Schiphol-Rijk, Netherlands. The fibers were coated with gold and observed under 25 kV accelerating voltage. Width of 100 randomly selected fibers was measured. The electrospinning of plain nylon-6 nanofibers resulted in a flat-sheet non-woven nanofibers membrane (). However, electrospinning of polymeric nano-composite () resulted in slight increase in the mean diameter and formation of some beads of the modified membrane also indicated the successful attachment of the dendrimer.
Removal of pathogens
The results showed that the removal of pathogens was good in both the cases i.e. with the plain nylon-6 nanofiber sheet as well as the membrane modified with PAMAM dendrimer. Both the membranes showed good removal capacity at lower microbial load. At higher microbial load the modified membrane showed better efficiency while there was a significant decrease in the removal efficacy of the plain nylone membrane beyond 3000 col/100 ml microbial concentration ().
Removal of Li+ ions
Removal of Li+ using plain and modified membranes is shown in . The maximum removal efficiency was more than 80% in case of modified membrane, while it was significantly low (˜55%) in case of plain nylon-6 membrane. There was a dramatic fall in the ion retention capacity of the modified membrane compared with the unmodified membrane.
Table I. Removal of Li+ using plane and modified membranes.
Renewal of membrane activity
After the saturation of the modified membrane there was a drastic fall in the ion-retaining capacity. Interestingly the membrane regained the activity after exposure to the low pH. We observed a slight downfall in the ion retention capacity even after acid treatment. After exposing for 30 min the membrane regained more than 90% of the initial ion retention capacity.
Discussion
The discharge of toxic metal ions into surface water, groundwater, and coastal water systems has caused a major water contamination problem throughout the world. In the past few years nanofibre microfiltration membranes received a special attention for water purification. Polyamide (PA) proved to be a suitable polymer for electrospinning and can be electrospun under steady-state conditions optimized by Chowdhury et al. (Citation2010). This production process resulted in a flat-sheet non-woven nanofibre membrane with the mean diameter between 50 and 100 nm and a thickness of 120 μm. To further increase the efficiency of the membrane we anchored the PAMAM dendrimer with the polymer before electrospinning. The filtration tests with modified nanofibers membranes showed better pathogen removal efficiency even at higher microbial concentration while the plain nylone membrane was unable to retain the capacity and there was a drastic decrease in pathogen removal percentage beyond a microbial load of 3000 col/100 ml. This increase in the efficacy after modification can be explained based upon the highly branched and charged surface of the PAMAM dendrimer. The lower removal of pathogens with plain nylon membrane can be explained by the fact that it can remove the pathogen by ultrafiltration and due to slighter density of the free charged functional groups. Therefore, a major fraction of bacteria may survive and may leak through the membrane. These modified membranes can be utilized to increase the efficacy of the existing water purifiers. Furthermore, ion retention study showed that modified membrane has maximum retention capacity. It may be due to the fact that PAMAM dendrimers have higher binding affinity for metal ions to their surface via the complexation reaction including Cu(II), Fe(III), Ag(I), and Zn(II), Li (I) (Crooks et al. Citation2001, Vassilev and Ford Citation1999, Kaczorowska and Cooper Citation2009). Therefore, the modified filter medium can be utilized for a number of metal ions not limited to the Li+. Here, we selected Li+ as a model to evaluate the effect of dendrimer anchoring on the ion retention capacity of the Nylon-6. There was a drastic decrease in the ion retention capacity of the modified membrane after 2nd cycle of water purification. It may be explained based upon the saturation of the terminal amine groups due to the presence of high ion concentration in the sample, while insignificant difference in the ion-trapping capacity of the plain nylon membrane may be due to involvement of physical adsorption.
Conclusion
For the water purification, it can be concluded that in the case of plain nylon membranes the retention efficiency for the ions (˜55%) and microbial retention is not satisfactory especially at higher concentration. Modification with PAMAM dendrimer retained the microbial retention capacity even at higher microbial load (> 3000 col/100 ml). We achieved ion retention more than 80% in case of modified membrane. Moreover, the retention capacity can be easily renewed by treatment with low pH solution. It can be concluded that nylon nanofibers filters have potential in water filtration applications due to high surface charge and very small pore size, but there are possibilities to improve the surface characteristics for optimum results. Modified membrane showed great potential and can be utilized to improve the efficacy of the existing water purifiers.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bjorge D, Daels N, Vrieze SD, Dejans P, Camp TV, Audenaert W, et al. 2010. Initial testing of electrospun nanofibre filters in water filtration applications. Water SA. 36:151–156.
- Chowdhury M, Stylios G. 2010. Effect of experimental parameters on the morphology of electrospun Nylon 6 fibers. IJBAS-IJENS. 10:116–131
- Crooks RM, Zhao M, Sun L, Chechik V, Yeung LK. 2001. Dendrimer-Encapsulated Metal Nanoparticles: Synthesis, Chacterization, and Applications to Catalysis. Acc Chem Res. 34:181–190
- Diallo MS, Christie S, Swaminathan P, Johnson JH, Goddard WA. 2005. Dendrimer Enhanced Ultrafiltration. Recovery of Cu (II) from Aqueous Solutions Using PAMAM Dendrimers with Ethylene Diamine Core and Terminal NH2 Groups. Environ Sci Technol. 39:1366–1377.
- Kaczorowska MA, Cooper HJ. 2009. Electron Capture Dissociation and Collision-Induced Dissociation of Metal Ion (Ag+, Cu2+, Zn2+, Fe2+, and Fe3+) Complexes of Polyamidoamine (PAMAM) Dendrimer. J Am Soc Mass Spectrom. 20:674–681.
- Sadr Ghayeni SB, Beatson PJ, Fane AJ, Schneider RP. 1999. Bacterial passage through microfiltration membranes in wastewater applications. J Memb Sci. 153:71–82
- Thavasi V, Singh G, Ramakrishna S. 2008. Electrospun nanofibers in energy and environmental applications. Energy Environ Sci. 1:205–221.
- Vassilev K, Ford WT. 1999. Poly (propylene imine) Dendrimer Complexes of Cu (II), Zn (II), and Co (III) As Catalysts of Hydrolysis of p-Nitrophenyl Diphenyl Phosphate. J Polym Sci Part A Polym Chem. 37:2727–2736.