Abstract
HEMA-Lactate-Dextran cryogel scaffolds were produced by cryogelation. Mesencyhmal stem cells (MSC) were isolated from rat bone marrow. Critical sized cranial bone defects were created in rat cranium. Stem cells were injected inside the macropores of the cryogel scaffolds prepared from HEMA-Lactate-Dextran possessing the same dimensions with the defect and placed in the cranial bone. The cryogels placed in the defect without stem cells served as control. After selected time intervals the experimental sites were removed from the animals and new bone formation and tissue integration were investigated by histological analysis. The in vivo results exhibited osseous tissue integration within the implant and mineralized functionally stable bone restoration of the cranial defects. Tissue formation started in the macrospores of the scaffold starting from periphery to the center. A significant ingrowth of connective tissue cells and new blood vessels allowed new bone formation. Histological data demonstrated that new bone per total defect area ratio, were not significantly different in “scaffold-stem cells” group compared to that of “scaffold only” group on all time points. However, the blood vessel density was significantly higher in “scaffold-stem cells” group comparing to that of the “scaffold only” group on day 30. “Scaffold-stem cells” given group gave better tissue response score when compared to “scaffold only” group on day 180.
Introduction
Cranial Region is a resistant configuration which consists of membranous bone surrounding the central nervous system. The protection and maintenance of the central nervous system with a bone is of vital importance. In the event that bone integrity in these regions are ruined and that traumatic or surgical defects occur, functional and/or aesthetic losses appear as a result of the disintegration of bone roof and restoration becomes compulsory.
Bone tissue has the remodeling potential with continuous activation – resorption cycles. Bone can restore itself by producing its original tissue in fractures or defects. However, restoration with this physiological mechanism in bone defects apart from the certain size and limits is not possible. Autogenous or allogenic grafts etc. obtained from other bony locations are used in cancer surgery, injuries with bone losses or in other reconstructive procedures. These bone restoration procedures can be performed by using grafts, as well as bone flaps, bone support materials or alloplastic materials. This is the one which is carried out by gold standard vascular bone grafts (bone flap) in surgical operations. However, it has disadvantages like having a morbidity-mortality increasing characteristic, being unable to find the appropriate bone of sufficient quantity and quality, requiring extra surgical skill and causing surgical difficulty (Sanan and Haines Citation1997). This situation has caused new searches but still sufficient solutions cannot be found for current problems.
Tissue engineering applications attracted attention in regenerative medicine, especially in regeneration of critical sized defects that do not heal themselves spontaneously (Alsberg et al. Citation2001). Tissue engineering presented new methods, for example, applying only cells as a suspension or combining them with scaffolds containing biologically active components. (Hutmacher and Cool Citation2007, Srouji et al. Citation2011, Rockwood et al. Citation2011). Scaffolds must have suitable pore size and porosity to allow cell growth and migration; adequate surface area and proper chemistry to promote cell adhesion and differentiation; and a degradation profile desired to guide the new tissue formation (Sharma and Elisseeff Citation2004, Caplan Citation2000). A wide range of scaffolds have been produced from polymers by using different scaffold preparation techniques such as electrospinning, salt leaching, molding, prototyping, etc. (Liu and Ma Citation2004, Hou et al. Citation2003, Lannutti et al. Citation2007).
Cryogelation is an alternative rather new scaffold production method and the three dimensional cryogel scaffolds prepared by using that technique have interconnected macroporosity and excellent mechanical properties including flexibility and elasticity in their swollen form. Non-degrading cryogels were synthesized previously from different type of polymers for chromatographic applications in biotechnology (Lozinsky et al. Citation2002). We, the first time in the literature, fabricated biodegradable cryogels to create a potential application area for them in regeneration of tissues as scaffolds (Bölgen et al. Citation2007). We evaluated the interaction of novel biodegradable cryogels with bone cells at different regimes in bioreactors for the restoration of bone tissue and with articular chondrocytes for cartilage tissue engineering (Bölgen et al. Citation2008, Bölgen et al. Citation2011). We have also demonstrated the biocompatibility of these cryogel scaffolds in different tissues via in vivo studies (Bölgen et al. Citation2009). These cryogels were produced from HEMA-Lactate- Dextran. L-Lactic acid and dextran are both natural materials. Poly(α-hydroxy acids), particularly polylactides, are among the most well-known (commercially available) biodegradable polymers and have been used for tissue engineering applications (Gürpınar et al. Citation2003, Valero-Cabre´ et al. Citation2001). Dextran, a polysaccharide, is degradable and shows comparatively good biocompatibility (VanDijk-Wolthuis et al. Citation1997). Poly(HEMA) is another important biomaterial that has been used to prepare biomaterials for wide kind of medical applications (Chirila et al. Citation1992, Quinn et al. Citation1995).
Stem cell-based therapies are promising new therapeutic approaches in regenerative medicine (Rosenbaum et al. Citation2008). Connective tissue cells including osteoblasts are all derived from their common precursors mesenchymal stem cells (MSCs). Due to the multipotent character and self renewal properties of MSCs, they aroused increasing attention particularly for repair of skeletal tissues. A number of studies have demonstrated that MSCs cultured in scaffolds can induce new bone formation and lead to improved healing of bone defects (Weinand et al. Citation2006, Kim et al. Citation2005). Our previous studies demonstrated the potential of biodegradable cryogels for tissue regeneration applications, therefore here, the use of biodegradable HEMA-Lactate-Dextran based cryogels with or without stem cells and their performance in regenerating critical sized bone defects was evaluated.
Materials and methods
Fabrication of cryogels
We have synthesized a series of HEMA-lactate-Dextran polymers carrying double bonds (coming from HEMA group) with different backbone compositions following the protocol described before (Bölgen et al. Citation2007). The following recipe and conditions were used to synthesize the polymer used in this study: L-lactide and HEMA were reacted in the presence of the catalyst, SnOct2, at 110°C. N,N’-carbonyldiimidazole (CDI) was dissolved in dimethylsulfoxide (DMSO) at 40°C in a nitrogen atmosphere and added to HEMA-LLA to obtain HEMA-lactate-imidazoyl carbamate (HEMA-LLA-CI). Dextran was dissolved in DMSO under nitrogen atmosphere and 4-(N,N-diethylamino)-pyridine (DMAP) was added to this solution, which is then mixed with the HEMA-LLA-CI solution to obtain HEMA-lactate-dextran (HEMA-LLA-D). Biodegradable cryogels with different compositions and pore morphologies were produced by cross-linking of this pre-polymer in syringe molds at cryo-conditions, using N,N’-methylene-bis(acrylamide) (MBAm) and N,N,N’,N’-tetramethylenediamine (TMDA) (both obtained from Sigma, Germany), as the crosslinker and catalyst (initiator) as described in our previous publication (Bölgen et al. Citation2007). The recipe and conditions selected to be used in this study were as follows: HEMA–LLA and HEMA–LLA–D were dissolved in a water: dioxane mixture (90: 10, v/v) to reach a final concentration of 6% w/v. The cross-linker, (MBAm), was dissolved in this mixture (25 wt % of total amount macromer). For initiation of reactions, first (TMDA) (1 wt %) was added and then ammonium persulfate (APS) (1 wt %) was added to the reaction mixture. 1 ml of the reaction mixture was injected into the glass mold. This reaction mixture was placed in a syringe mold and frozen at − 20°C for about 1 h and the samples were then kept at − 12°C for 16 h. The cryogels were cut into disks (Diameter: 8 mm and height: 1 mm), dried at room temperature, and sterilized by ethylene oxide before animal experiments.
Preparation of rat bone-marrow mesenchymal stromal/stem cells (MSC)
Preparation of the stem cells used in the animal test were as follows (Ayatollahi et al. Citation2012): Femur and tibia samples were isolated from Sprague Dawley rats under general anesthesia and placed into the cell culture in Dulbecco's Modified Eagle's Medium – Low glucose (DMEM-LG) medium (Sigma, Germany) containing 3% penicillin/streptomycin (Sigma, Germany). Under sterile conditions, femur and tibia samples were flushed with this incubation medium and the isolated cells were washed twice at 1500 rpm for 5 min. Cells (3 × 105 cells/cm2) were incubated in 75 cm2 petri dishes in DMEM-LG medium containing also 20% FBS (Fetal Bovine Serum) (Sigma, Germany), 1% penicillin/streptomycin, and 2 mM glutamine (Sigma, Germany) at 37°C and 5% CO2. Cell culture medium was changed in every 3–4 days. Non-adherent cells were discarded and confluent cells were detached by using 0.25% Tripsin/EDTA (Sigma, Germany), counted by using Trypan blue, frozen in cell culture medium containing 10% DMSO (Fluka, Germany) and stored in liquid nitrogen. Cell pellets were re-suspended within PBS to the concentration of 1 × 106 cells per 50 μL and transferred to insulin injectors for in vivo experiments. Differentiation assays were performed in order to demonstrate in-vitro osteogenic differentiation capacity of the expanded cells. Differentiation media composed of DMEM-LG, FBS, dexamethasone (Sigma, Germany), beta-glycerophosphate (Sigma, Germany) and ascorbic acid (Sigma, UK) was supplemented to the cell culture plates containing culture-expanded mesenchymal stem cells. Differentiation was monitored for 21 days. Osteogenic differentiation was confirmed by Alizarin Red staining at the end of this period. Extracellular matrix calcification was evident by appearance of calcium deposits in culture. Images were obtained by Olympus microscope CKX41 (Tokyo, Japan).
Animal model
In order to present the effects of stem cells on bone regeneration a nonload-bearing cranial bone defect model was selected in which we have a quite long experience in our previous studies related to development of scaffold for bone repair (Pişkin et al. Citation2009, Aydın et al. Citation2011). Fifty seven Sprague Dawley rats weighing between 250–300 g fed ad libitum under temperature- and humidity-controlled conditions at the Animal Research Center of Hacettepe University. This study was performed under permission/approval from the Animal Ethical Committee of Hacettepe University (Approval number: 2007/31-13, Approval date: 26.03.2007).
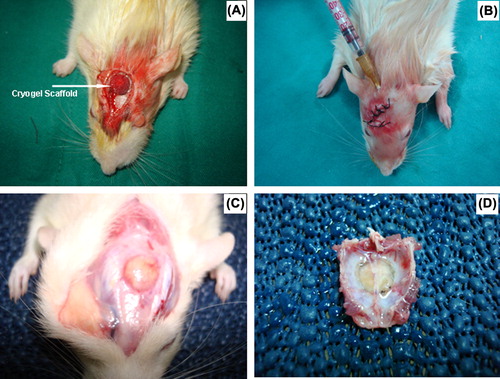
Implantation studies were performed as follows: Animals were anesthetized with a mixture of ketamine HCl (Parke Davis, 50 mg/ml, Taiwan) and Rompun (Bayer, 2%, Germany). The cranial site of the rats was shaved and sterilized with anticeptic Baticon solution (Droksan, 10%, Turkey). The periosteum on the cranial surface was carefully cut and pulled to one side of the defect (Bölgen et al. Citation2009). A disk shaped cranial bone with an 8 mm in diameter (critical sized defect) was removed by using a circular saw carefully while keeping the duramater intact (). The cryogel scaffold prepared in the previous steps (a disk with 8 mm in diameter) was placed in the defect and the periosteum was put back to its original position, and the incision was closed by suturing. In the stem-cell group, a stem cell suspension with about 1 × 106 cells were incorporated inside the cryogel scaffolds by injection (). 29 and 28 test animals were used in the “scaffold only” and “scaffold-stem cell” groups, respectively.
Figure 1. Animal model: (A) Cryogel scaffold implanted in the defect; (B) stem cells injected inside the scaffold which was already implanted in the defect; (C) The cryogel scaffold (loaded with stem cells) in the animal cranial defect after 90 days; (D) the tissue sample harvested from the defect area (after 90 days) for further histological and histomorphometric analysis.
Histology and histomorphometry
The following test methodology was applied as also described in our earlier publications (Pişkin et al. Citation2009, Aydın et al. Citation2011). The test animals were sacrificed at the selected time intervals (after 30, 90 and 180 days of post-implantation) and bone tissue specimens were removed (Figures 1C, 1D) and, placed in 10% neutral buffered formalin at room temperature, all specimens were decalcified in De Castro solution (chloral hydrate, nitric acid, distilled water) and embedded in paraffin by using an automated tissue processor with vacuum. About 3–5 micrometer thick sections were stained with hematoxylin & eosin (HE), Masson's trichrome (MT). Note that MT produces high contrast images with red bone, green osteoid-cartilage and purple cell cytoplasm. Photomicrographs of each cranial defect area were generated by a light microscope (Leica, DMR, Germany) attached computerized digital camera (Model DFC 480, Leica Westlar, Germany). The entire defect area was visible at the lowest magnification. Bright-field images were captured and analyzed quantitatively by image-processing program (Qwin Plus, Leica Inc.Westlar Germany). Number of pixels corresponding to new trabecular bone area in each image was quantified, divided by the total number of pixels corresponding to total defect area and converted to μm2 in each specimen. Osteoblasts were quantified based on their morphology on HE stained sections for length of their linear apposition along osteoid-new bone surfaces relative to total new bone-osteoid surface length for 3 randomly selected high power fields (200×) and are reported as a fraction (%) average for each sample. New blood vessels within the defect were similarly digitally counted at 4 randomly selected high power fields (200×) and are reported as average for each sample (Lu and Rabie Citation2004, Aronin et al. Citation2009).
A total tissue response score was given to each specimen regarding the presence of fibrous connective tissue formation and inflammatory cellular infiltration. Regarding fibrous connective tissue formation (4) was given for severe deposition of dense collagenous connective tissue around implant (3), for disruption of normal tissue architecture and presence of moderately dense fibrous connective tissue (2), for presence of moderate connective tissue (1), for presence of delicate spindle-shaped cells or mild fibroplasia (0), for no difference from normal control tissue, no presence of connective tissue at or around implant site. Inflammatory cellular infiltration was scored as (4), for severe cellular infiltrate response to implant or tissue necrosis at or around the site (3), for the presence of large numbers of lymphocytes, macrophages and foreign body giant cells, also notable presence of eosinophils and neutrophils (2), for presence of several lymphocytes, macrophages with a few foreign body giant cells and a small foci of neutrophils (1), presence of a few lymphocytes or macrophages, no presence of foreign body giant cells, eosinophil or neutrophils (0), for no difference from normal control tissue, no presence of macrophages, foreign body cells, lymphocytes, eosinophils or neutrophils at or around implant site (Pişkin et al. Citation2009).
Statistical analysis
Independent variables were the groups and the dependent variables were the histology parameters. The normality of distribution and the homogeneity of variances of the samples were established using the Shapiro–Wilk test. All parameters were analyzed by nonparametric tests Kruskal–Wallis was used for multiple comparison and Mann–Whitney U as post-hoc test. Descriptive statistical values were expressed as median, minimum and maximum. The difference was considered significant if p < 0.05.
Results
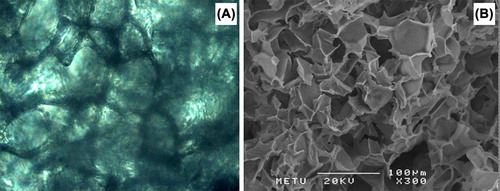
shows representative optical and scanning electron microscopy (SEM) pictures of the disk shape biodegradable cryogels made of HEMA-lactate-Dextran produced/used in this study. As shown in the SEM micrograph, these cryogels have highly opened and interconnected pore morphologies which are very good for tissue engineering applications because they do allow a nice surrounding for cell attachment, migration and growth. Besides their pore structures these scaffolds exhibit extraordinary properties comparing to other similar hydrogels as discussed in detail in our previous articles (Bölgen et al. Citation2007). Briefly, they do swell in aqueous media very rapidly (in few minutes) and reach equilibrium. The swelling ratio of the scaffold produced and used in this study was about 15 (Swelling ratio = (mwet−mdry)/mdry). Note that in our animal studies we have used dried cryogels. When we have implanted them in the cranial defects, they did swell by absorbing surrounding aqueous media and thus created a very good environment for stem cells injected within the pores of the cryogels and/or the cells migrating from the surrounding tissue. Note that our previous studies have exhibited that these scaffolds are highly biocompatible and allow nice cell attachment and growth (Bölgen et al. Citation2008, Bölgen et al. Citation2009, Bölgen et al. Citation2011). Here in this study we have designed a further strategy in which stem cells were used together with the cryogel scaffolds in order to investigate their role in the regeneration. Note that here we have used cryogels as typical biomaterials, and then stem cells were injected within the matrix when the cryogels were implanted.
shows the results of histological and histomorphometric analysis which may be discussed as follows: New bone per total defect area ratio significantly increased in time (0.12 ± 0.03 day 30, 0.21 ± 0.08 day 90, 0.34 ± 0.17 day 180 and 0.15 ± 0.05 day 30, 0.24 ± 0.07 day 90, 0.27 ± 0.11 day 180) in both the “scaffold only” group and “scaffold-stem cells” group, respectively (p < 0.05, ). New bone per total defect area ratio, osteoblast-lined active bone length were not significantly different in scaffold-stem cell group comparing to that of “scaffold only” group on day 30, 90 and 180 (Figures 3A, 3B). The blood vessel density was significantly higher in scaffold-stem cell group comparing to that of the “scaffold only” group on day 30 (p < 0.05, ). Blood density did not differ between groups on days 90 and 180 (). The new bone formation significantly increased in time in all groups (p < 0.05).
Figure 3. Histograms from A to D present the descriptive statistical data (means and standard deviations) belonging to control and the experiment groups on time. The white and black columns show the “scaffold only” and “scaffold-stem cells” groups, respectively. A: New bone/total cavity ratio, B: Active osteoblast lining length ratio, C: Blood vessel density, D: Tissue response to the implant. The groups where the difference within them is statistically significant are indicated with a (*) and (*).
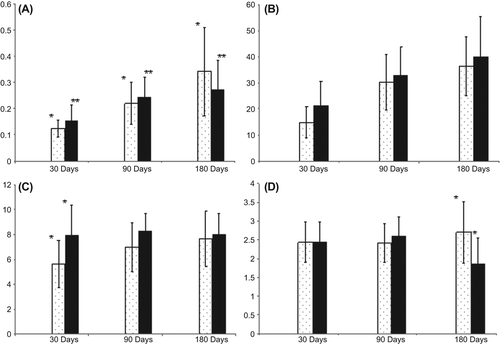
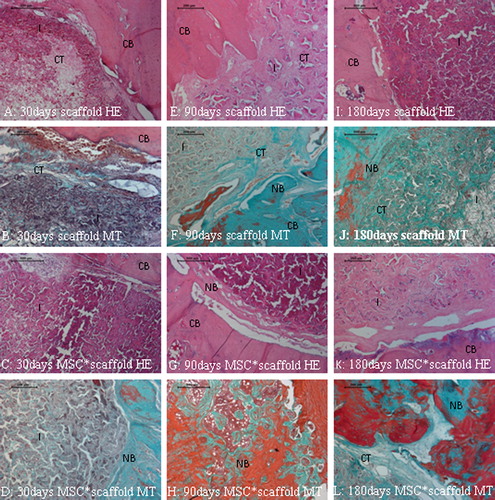
Tissue response to the implant was mild and tissue response scores remained low (). The polymer allowed a guided new bone formation within its empty channels from both peripheric and central regions of the cavity. Tissue response to “scaffold only” group was similar to the tissue response to “scaffold-stem cells” group at 30 and 90 days. “Scaffold-stem cells”-implanted group gave better tissue response score when compared to “scaffold only” group on day 180 (p < 0.05) (). This proved that the polymer was biocompatible. Neither fibrosis (scar tissue), nor necrosis, nor foreign body reaction was noted in any of the samples at any time point. Degradation of the polymer started minimally from the periphery to the center from day 30. However, biomaterial kept its circular shape and guided the remodeling of new bone trabecules in its channels on day 180. None of the cavities were totally ossified on day 180 (). According to these data, the new scaffold was biocompatible and cell-scaffold accelerated intramembranous ossification of the critical size calvarial defect.
Figure 4. A–L show the healing stages of the critical sized defect (cavity) filled with the biocompatible polymeric biomaterial guided well organized fibrous callus. Note that the implant allow an intramembranous bone formation through this callus/connective tissue witihin its 3D pores from the 90th to 180th days in E–L. New bone trabecules are apparent in green with MT stain in F, J; and L. CB: Compact bone, NB: New bone, I: Implant, CT: Connective tissue, HE: Haematoxylin & Eosin, MSC*: mesenchymal stem cell, MT: Masson's Trichrome.
Discussion
In this study we demonstrated that the new scaffold was biocompatible and the “scaffold-stem cells” composite accelerated vascularization during the ossification of the critical size calvarial defect. The new bone formation and vascularization significantly increased in time in all groups. In our previous studies we have demonstrated that the critical sized bone defects in rats (8 mm diameter) can not heal themselves spontaneously (Pişkin et al. Citation2009). Therefore we did not use control group in the present study. Our preliminary investigation related to the tissue response and biocompatibility of the cryogel scaffolds showed that cryogels may be potentially used in critical sized bone defect regeneration (Bölgen et al. Citation2009). We recently demonstrated the competence of the MSCs on the calvarial critical sized defects also mixed them with poly(L-lactide) and poly (ϵ-caprolactone) type scaffolds (Aydın et al. Citation2011). Therefore here we aimed to enhance the capacity of our biocompatible cryogels by combining them with the MSCs for their potential on critical sized cranial defect regeneration model.
Mesenchymal stem cells can be used for xenogenic transplantation due to their multi-differentiation and regeneration potential, high proliferation rate, and immune suppressive effects (Niemeyer et al. Citation2010, Zong et al. Citation2010). Mesenchymal stem cells are currently in operation in clinical trials in several indications such as the facilitation of bone marrow reconstitution after hematopoietic stem cell transplantation, graft versus host disease (Koc et al. Citation2000, Lazarus et al. Citation2005), construction of the different organ walls (Gong and Niklason Citation2008), as well as the musculoskeletal regeneration by using tissue engineering techniques (Godeladze et al. Citation2009).
It was demonstrated that MSCs cultured in different scaffolds can induce new bone formation in vitro and/or in vivo conditions (Yamada et al. Citation2004, Meinel et al. Citation2005, Miura et al. Citation2006, Mankani et al. Citation2006, Aydın et al. Citation2011). MSCs have the ability to enhance healing of critical-size defects at varying ranges (Yamada et al. Citation2004, Meinel et al. Citation2005, Miura et al. Citation2006, Mankani et al. Citation2006). We recently reported the capability of the MSCs on calvarial defect healing after proper culturing and characterization steps. However the MSC performance may diverge on the subject of the scaffold behavior, animal model or surgical procedure (Aydın et al. Citation2011).
Zong et al. used the differentiated osteoblastic cells and the undifferentiated MSCs with PLGA scaffolds on critical sized calvarial defects (Zong et al. Citation2010). They reported fibrous connective tissue formation and the degradation of the scaffold in the defect area grafted with scaffold, human MSC construct or osteoblast construct at 10 weeks after transplantation. At 20 weeks, scaffolds could not be observed and the bone-like tissue in the area grafted with human MSC construct was thicker than the tissue in the area grafted with osteoblast construct, the area grafted with blank scaffold was still filled with a fibrous-like tissue. In our study the scaffold and the scaffold-MSCs applications accelerated the new bone formation stronger and earlier than the Zongs’ teams’ constructs. In our study, dense connective tissue in which the porous scaffold allowed the healthy expansion of bone precursor cells, fibers and the growing new blood vessels throughout cryogel particles was gradually replaced by original osseous elements. These data are consistent with Schantz et al.s’ who reported that cranial defects with PCL scaffolds showed variable amounts of newly generated bone in the form of bony islands; however, complete osseous union was not present after 90 days. However neovascularization occurred, with multiple capillaries branching throughout the entire PCL scaffold architecture (Schantz et al. Citation2003). Our cryogel-MSC composites exhibited similar accelerating capacity for neovascularization and the mild tissue response guiding the new bone formation within its interconnected pores.
Conclusion
This study demonstrated that HEMA-lactate-Dextran-based cryogels exhibit sufficient properties to be considered as tissue engineering scaffolds for cranial bone defect repair. As a conclusion both the cryogel scaffold itself and MSCs-cryogel composite can enhance the repair of critical sized calvarial defects mainly by accelerating the vascularization. The in vivo results demonstrated osseous tissue integration within the implant and mineralized functionally stable bone restoration of the cranial defects. Histological data revealed that new bone per total defect area ratio, were not significantly different in “scaffold-stem cells” group comparing to that of “scaffold only” group on all time points. However, the blood vessel density was significantly higher in “scaffold-stem cells” group compared to that of the “scaffold only” group on day 30. The latest developments in stem cell research demonstrated that stem cells were transformed considering the signals in the micro environment. In this study, it was expected that the stem cells would play the leading role in the formation of active osteoblasts; however, the results showed that the angiogenesis was enhanced. Sufficient/necessary perfusion and for this reason the formation of new vascular constructs is needed for the regeneration of bone defects exposed to surgical trauma and the micro environment that its vascular support is corrupted by surgical operation. The results showed that the stem cell added fronted to angioblastic transformation (vasculogenesis) instead of transformation to osteoblasts producing bone matrix. This orientation of the stem cells can be followed by prior marking and this research can be the evaluation step of another study. For our future study, it is concluded that use of the scaffold with these cells that take role in the formation of new vascular constructs and the cells directed to osteoblastic transformation would give enhanced results in the regeneration of cranial bone defects.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This study was performed in the context of the EU-FP6-NoE Expertissues Project. Erhan Pişkin was supported by the Turkish Academy of Sciences (TUBA) as a full member.
References
- Alsberg E, Hill EE, Mooney DJ. 2001. Craniofacial tissue engineering. Crit Rev Oral Biol Med. 12:64–75.
- Aronin CEP, Sadik KW, Lay LA, Rion DB, Tholpady SS, Ogle RC, Botchwey EA. 2009. Comparative effects of scaffold pore size, pore volume, and total void volume on cranial bone healing patterns using microsphere-based scaffolds. J Biomed Mater Res. 89A: 632–641.
- Ayatollahi M, Salmani MK, Geramizadeh B, Tabei SZ, Soleimani M, Sanati MH. 2012. Conditions to improve expansion of human mesenchymal stem cells based on rat samples. World J Stem Cells. 26:1–8.
- Aydın HM, Korkusuz P, Vargel İ, Kılıç E, Güzel E, Çavusoğlu T, et al. 2011. A 6-month in vivo study of polymer/mesenhymal stem cell constructs for cranial defects. J Bioact Comp Pol. 26:207–221.
- Bölgen N, Plieva F, Galaev IY, Piskin E, Mattiasson B. 2007. “Cryogelation” for preparation of novel biodegradable tissue engineering scaffolds. J Biomater Sci Polymer Ed. 18:1165–1179.
- Bölgen N, Yang Y, Korkusuz P, Güzel E, El-Haj A, Pişkin E. 2008. Three-dimensional ingrowth of bone cells within biodegradable cryogel scaffolds in bioreactors at different regimes. Tissue Eng: Part A. 14:1743–1750.
- Bölgen N, Yang Y, Korkusuz P, Güzel E, El-Haj A, Pişkin E. 2011. 3D ingrowth of bovine articular chondrocytes in biodegradable cryogel scaffolds for cartilage tissue engineering. J Tissue Eng Regen Med. 5:770–779.
- Bölgen N, Vargel İ, Korkusuz P, Güzel E, Pişkin E. 2009. Tissue responses to novel tissue engineering biodegradable cryogel-scaffolds: an animal model. J Biomed Mater Res Part A. 91:60–68.
- Caplan AI. 2000. Tissue engineering designs for the future: new logics, old molecules. Tissue Eng. 6:1–8.
- Chirila TV, Thompson DE, Constable IJ. 1992. In vitro cytotoxicity of melanized poly(2-hydroxyethyl methacrylate) cryogels, a novel class of ocular biomaterials. J Biomater Sci Polym Ed. 3:481–498.
- Godeladze JO, Reseland JE, Duroux-Richard I, Apparailly F, Jorgensen C. 2009. From stem cells to bone: phenotype acquisition, stabilization, and tissue engineering in animal models. Ilar J. 51:42–61.
- Gong Z, Niklason LE. 2008. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J. 22:1635–1648.
- Gürpınar ÖA, Tuzlakoğlu K, Onur MA, Tümer A, Serdar MA, Ünal N, Piskin E. 2003. BHK cell attachment and growth on EDA-plasma treated poly(L-lactide/caprolactone) biodegradable films. J Biomater Sci Polym Ed. 14:589–600.
- Hou QP, Grijpma DW, Feijen J. 2003. Porous polymeric structures for tissue engineering prepared by a coagulation, compression molding and salt leaching technique. Biomaterials. 24:1937–1947.
- Hutmacher D, Cool S. 2007. Concepts of scaffold-based tissue engineering: the rationale to use solid free-form fabrication techniques. J Cell Mol Med. 11:654–669.
- Kim HJ, Kim UJ, Vunjak-Novakovic G, Min BH, Kaplan DL. 2005. Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials. 26i:4442–4452.
- Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. 2000. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 18:307–316.
- Lannutti J, Reneker D, Ma T, Tomasko D, Farson D. 2007. Electrospinning for tissue engineering scaffolds. Mater Sci Eng C. 27:504–509.
- Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, et al. 2005. Cotransplantation of HLA-identical sibling culture- expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 33:389–398.
- Liu X, Ma PX. 2004. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 32:477–486.
- Lozinsky V, Plieva FM, Galaev YU, Mattiasson B. 2002. The potential of polymeric cryogels in bioseparation. Bioseparation. 10:163–188.
- Lu M, Rabie ABM. 2004. Quantitative assessment of early healing of intramembranous and endochondral autogenous bone grafts using micro-computed tomography and Qwin image analyzer. Int J Oral Maxillofac Surg. 33:369–376.
- Mankani MH, Kuznetsov SA, Wolfe RM, Marshall GW, Robey PG. 2006. In vivo bone formation by human bone marrow stromal cells: reconstruction of the Mouse calvarium and mandible. Stem Cells. 24:2140–2149.
- Miura M, Miura Y, Sonoyama W, Yamaza T, Gronthos S, Shi S. 2006. Bone marrow-derived mesenchymal stem cells for regenerative medicine in craniofacial region. Oral Dis. 12:514–522.
- Meinel L, Fajardo R, Hofmann S, Langer R, Chen J, Snyder B, et al. 2005. Silk implants for the healing of critical size bone defects. Bone. 37:688–698.
- Niemeyer P, Schönberger TS, Hahn J, Kasten P, Fellenberg J, Suedkamp N, et al. 2010. Xenogenic transplantation of human mesenchymal stem cells in a critical size defect of the sheep tibia for bone regeneration. Tissue Eng Part A. 16:33–43.
- Quinn CP, Pathak CP, Heler A, Hubbell JA. 1995. Photo-crosslinked copolymers of 2-hydroxyethyl methacrylate, poly(ethylene glycol) tetra-acrylate and ethylene dimethacrylate for improving biocompatibility of biosensors. Biomaterials. 16:389–396.
- Pişkin E, İşoğlu İA, Bölgen N, Griffiths S, Vargel İ, Çavuşoğlu T, et al. 2009. In vivo performance of simvastatin loaded electrospun spiral-wounded polycaprolactone scaffolds in reconstruction of cranial bone defects in a rat model. J Biomed Mater Res Part A. 90:1137–1151.
- Rockwood DN, Gil ES, Park SH, Kluge JA, Grayson W, Bhumiratana S, et al. 2011. Ingrowth of human mesenchymal stem cells into porous silk particle reinforced silk composite scaffolds: An in vitro study. Acta Biomater. 7:144–151.
- Rosenbaum AJ, Grande DA, Dines JS. 2008. The use of mesenchymal stem cells in tissue engineering. Organogenesis. 4:23–27.
- Sanan A, Haines SJ. 1997. Repairing holes in the head: a history of cranioplasty. Neurosurgery. 40:588–603.
- Schantz JT, Hutmacher DW, Lam CXF, Brinkmann M, Wong KM, Lim TC, et al. 2003. Repair of calvarial defects with customised tissue-engineered bone grafts II. Evaluation of cellular efficiency and efficacy in vivo. Tissue Eng. 9:127–139.
- Sharma B, Elisseeff JH. 2004. Engineering structurally organized cartilage and bone tissues. Ann Biomed Eng. 32:148–159.
- Srouji S, Ben-David D, Lotan R, Livne E, Avrahami R, Zussman E. 2011. Slow-release human recombinant bone morphogenetic protein-2 embedded within electrospun scaffolds for regeneration of bone defect: in vitro and in vivo evaluation. Tissue Eng Part A. 17:269–277.
- Weinand C, Pomerantseva I, Neville CM, Gupta R, Weinberg E, Madisch I, et al. 2006. Hydrogel-β-TCP scaffolds and stem cells for tissue engineering bone. Bone. 38:555–563.
- Valero-Cabre´ A, Tsironis K, Skouras E, Perego G, Navarro X, Neiss WF. 2001. Superior muscle reinnervation after autologous nerve graft and poly-L-lactide-e-caprolactone (PLC) tube implantation in comparison to silicone tube repair. J Neurosci Res. 63:214–223.
- VanDijk-Wolthuis WNE, Hoogeboom JAM, vanSteenbergen MJ, Tsang SKY, Hennink WE. 1997. Degradation and release behaviour of dextran-based hyrogels. Biomacromolecules. 30:4639–4645.
- Yamada Y, Ueda M, Naiki T, Takahashi M, Hata K, Nagasaka T. 2004. Autogenous injectable bone for regeneration with mesenchymal stem cells and plateletrich plasma: tissue-engineered bone regeneration. Tissue Eng. 10:955–964.
- Zong C, Xue D, Yuan W, Wang W, Shen D, Tong X, et al. 2010. Reconstruction of rat calvarial defects with human mesenchymal stem cells and osteoblast-like cells in poly-lactic-co-glycolic acid scaffolds. Eur Cell Mater. 20:109–120.