Abstract
Unrestricted somatic stem cells (USSCs) loaded in nanofibrous PHBV scaffold can be used for skin regeneration when grafted into full-thickness skin defects of rats. Nanofibrous PHBV scaffolds were designed using electrospinning method and then, modified with the immobilized collagen via the plasma method. Afterward, the scaffolds were evaluated using scanning electron microscopy, physical and mechanical assays. In this study; nanofibrous PHBV scaffolds loaded with and without USSCs were grafted into the skin defects. The wounds were subsequently investigated at 21 days after grafting. Results of mechanical and physical analyses showed good resilience and compliance to movement as a skin graft. In animal models; all study groups excluding the control group exhibited the most pronounced effect on wound closure, with the statistically significant improvement in wound healing being seen on post-operative Day 21. Histological and immunostaining examinations of healed wounds from all groups, especially the groups treated with stem cells, showed a thin epidermis plus recovered skin appendages in the dermal layer. Thus, the graft of collagen-coated nanofibrous PHBV scaffold loaded with USSC showed better results during the healing process of skin defects in rat model.
Introduction
The final goal of tissue engineering should be the functional recovery of damaged tissue in vivo and in vitro reconstructions of tissue architecture while realizing exquisite tissue-specific functions (Raf and Singer Citation2000). The reconstruction of skin defects remains a major concern when the defective area is widespread, severely contaminated by microorganisms, or poorly vascularized, as can be the case with irradiation defects, congenital skin disorders, or extensive burns. Numerous materials for skin regeneration, including temporary substitutes, such as porcine xenografts, synthetic membranes, and allogeneic substitutes or permanent skin substitutes, such as cultured epidermis and dermal substitutes, have been investigated (Sheridan and Tompkins Citation1990). Among these materials, artificial dermal substitutes are structurally optimized to be incorporated into the surrounding tissues and to allow cell invasion by fibroblasts and capillaries for subsequent dermal remodeling (Suzuki et al. Citation1990). Mesenchymal stem cells (MSCs) or epidermal cells have been demonstrated playing an effective role in promoting wound healing when injected into the skin defect, either alone or in combination with human amniotic membrane (Kim et al. Citation2008). Kim et al. (Citation2009) shown that HAM loaded with MSCs improves wound healing. Umbilical cord blood contains hematopoietic as well as non-hematopoietic mesenchymal stem cells, these latter also named as cord blood embryonic-like stem cells (CBEs) (Branski et al. Citation2009). CBEs have been shown to differentiate into neural, hepatobiliary, pancreatic-like precursors, and potentially others (Branski et al. Citation2009). It was also suggested that stem cells from umbilical cord blood are able to differentiate into epithelial cells under in vitro conditions and could therefore be used as a starting material for isolation and expansion of cells in large skin defects (Kamolz et al. Citation2006). Human umbilical cord blood is a rich source of hemopoietic stem cells for clinical application and may be one of the largest sources of stem cells with naive immune status (Wilson et al. Citation2011). Cord lining-mesenchymal stem cells express CD23, CD14 and low amounts of CD34 and CD35; they do not express endothelial marker CD31 and have greater in vitro expansion than Wharton's jelly-derived MSCs (Ishige et al. Citation2009). CD14 inhibits T cells. Wharton's jelly-derived MSCs do not express CD14 or CD23. Despite those descriptions, the cell markers of umbilical cord-derived MSCs are under great debate (Kita et al. Citation2010, Wu et al. Citation2007). One of the key factors of tissue engineering is to create a three-dimensional scaffold with suitable properties such as degradation rate, high porosity, and interconnected pores. Typically, biodegradable polymeric scaffolds are fabricated using different methods (Mikos et al. Citation1993, Nam et al. Citation2000). In natural tissues, cells are surrounded by extracellular matrix, which has physical structural features ranging from nanometer scale to micrometer scale. Hence, a nanostructured porous and large surface area is needed as an alternate to natural ECM. To mimic the natural ECM, many research groups tried to fabricate nanofibrous scaffold by different methods like electrospinning (Hartgerink et al. Citation2002, Fong et al. Citation2002, Zhang et al. Citation2007, Meng et al. Citation2007, Ndreu et al. Citation2008). Wide variety of natural materials, such as collagen [Biobrane™, Integra®,Alloderm™], fibrin [Bioseed™], HA [Laserskin™] and GAGs [Integra®], have been used in commercialized skin grafts (Metcalfe and Ferguson Citation2007, Yannas Citation2004, Ruszczak Citation2003). Nylon [Transcyte™] and biodegradable polymers such as polyglactin [Dermgraft®], polycaprolactone (PCL) (Dai et al. Citation2004), and PLGA (Yang et al. Citation2006) were used for fabricating skin substitutes. The combined application of both cell sources (USSCs and scaffold) is expected to have a wide clinical use for the treatment of skin lacerations and warrants further analysis. Poly 3-hydroxybutyrate-co-3-hydroxyvalerate (PHBV) scaffold has shown good biocompatibility, biodegradable, and also piezoelectric properties. Nanofibers have improved the performance of biomaterials. Electrospinning is one of the most important methods for the fabrication of nanofibrous scaffolds (Ruszczak Citation2003, Dai et al. Citation2004, Yang et al. Citation2006). Some natural materials such as collagen and fibronectin and some peptides have been reported as scaffold modifiers (Ito et al. Citation2003, Kapur et al. Citation2003). Controlling surface properties is very important for the high performance of adhesion. Biomaterials wettability is an important factor in the surface modification of materials. Surface modification of hydrophobic polymer surfaces can be achieved by wet (acid, alkali), dry (plasma), and radiation treatments (ultraviolet radiation and laser) (Lu et al. Citation2004, Roucoules et al. Citation2002, Hay et al. Citation2008, Kogelschatz Citation2003). In this study, nanofibrous poly (3-hydroxybutyrate-co-3-hydroxyvalerate scaffold were fabricated by electrospinning and were modified with collagen by plasma radiation. The samples were evaluated using scanning electron microscope (SEM), physical and mechanical analysis, and in vitro assays then loaded with to USSCs and implanted in damaged rat and investigated by different analyses.
Materials and methods
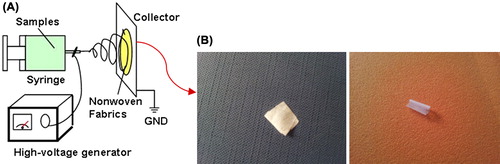
A PHBV containing 5 mol% of 3-hydroxyvalerate with a molecular weight of 680,000 Daltons was purchased from Sigma Chemical Co. 2, 2, 2-Trifluoroethanol (TFE) to prepare PHBV solution was also purchased from Sigma-Aldrich Chemicals and was used as received without further purification. Electrospinning apparatus used in this study was purchased from asia nano meghyas company (Iran). In our previous study, nanofibrous PHBV was designed with constant parameters and was investigated using the different physical and chemical analyses (Ai et al. Citation2011) which are briefly described below. PHBV was dissolved at determined concentration in TFE. The PHBV solution (2%w) was contained in a glass syringe controlled by syringe pump. A positive high-voltage source was applied at the tip of a syringe needle through a wire. The nanofibers were designed with determined parameters (syringe size, 17 mm; collector speed, 1000 rpm; injected speed, 2 mL/min; distance from syringe tip to collector, 75 mm; voltage, 20 kv; temperature, 30°C; and time, 7 h). We used a microwave plasma source to modify the surface of the nanofibrous samples. The microwave plasma has significant advantage over other techniques like Radio Frequency Glow Discharge (RFGD), frequently used in polymer surface modifications. Microwave sources could be operated at low pressures (10–3 to 10–1 mbar), which reduced the risk of gas phase contamination during processing. Moreover, the plasma properties could be controlled conveniently by adjusting the microwave power. Hence, the surface modification of this polymer became important. Before exposure, the nanofibrous mats were cut into 1 × 1 cm sections. Then, they were placed within the ethanol solution for 24 hours to eliminate pollution and fats on the films. After removing the mats from the solution, they were well washed in distilled water. The samples were placed in the plasma chamber and exposed to oxygen gas during 60 sec. The plasma surface treatment was reached in the microwave-induced plasma with a surface wave at 100W. Collagen (type I, from bovine Achilles tendon, purchased from Sigma) was immobilized onto the nanofibrous surface based on the following protocol. The modified nanofibrous mats were rinsed into PBS containing 10 mM EDC and 10 mM sulfo-NHS to activate the carboxyl groups on the surfaces. Collagen solution (15 mg/ml in acetic acid buffer solution, 50 mM, pH = 5) was injected into the previous solution and was shaken gently for 24 h at 4°C. The obtained samples were placed inside a vacuum oven so that they would fully lose humidity. The surface characteristics of nanofibrous films were studied using SEM (Cambridge Stereo-scan, model S-360; Cambridge Instruments, Wetzlar, Germany) to analyze the changes in the surface morphology. The sample surface's static contact angles were investigated using a contact-angle-measuring device (Krüss G10; Krüss, Matthews, NC), following the sessile drop method. The electrospun PHBV mats were subjected to stress–strain analysis using a universal testing machine under an extension rate of 5 mm/min and 100 N load cell. Specific surface area of the electrospun nanofibrous PHBV mat was determined using a surface area and pore size analyzer. The electro-spinning set has been shown in .
In vivo Study
Cell culture and Surgical procedures
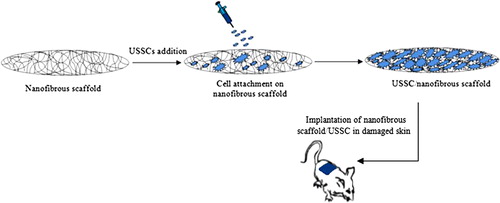
Culture and isolation protocol of the USSC from fresh umbilical cord blood was previously described by Kogler et al. (Citation2004) and also Heidari et al. (Citation2012). Fifty male Wistar white rats aged approximately 4–8 weeks and weighed 180–220 g at the beginning of the experiment were divided into five groups. The protocol for the experiment was approved by the Institutional Animal Care and Use Committee of Beheshti University. Animals were handled according to the guidelines established for animal care at the center. Each rat had free access to both sterile water and standard rodent soft chow ad libitum. Animals were anesthetized using 80 mg/kg ketamine and 10 mg/kg xylazin injection, and their back was shaved and swabbed with povidine-iodine followed by 70% ethanol.The scrubbing was then repeated two more times. Then, a sterile template measuring 1.5 cm × 1.5 cm was placed on their skin and the outline was traced using a sterile fine felt-tipped pen. The medial border of the template was oriented parallel to the sagittal axis of the animal. Full-thickness wounds of 1.5 cm × 1.5 cm were made by excising the skin within the confines of the square down to the level of subcutaneous panniculus carnosus. The wound cavity was covered with the designated scaffold and was sutured using 10–0 nylon at 0.75-cm intervals. Among the 50 skin defects, ten defects were grafted with un-modified nanofibrous scaffolds without stem cells, ten defects were grafted with un-modified nanofibrous scaffolds loaded with about 2 × 106 USSCs, ten defects were grafted with collagen-cross linked nanofibrous scaffolds without stem cells, ten defects were grafted with collagen-cross linked nanofibrous scaffolds loaded with about 2 × 106 USSCs, and then ten defects were grafted with control group. The wounds were covered with a standard wet compress to prevent scaffold detachment and desiccation, and then, each animal was housed in its own cage to avoid damage to the wound. Post-surgery care included analgesic and antibiotic (cyclosporine 10 mg/kg) injections. The immunosuppressive drug, cyclosporine, was given to animals every day at a concentration of 10 mg/kg by subcutaneous injection. Checking any post-surgery pain, distress or complications was done 24 h after surgery and daily afterward. The process of reconstruction of defected skin with USSCs and nanofibrous scaffold is shown in .
Histological and Immunostaining assessments
The wounds were harvested 21 days after grafting, then stained, and investigated for histological assessment. The reconstituted skin was cut to the control depth determined as the excision down to the level of panniculus carnosus (15 mm wide, 15 mm long, and a 5 mm deep), and fixed in 10% formalin at 4°C for 5 days, dehydrated, and then paraffin-embedded. Serial 2-μm paraffin sections were cut with a rotating microtome (MICROM) and stained with hematoxylin and eosin (H&E) according to the routine histology protocol. The wounds were evaluated for the presence of smooth muscle, number of sebaceous units and hair follicles, epithelialization, fibroplasias, and vascular hyperplasia etc. Keratinocytes from the rat skin equivalents were analyzed for their specific expression of cytokeratin 10 (K10) by means of immunohistochemical analysis. The results of each group were statistically analyzed using the ANOVA analysis. The quantitative data analyses were carried out with the statistical procedures of Excel program.
Results and discussion
Characterization
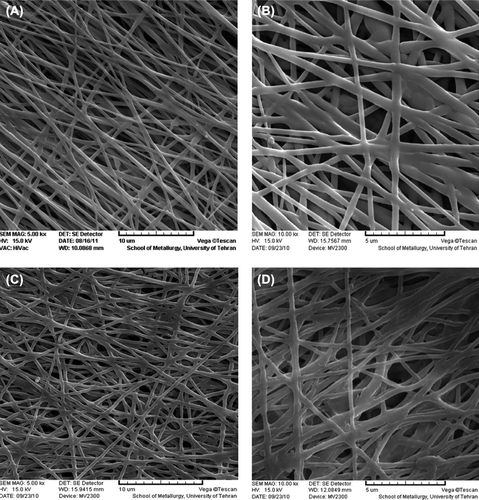
shows the SEM images of the un-modified nanofibrous mats at different magnifications. The smooth and homoloous nanofibers are clearly shown in . The average size for the un-modified and the collagen-coated nanofibers were about 100 and 400 nm, respectively.
Figure 3. SEM images of the un-cross linked nanofibrous PHBV mat (A: 5000× – B: 10000×) and the collagen-coated nanofibrous PHBV mat (C: 5000× – D: 10000×).
Mechanical and physical properties of nanofibrous PHBV scaffolds are presented in . The contact angles of 105° and 56° was measured for the un-modified and the collagen-coated nanofibers, respectively. The 49° difference in the contact angle shows a better hydrophilicity of the collagen-coated nanofibers compared to the un-cross linked samples. Porosity of un-modified nanofibrous and the collagen-coated scaffolds were calculated as 91.62%, and 80.31% and their pore size was also measured as 0.45 ± 0.25 μm and 0.30 ± 0.11 μm, respectively. The specific surface area for the un-modified electrospun nanofibrous scaffolds was about 138 m2/g and the collagen-coated nanofibrous ones was about 108 m2/g. It is discernible that the electrospun nanofibrous mat has high porosity and high level of specific surface area, as well. The tensile modulus of the nanofibrous scaffolds is equal to that of the human skin. Moreover, the ultimate tensile stress value of the nanofibrous PHBV scaffolds is suitable compared with that of the human skin (tensile modulus of the human skin is 15–150 MPa, and its ultimate tensile stress is 5–30 MPa).
Table I. The mechanical and physical properties of electrospun nanofibrous PHBV mats.
In vivo Results
Wound-size measurement
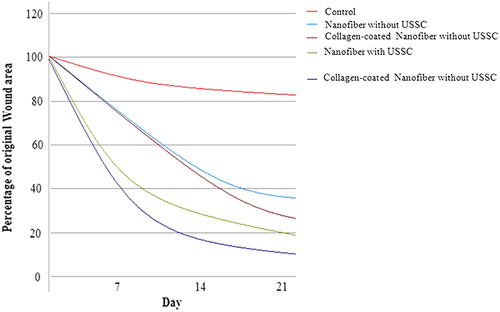
Any inflammation symptoms were not seen in the wound surface of the study groups. The study groups showed the most pronounced effect on wound closure, with statistically significant improvements in wound healing being seen at 21 day after grafting (). The earliest difference was observed between the collagen-coated nanofibrous mat/USSCs, when they demonstrated ≥ 82% and ≥ 89% wound closure, respectively. By contrast, the study control group demonstrated ≤ 20% wound closure and was still poorly healed (P < 0.05).
Histological assessment
Histological images related to the control, grafted and normal skin groups are shown in .
Figure 5. Histology of wounds by H&E staining in the different groups on post-operative day 21. A) The nanofibrous PHBV scaffold without USSCs. B) The nanofibrous PHBV scaffold with USSCs. C) The collagen-coated nanofibrous PHBV scaffold without USSCs. D) The collagen-coated nanofibrous PHBV scaffold with USSCs. E) The normal skin. F) control. (Abbreviations: FD fibrinous debris; EP – epithelialization; HF – hair follicle; HB – hair bulb; SB – sebaceous gland; VS – vascularized section). Scale bars: 200 m.
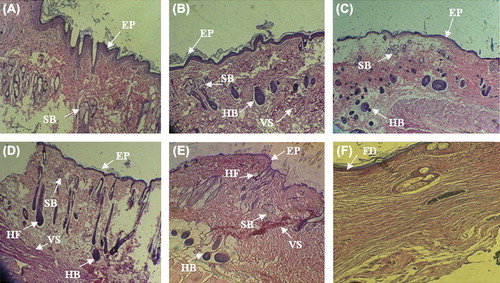
Histological examination of wounds in Group B, 21 days after grafting, exhibited the well-recovered epidermal and dermal layers. The epidermis was thin and showed a mature differentiation, and the dermal layer showed well-recovered skin appendages at this time point. All the study groups well-showed the formation of epidermal layer, but skin appendages were not formed in the dermal section in all groups. Formation of epidermal and dermal layers and, especially, formation of sebaceous gland was well-seen in the study groups with USSCs. But a thickened epidermis with immature epithelial cells and recovered skin appendages and also hair follicles were formed in the dermal layer in the with collagen-coated nanofibers/USSCs, similar to the histological features of the normal group. The formation of hair bulbs was well seen in the groups. Skin appendages were partially recovered in groups with cells.
Immunostaining results
The identification of keratinocytes of the skin equivalents was performed by the immunodetection of cytokeratins, which are specific markers for the epithelial differentiation.
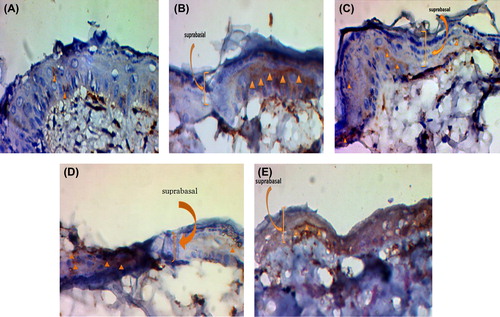
On the other hand, suprabasal cell layers of the skin equivalents expressed K10, a marker for terminal differentiation, which were seen at the Day 21. shows the formation of epithelial layer and detection or presentation of keratinocytes for the normal skin and the study groups with USSCs. In contrast to the groups without cells, the groups with the USSCs demonstrated an increased amount of epidermis formation and the presence of keratinocytes.
Figure 6. Immunostaining or epidermal markers expression (cytokeratin-10) on day 21. A) The un-modified nanofibrous scaffold without USSCs. B) The un-modified nanofibrous scaffold with USSCs. C) The collagen-coated nanofibrous scaffold without USSCs. D) The collagen-coated nanofibrous scaffold with USSCs. E) The normal skin. Scale bars: 50 μm.
Conclusion
Due to the complete loss of skin tissue including epidermis, skin appendages and dermis in the full thickness skin defects, the skin tissue is not capable of self-repairing and restoring functions. This study reveals the great efficacy of collagen-coated nanofibrous PHBV scaffold loaded with USSCs as skin grafts for treating the acute full-thickness skin wounds in a rat model. The combined use of nanofibers and USSCs for repairing the acute full-thickness wound of 1.5 cm × 1.5 cm gave rise to a successful wound healing and skin regeneration in rats. On the post-operative Day 21, the reconstructed skin in the nanofibrous scaffolds, especially ones loaded with USSCs, demonstrated an intact epithelium together with the formation of new hair follicles and sebaceous glands, which were reminiscent of the structures of the natural skin. The results of immunostainning with cytokeratin showed that implanting the cell-laden scaffolds lead to the formation of a thicker epidermis layer due to the differentiation of stem cells into keratinocytes and fibroblasts. USSCs exhibited a specific effect on wound healing, especially on the regeneration of skin appendages. Taken together, these findings suggest a great potential of the collagen-coated nanofibrous scaffolds loaded with USSCs as efficient skin grafts for the treatment of acute full-thickness skin wounds.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ai J, Heidari S, Ghorbani F, Ejazi F, Biazar E, Asefnejad A, et al. 2011. Fabrication of coated-collagen electrospun PHBV nanofiber film by plasma method and its cellular study. J Nanomaterials. Article ID 123724, doi:10.1155/2011/123724.
- Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. 2009. A review of gene and stem cell therapy in cutaneous wound healing. Burns. 35:171–180.
- Dai NT, Williamson MR, Khammo N, Adams EF, Coombes AGA. 2004. Composite cell support membranes based on collagen and polycaprolactone for tissue engineering of skin. Biomaterials. 25:4263–4271.
- Fong H, Weidong L, Wang CS, Vaia RA. 2002. Generation of electrospun fibers of nylon 6 and nylon 6-montmorillonite nanocomposite. Polymer. 43:775–780.
- Hartgerink JD, Beniash E, Stupp SI. 2002. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci. 99:5133–5138.
- Hay KM, Dragila MI, Liburdy J. 2008. Theoretical model for the wetting of a rough surface. J Coll Inter Sci. 325:472–477.
- Heidari S, Soleimani M, Tavirani MR, Ebrahimi M, Raeisossadati R, Yasaei H, et al. 2012. Evaluation of unrestricted somatic stem cells as a feeder layer to support undifferentiated embryonic stem cells. Mol Rep Dev. 79:709–718.
- Ishige I, Nagamura-Inoue T, Honda MJ, Harnprasopwat R, Kido M, Sugimoto M, et al. 2009. Comparison of mesenchymal stem cells derived from arterial, venous,and Wharton's jelly explants of human umbilical cord. Int J Hematol. 90:261–269.
- Ito T, Nakamura T, Suzuki K, et al. 2003. Regeneration of hypogastric nerve using a polyglycolic acid (PGA)-collagen nerve conduit filled with collagen sponge proved electrophysiologically in a caninemodel. Int J Art Org. 26:245–251.
- Kamolz LP, Kolbus A, Wick N, Mazal PR, Eisenbock B, Burjak S, Meissl G. 2006. Cultured human epithelium: human umbilical cord blood stem cells differentiate into keratinocytes under invitro conditions. Burns. 32:16–9.
- Kapur TA, Schoichet MS. 2003. Chemically-bound nerve growth factor for neural tissue engineering applications. J. Biomats Sci, Polymer Edition. 14:383–394.
- Kim CH, Kim SS, Sohn SK, Kim DH, Song CG, Kim HJ. 2008. The effect of human amniotic membrane, epidermal cells and marrow mesenchymal stem cells in healing a skin defect. J Korean Orthop Assoc. 43:276–286.
- Kim SS, Song CK, Shon SK, Lee KY, Kim CH, Lee MJ, Wang L. 2009. Effects of human amniotic membrane grafts combined with marrow mesenchymal stem cells on healing of full-thickness skin defects in rabbits. Cell Tissue Res. 336:59–66.
- Kita K, Gauglitz GG, Phan TT, Herndon DN, Jeschke MG. 2010. Isolation and characterization of mesenchymal stem cells from the sub-amniotic human umbilical cord lining membrane. Stem Cells Dev. 19:491–502.
- Kogelschatz U. 2003. Dielectric-barrier discharges: their history, discharge physics, and industrial applications. Pl Chem Pl Proc. 23:1–46.
- Kogler G, Sensken S, Airey JA, Trapp T, Müschen M, Feldhahn N, et al. 2004. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 200:123–135.
- Lu HW, Lu QH, Chen WT, Xu HJ, Yin J. 2004. Cell culturing on nanogrooved polystyrene petri dish induced by ultraviolet laser irradiation. Materials Letters. 58:29–32.
- Meng W, Kim SY, Yuan J, Kim JC, Kwon OH, Kawazoe N, et al. 2007. Electrospun PHBV/collagen composite nanofibrous scaffolds for tissue engineering. J Biomater Sci Polym Ed. 18:81–94.
- Metcalfe AD, Ferguson MWJ. 2007. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface. 4:413–437.
- Mikos AG, Sarakinos G, Lyman MD, Ingber DE, Vacanti JP, Langer R. 1993. Prevascularization of porous biodegradable polymers. Biotechnol Bioeng. 42:716–723.
- Nam YS, Yoon JJ, Park TGJ. 2000. A novel fabrication method of macroporous biodegradable polymer scaffolds using gas foaming salt as a porogen additive. Biomed Mater Res. 53:1–7.
- Ndreu A, Nikkola L, Ylikauppila H, Ashammakhi N, Hasirci V. 2008. Electrospun biodegradable nanofibrous mats for tissue engineering. Nanomedicine. 3:45–60.
- Raf C, Singer AJ. 2000. Wound repair: basic biology to tissue engineering. In: Lanza RP, Langer R, Vacanti J, Eds. Principles of Tissue Engineering, 2nd ed. New York: Academic Press, pp. 857–878.
- Roucoules V, Gaillard F, Mathia TG, Lanteri P. 2002. Hydrophobic mechano chemical treatment of metallic surfaces.Wettability measurements as a means of assessing homogeneity. Adv Coll Inter Sci. 97:177–201.
- Ruszczak Z. 2003. Effect of collagen matrices on dermal wound healing. Adv Drug Deliv Rev. 55:1595–1611.
- Sheridan RL, Tompkins RG. 1990. Skin substitutes in burns. Burns. 25:97–103.
- Suzuki S, Matsuda K, Isshiki N, Tamada Y, Ikada Y. 1990. Experimental study of a newly developed bilayer artificial skin. Biomaterials. 11:356–360.
- Wilson A, Butler PE, Seifalian AM. 2011. Adipose-derived stem cells for clinical applications: a review. Cell Prolif. 44:86–98.
- Wu KH, Zhou B, Lu SH, Feng B, Yang SG, Du WT, et al. 2007. In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J Cell Biochem. 100:608–616.
- Yang WS, Roh HW, Lee WK, Ryu GH. 2006. Evaluation of functions and tissue compatibility of poly (D,L-lactic-co-glycolic acid) seeded with human dermal fibroblasts. J Biomater Sci Polym Ed. 17: 151–162.
- Yannas IV. 2004. Synthesis of tissues and organs. Chembiochem. 5: 26–39.
- Zhang YZ, Su B, Venugopal J, Ramakrishna S, Lim CT. 2007. Biomimetic and bioactive nanofibrous scaffolds from electrospun composite nanofibers. Int J Nanomedicine. 2:623–638.