Abstract
A new approach to albumin removal for proteome studies involving human plasma samples was presented with dye affinity poly glycidyl methacrylate (PGMA) nanobeads in an average size of 45 nm. The specific surface area of PGMA nanobeads was calculated as 2616 m2/g. Cibacron Blue F3GA (CB) was immobilized onto PGMA nanobeads as dye ligand, and CB immobilized PGMA (CB-PGMA) nanobeads were characterized by a serial processing. Albumin depletion efficiency of CB-PGMA nanobeads was investigated in human plasma sample and confirmed by two-dimensional gel electrophoresis.
Introduction
Dye-ligand affinity systems have been utilized as a powerful technique, as they react with a wide variety of biological materials (CitationDenizli and Pişkin 2001). A number of synthetic dyes, particularly Cibacron Blue F3GA (CB), which could be used as affinity ligands, were the most widely used in protein separation and purification for about 30 years (CitationClonis et al. 2000). CitationDean and Watson (1979) first used the dye affinity ligand CB for extensive purification of a variety of enzymes, such as kinases, phosphatases, and dehydrogenases (e.g. alcohol, lactate, and malate dehydrogenases). Later it was used for isolation of serum albumin (CitationTravis and Pannell 1973, CitationLeatherbarrow and Dean 1980), as well as other serum proteins (CitationBirkenmeier et al. 1983, CitationGee et al. 1979). Gianazza and Arnaud had studied the fractionation of plasma proteins on immobilized CB and had performed its mechanism of interaction with proteins. They recovered > 80% of the albumin present in the initial serum sample. They also studied extensively the dye–protein interaction mechanisms and concluded that ionic forces were the major driving force in the binding event and that hydrophobic interactions played an accessory role (CitationGianazza and Arnaud 1982a, Citation1982b). Recently, dye affinity ligand CB have been used for removing high abundant plasma albumin, which constitutes > 60% of the total amount of plasma proteins, prior to detection of low-abundance plasma proteome, in which most of biomarkers of any possible disease could be found (CitationGovorukhina et al. 2003, CitationYoung et al. 2005, CitationAhmed et al. 2003, CitationGirolamo et al. 2011). CB is an inexpensive commercially available dye ligand and can be easily immobilized on matrices, especially bearing hydroxyl groups (CitationDenizli and Pişkin 2001). After fixation to appropriate insoluble supports, CB has been used as ligand in affinity chromatography to isolate and remove albumin from human plasma (CitationAndac et al. 2012). In literature, immobilized CB on different matrices had been used for albumin separation and purification from various sources including human serum. Altintas and Denizli prepared CB immobilized poly(glycidyl methacrylate) [poly(GMA)] monosize microbeads and reached 99.3% human serum albumin (HSA) depletion efficiency (CitationAltıntaş and Denizli 2006a). CitationDemiryas et al. (2007) used poly(acrylamide-allyl glycidyl ether) cryogel for CB immobilization and used the matrix for the separation of albumin from aqueous solutions and human plasma. CitationKumar et al. (2009) generated a systematic library of 96 affinity resins which were prepared as novel analogs of CB and tested in a batch binding and elution mode using seven different proteins, including four Aspergillus enzymes, bovine pancreatic trypsin, and the two serum proteins, albumin and IgG. CitationSteel et al. (2003) used CB-based and immune-affinity resin to remove albumin and IgG from human serum samples in order to simplify the serum proteome in a single step. CitationOdabaşı and Denizli (2004) prepared CB immobilized magnetic poly(2-hydroxyethyl methacrylate) [PHEMA] beads and observed an increase in the level of HSA adsorption. CitationColantonio et al. (2005) reported CB based extraction method for the effective and specific removal of albumin from serum with increased detection of low abundant proteins in electrophoresis. CitationMorais-Zani et al. (2011) performed the depletion of albumin from Bothrops jaraca plasma using the HiTrap Blue high-performance column (GE Healthcare Life Sciences, Piscataway, NJ, USA) and the kit Albumin & IgG Depletion SpinTrap column (GE Healthcare Life Sciences).
In this study, we have attempted to prepare and characterize CB immobilized PGMA nanobeads (CB-PGMA), which offer promising approach with good depletion efficiency of albumin from human serum. Recently, the synthesis of nano-sized matrices has been used due to the advantages of their exceptionally small dimensions in application of special purposes (CitationLopez et al. 2001, CitationSaka et al. 2013, CitationJang et al., 2005). While PHEMA is well known for its nontoxicity, biocompatibility, and wide spread biomedical applications, PGMA has an advantage in the presence of reactive oxirane groups which can be effectively modified (CitationHorák and Shapoval 2000). Therefore, PGMA was chosen as the main matrix due to the convertible oxirane groups (CitationHorák 2001). By alkalization with sodium hydroxide, oxirane groups can be transformed to hydroxyl groups that easily immobilize CB via nucleophilic substitution reaction (CitationAltıntaş and Denizli 2006a). The present work was accomplished in three main parts. Firstly, PGMA nanobeads were synthesized by mini emulsion polymerization method (45 nm in average diameter) and CB was immobilized on these PGMA nanobeads under alkali conditions. Secondly, CB-PGMA were characterized by atomic force microscopy (AFM), scanning electron microscopy (SEM), fourier transform infrared spectroscopy (FTIR), and zeta size analysis. Finally, the depletion efficiency of CB-PGMA nanobeads was studied with human plasma and confirmed by 2D-gel electrophoresis.
Experimental
Materials
CB was obtained from Polyscience (Warrington, USA) and used without further purification. For the synthesis of PGMA nanobeads, glycidyl methacrylate (GMA) (Fluka A.G., Buchs, Switzerland) was purified by vacuum distillation and stored in a refrigerator until use. Polyvinyl alcohol (PVAL) (MW: 30,000–70,000, Sigma, St. Louis, MO, USA) was selected as the steric stabilizer. Human plasma (Cat No: P4639) was purchased from Sigma (St. Louis, MO, USA). All other chemicals were of reagent grade and were purchased from Merck AG (Darmstadt, Germany). All water used in the experiments was purified using a Barnstead (Dubuque, IA) ROpure LP® reverse osmosis unit. Buffer and sample solutions were prefiltered through a 0.2 mm membrane (Sartorius, Gottingen, Germany).
Synthesis of CB-PGMA
The miniemulsion polymerization method was adapted from the reference reported elsewhere (CitationTan et al. 2008). A mixture of sodium dodecyl sulfate (SDS) and PVAL had been used as the surfactant of the polymerization system. Briefly, two aqueous phases were prepared. Dissolving 0.4 g of PVAL and SDS in 20 mL of deionized water formed the first aqueous phase. A second aqueous phase of 0.2 g of PVAL and SDS were dissolved in 400 mL of deionized water. A 0.8 mL of glycol methacrylate (GMA) and 4.2 mL of ethylene glycol dimethacrylate were then mixed for the monomer phase. The monomer mixture was slowly added into the first aqueous phase, followed by homogenization at 24,000 rpm with a homogenizer (T10 basic, Ika-Ultra Turrax, Germany) to form miniemulsion. The sodium bisulfite (0.2 g) and ammonium per sulfate initiated (0.3 g) miniemulsion polymerization of GMA was carried out in a fixed reaction volume of 250 mL at 40°C within a mixing rate of 500 rpm for 24 h. Subsequently the reactor was cooled and the synthesized nanobeads were separated from the medium by centrifugation at 30,000 rpm. Washing out process with ethanol and water reciprocally provided removal of the soluble components. The procedure for covalent attachment of CB to PGMA nanobeads was applied according to the following reference (CitationAltıntaş and Denizli 2006b). Briefly, 1.0 mg/mL of CB was mixed with 0.1 g of PGMA nanobeads, which was suspended in 100 mL of 1.0 M sodium hydroxide solution. The reaction conditions were maintained at constant temperature value of 80°C with 400 rpm mixing rate for 4 h. Under these experimental conditions, a chemical reaction took place between the chlorine atom of the CB and the epoxy groups of the PGMA nanobeads. After incubation, CB-PGMA nanobeads were centrifuged and dispersed in 50% ethanol solution, and the dispersion was sonicated for 2 h in an ultrasonic bath in order to remove physically adsorbed CB molecules. Then, CB-PGMA nanobeads were extensively washed with deionized water and stored at 4°C with 0.02% sodium azide against microbial contamination.
Characterization of CB-PGMA
The nanobead size distribution and polydispersity index (PDI) of CB-PGMA nanobeads were measured by Nano Zetasizer (NanoS, Malvern Instruments, London, UK). The surface area of CB-PGMA nanobeads was calculated in terms of m2/g corresponding to the following relationships (CitationBangs 1987).
Here, A, S, d, ρL, and ρs are referred as the surface area/mL for suspensions in water (m2/g), the weight of solids (S, %), mean diameter (d, μm), density of bead suspension (ρL = 100.S/[S.(1−ρs)+ 100.ρs], g/mL), and density of solid bead (ρS, g/mL), respectively.
The topographic images of CB-PGMA were taken by atomic force microscope (AFM, Nanomagnetics Instruments, Oxford, UK). The surface morphology of CB-PGMA nanobeads was characterized by scanning electron microscope (SEM, JEOL JSM 5600, Jeol Co., Tokyo, Japan). The content of CB was calculated from stoichiometric ratio of sulfur by performing elemental analysis (Leco, CHNS–932, USA). All the infrared spectra were collected by a FTIR spectrometer (Thermo Fisher Scientific, Nicolet iS10, Waltham, MA, USA) to identify the functional groups in the range of 4000–600 cm–1.
Depletion efficiency of albumin from human plasma
In vitro depletion experiments were carried out using human plasma. 4.0 mL of phosphate buffer saline (10 mM PBS pH:7.4, NaCl: 0.9%) was used for dilution of human plasma by 1:10. Briefly, 100 μL of diluted human plasma was added to 1.0 mL of CB-PGMA nanobeads suspension in a concentration of 2.5 mg/mL. The incubation process was performed with a rotary shaker for 2 h at room temperature. The amount of albumin adsorbed by CB-PGMA nanobeads was determined by measuring the initial and final concentration of albumin in plasma (TOBB Hospital, Ankara, Turkey). Desorption of the adsorbed albumin from CB-PGMA nanobeads was studied with 10 mM pH 7.4 phosphate buffer containing 1 M NaCl solution. Desorption medium was maintained for 2 h at room temperature. In order to show the reusability, adsorption–desorption cycle was repeated ten times using the same batch.
2D electrophoresis was carried out at Multiphore II Electrophoresis Unit (Amersham Biosciences). 2D clean up kit was used to remove contaminants prior to sample preparation. Untreated and treated human plasma samples were redissolved in sample buffer containing 7 M urea, 2 M thiourea, 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, 65 mM dithiothreitol, 5 mM tributylphosphine, and 0.4% ampholytes immobilized pH gradient buffer (IPG Buffer). Untreated and treated human plasma samples (10 μL, 10–200 mg/mL protein concentration) were focused in an 13-cm pH 3–10 immobilized pH gradient strip (Amersham Biosciences) after rehydration in DeStreak Reagent with 2.0% IPG buffer (Amersham Biosciences) for 14 h. Gel strips were equilibrated in 6 M urea, 2% SDS, 1.5% dithiothreitol, 30% glycerol, 50 mM Tris, pH 8.8, for 10 min followed by incubation in the same solution, but replacing dithiothreitol with 3% iodoacetamide, for an additional 10 min. The gel strips were placed on a 10% polyacrylamide gel for resolution in the second dimension. Gels were stained with colloidal Coomassie blue and imaged with Image Quant 300 (Amersham, USA).
Results and discussion
Characterization of CB-PGMA
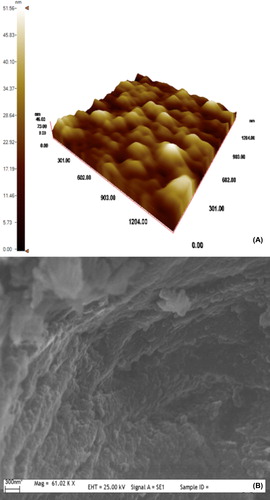
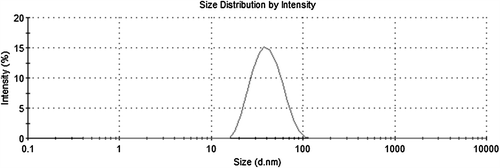
By adjusting various reaction parameters, such as selection of the reaction medium, stabilizer/emulsifier concentration, initiator concentration, and polymerization temperature, it is possible to control the bead size within a range of 30 nm to 4 μm (CitationRittich et al. 2009). It is always crucial to find optimum reaction conditions for monodisperse particles, which are uniform in size. The beads are generally considered monodisperse if their PDI is less than 1.03 (CitationHorák et al. 2003). and shows the surface morphology and size characteristics of PGMA nanobeads by means of AFM and SEM pictures, respectively. In , PGMA nanobeads are spherical and monodisperse with maximum average size recorded as 45 nm. confirmed the spherical morphology of PGMA nanobeads by SEM. The narrow size distribution of PGMA nanobeads was one of the most important parameters affecting the adsorption dynamics (CitationSaka et al. 2013). The average size and PDI of PGMA nanobeads were recorded as 42.5 nm and 0.118, respectively, with zeta size analyzer, which was in conformity with AFM results and supporting the repeatable adsorption processes ().
According to the sulfur stoichiometry, the quantity of CB immobilized onto PGMA nanobeads was calculated as 50.3 μmol/g. In the meantime, the specific surface area of PGMA nanobeads was calculated as 2616 m2/g. On account of the fact that this calculated high surface area yields low mass transfer restrictions, the prepared nanobeads may lead to extremely high HSA adsorption capacities (CitationSaka et al. 2013). Some physical properties of CB-PGMA nanobeads are summarized in .
Table I. Some physical properties of the CB-PGMA nanobeads.
After size distribution and surface characterization, CB was easily immobilized on PGMA nanobeads via reactive oxirane groups under alkaline conditions. Although, PHEMA based matrices offer a number of properties important for bioseparation, such as biocompatibility, hydrophilicity, and protein friendly surfaces with low nonspecific interaction (CitationDenizli et al. 1988, CitationAndac et al. 2008, Citation2012, CitationBaşar et al. 2007, CitationKarakoç et al. 2009), a great advantage of PGMA based supports is their easy chemical modification (CitationHorák et al. 2003).
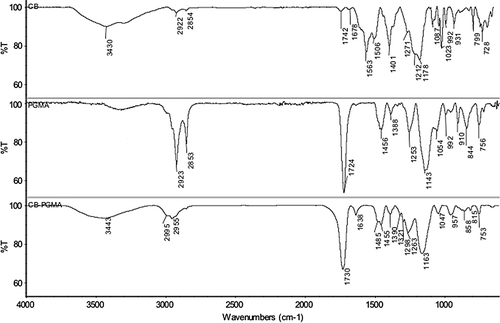
The confirmation of CB immobilization on CB-PGMA nanobeads was determined by FTIR spectra (). The presence of oxirane groups in PGMA nanobeads was observed by a characteristic band at 910 cm− 1 (CitationHorák 2001). The adsorption bands at 756 cm− 1 (C-H bending vibration of epoxide ring), 1143–1253 cm− 1 (C-O stretch in ester), 1724 cm− 1 (C = O stretching), and 2853–2923 cm− 1 (C-H aliphatic) were attributed to other characteristic groups in PGMA nanobeads (CitationSattarzadeh and Golipour 2012). The FTIR spectrum of CB-PGMA nanobeads shows the characteristic bands at 1047 cm− 1 (aromatic C = C stretching), 1730 cm− 1 (C = O stretching), 2955–2995 cm− 1 (C-H aliphatic), and 3443 cm− 1 (aromatic C-N stretching). In addition to these bands, the new peaks come out at 1455–1638 cm− 1 (N-H bending of CB) and 1163–1298 cm− 1 (asymmetric and symmetric S = O stretching), which were considered as an indication of the presence of CB within PGMA nanobeads. The visual observations (the color of the nanobeads) also ensured the attachment of dye molecules on PGMA nanobeads. It should be noted that CB immobilized PGMA nanobeads were extensively washed with ethanol until no release was observed.
Depletion efficiency of albumin from human plasma
CB is a derivative of monochlorotriazine dye (CitationAndac et al. 2007), containing three titratable acidic sulfonate groups and four basic primary and secondary aromatic amine groups, which enables it to bind with considerable specificity and affinity to nucleotide dependent enzymes and to a series of other proteins including serum albumin (CitationKopperschlager et al. 1982). The interaction of albumin with immobilized CB to PGMA nanobeads is possibly with mixed mode behavior where both electrostatic and aromatic hydrophobic interactions can occurr (CitationAndac et al. 2007). The depletion efficiency of albumin from human plasma was investigated by treating 1:10 diluted human plasma with CB-PGMA nanobeads, and the results showed that 1672 mg/g of albumin were depleted with 95% of removal efficiency (). The results are considerably high compared with the ones present in the literature (CitationAndac et al. 2012, CitationSaka et al. 2013, CitationDemiryas et al. 2007, CitationAltıntaş and Denizli 2006a). For instance, Andaç et al. used CB immobilized PHEMA cryogel and reached 950 mg/g dry cryogel for nondiluted serum with 77% albumin depletion efficiency (CitationAndac et al., 2012). CitationBjörhall et al. (2005) used five commercially available columns including Aurum Serum Protein Minikit (Bio-Rad, USA) consisting of CB as a ligand and they achieved 96.3% of albumin depletion. CitationDenizli et al. (1999) prepared CB attached PHEMA microbeads and 109.6 mg/g HSA adsorption was achieved from human plasma. CitationAltıntaş and Denizli (2006a) reported 99.3% albumin depletion amount with dye-affinity monosize poly(GMA) beads. CitationKarataş et al. (2007) used anti-human serum albumin antibody-sepharose 4B column and they reached 99.4% of depletion efficiency for albumin prior to immunoglobulin depletion in serum sample.
Table II. Albumin depletion efficiency of CB-PGMA nanobeads.
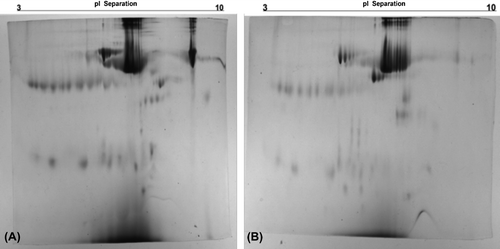
Removal of high-abundant proteins in serum was expected to improve the resolution on 2D gels in two major ways: (i) by enabling visualization of proteins that comigrate with the high-abundant proteins and (ii) by increasing the protein load of the less-abundant proteins (CitationBjörhall et al. 2005). Depleted human plasma samples from CB-PGMA nanobeads were selected to investigate the improvement in resolution and the appearance of new protein spots on 2D gels as compared to untreated plasma samples. In , the optical density of albumin spot masks the protein pattern on untreated plasma in 2D gel, resulting in a bulky protein region, particularly in the high-abundant protein area (). The treatment of human plasma with CB-PGMA nanobeads enabled the depletion of albumin, resulting in the detection of several new spots in the high-abundant protein region (). In general, the regeneration is an important part to generate these supports more affordable for the laboratory and commercial applications. Desorption ratios of CB-PGMA nanobeads have been achieved as high as 96% and after ten adsorption–desorption cycles, it was maintained at about 92% of initial binding capacity (data not shown).
Conclusions
PGMA nanobeads are a new, inexpensive, and modifiable support suitable for immobilization of ligands such as CB that has extensive potential for selective albumin removal in applications prior to proteome studies (CitationHorák 2001). The CB-PGMA nanobeads can easily process available amount of human plasma and it can be reused several times, which allows these nanobeads use at low cost. The focus of this study was to investigate a cost effective and reusable dye affinity PGMA nanobeads having high adsorption capacity for depletion of albumin for proteome studies involving plasma/serum samples. According to adsorption capacity, recovery, and binding specificity results, dye affinity PGMA nanobeads offered a promising albumin depletion approach.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahmed N, Barker G, Oliva K, Garfin D, Talmadge K, Georgiou H. 2003. An approach to remove albumin for the proteomic analysis of low abundance biomarkers in human serum. Proteomics. 3:1980–1987.
- Altıntaş EB, Denizli A. 2006a. Efficient removal of albumin from human serum by monosize dye-affinity beads. J Chromatogr B. 832:216–223.
- Altıntaş EB, Denizli A. 2006b. Monosize poly(glycidyl methacrylate) beads for dye-affinity purification of lysozyme. Int J Biol Macromol. 38:99–106.
- Andac CA, Andac M, Denizli A. 2007. Predicting the binding properties of cibacron blue F3GA in affinity separation systems. Int J Biol Macromol. 41:430–438.
- Andac M, Plieva FM, Denizli A, Galaev IY, Mattiasson B. 2008. Poly(hydroxyethyl methacrylate)-based macroporous hydrogels with disulfide cross-linker. Macromol Chem Phys. 209: 577–584.
- Andac M, Galaev IY, Denizli A. 2012. Dye attached poly(hydroxyethyl methacrylate) cryogel for albumin depletion from human serum. J Sep Sci. 35:1173–1182.
- Bangs LB. 1987. Uniform Latex Particles. Indianapolis, USA: Seradyn Inc., Seragen Diagnostics.
- Başar N, Uzun L, Güner A, Denizli A. 2007. Lysozyme purification with dye-affinity beads under magnetic field. Int J Biol Macromol. 41:234–242.
- Birkenmeier G, Usbeck E, Saro L, Kopperschläger G. 1983. Triazine dye binding of human alpha-fetoprotein and albumin. J Chromatogr. 265:27–35.
- Björhall K, Miliotis T, Davidsson P. 2005. Comparison of different depletion strategies for improved resolution in proteomic analysis of human serum samples. Proteomics. 5:307–317.
- Clonis YD, Labrou NE, Kotsira VPh, Mazitsos C, Melissis S, Gogolas G. 2000. Biomimetic dyes as affinity chromatography tools in enzyme purification. J Chromatogr A. 891:33–44.
- Colantonio DA, Dunkinson C, Bovenkamp DE, Van Eyk JE. 2005. Effective removal of albumin from serum. Proteomics. 5: 3831–3835.
- Dean PD, Watson DH. 1979. Protein purification using immobilised triazine dyes. J Chromatogr. 165:301–319.
- Demiryas N, Tuzmen N, Galaev IY, Piskin E, Denizli A. 2007. Poly(acrylamide-allyl glycidyl ether) cryogel as a novel stationary phase in dye-affinity chromatography. J Appl Polym Sci. 105: 1808–1816.
- Denizli A, Pişkin E. 2001. Dye-ligand affinity systems. J Biochem Biophys Methods. 49:391–416.
- Denizli A, Kiremitçi M, Pişkin E. 1988. Subcutaneous polymeric matrix system p(HEMA-BGA) for controlled release of an anticancer drug (5-fluorouracil): II: Release kinetics. Biomaterials. 9:363–366.
- Denizli A, Kokturk G, Yavuz H, Piskin E. 1999. Dye-ligand column chromatography: albumin adsorption from aqueous media and human plasma with dye-affinity microbeads. J Appl Polym Sci. 74:2803–2810.
- Gee AP, Borsos T, Boyle MD. 1979. Interaction between components of the human classical complement pathway and immobilized Cibacron Blue F3GA. J Immunol Methods. 30:119–126.
- Gianazza E, Arnaud P. 1982a. A general method for fractionation of plasma proteins. Dye-ligand affinity chromatography on immobilized Cibacron Blue F3GA. Biochem J. 201:129–136.
- Gianazza E, Arnaud P. 1982b. Chromatography of plasma proteins on immobilized Cibacron Blue F3-GA. Mechanism of the molecular interaction. Biochem J. 203:637–641.
- Girolamo FD, Righetti PG, D’Amato A, Chung MCM. 2011. Cibacron Blue and proteomics: the mystery of the platoon missing in action. J Proteomics. 74:2856–2865.
- Govorukhina NI, Keizer-Gunnink A, van der Zee AAG, de Jong S, de Bruijn HWA, Bischof R. 2003. Sample preparation of human serum for the analysis of tumor markers: comparison of different approaches for albumin and γ-globulin depletion. J Chromatogr A. 1009:171–178.
- Horák D, Shapoval P. 2000. Reactive poly(glycidyl methacrylate) microspheres prepared by dispersion polymerization. J Polym Sci A Polym Chem. 38:3855–3863.
- Horák D. 2001. Magnetic poly glycidyl methacrylate microspheres by dispersion polymerization. J Polym Sci A Polym Chem. 39: 3707–3715.
- Horák D, Semenyuk N, Lednicky F. 2003. Effect of the reaction parameters on the particle size in the dispersion polymerization of 2-hydroxyethyl and glycidyl methacrylate in the presence of a ferrofluid. J Polym Sci A Polym Chem. 41:1848–1863.
- Jang J, Bae J, Ko S. 2005. Synthesis and Curing of Poly(glycidyl methacrylate) Nanoparticles. J. Polym. Sci: Part A: Polym. Chem. 43:2258–2265.
- Karakoç V, Yılmaz E, Türkmen D, Özturk N, Akgöl S, Denizli A. 2009. Selective separation of human serum albumin with Cu(II) chelated PHEMA based nanoparticles. Int J Biol Macromol. 45:188–193.
- Karataş M, Akgıl S, Yavuz H, Say R, Denizli A. 2007. Immunoglobulin G depletion from human serum with metal-chelated beads under magnetic field. Int. J. Biol.Macromol. 40:254–260.
- Kopperschlager G, Böhme HJ, Hofmann E. 1982. Cibacron blue F3GA and related dyes as ligands in affinity chromatography. Adv Biochem Eng. 25:101–138.
- Kumar S, Dalvi DB, Moorthy M, Korde SS, Fondekar KP, Sahasrabudhe SD, et al. 2009. Discriminatory protein binding by a library of 96 new affinity resins: a novel dye-affinity chromatography tool-kit. J Chromatogr B. 877:3610–3618.
- Leatherbarrow RJ, Dean PD. 1980. Studies on the mechanism of binding of serum albumins to immobilized Cibacron Blue F3GA. Biochem J. 189:27–34.
- Lopez D, Cendoya I, Torres F, Tejada J, Mijangos C. 2001. Preparation and characterization of poly(vinyl alcohol)-based magnetic nanocomposites. 1. Thermal and mechanical properties. J Appl Polym Sci. 82:3215–3222.
- Morais-Zani K, Grego KF, Tanaka AS, Tanaka-Azevedo AM. 2011. Depletion of plasma albumin for proteomic analysis of bothrops jararaca snake plasma. J Biomol Tech. 22:67–73.
- Odabaşı M, Denizli A. 2004. Cibacron Blue F3GA-attached magnetic poly(2-hydroxyethyl methacrylate) beads for human serum albumin adsorption. Polym Int. 53:332–338.
- Rittich B, Španová A, Horák D. 2009. Functionalised magnetic microspheres with hydrophilic properties for molecular diagnostic applications. Food Res Int. 42:493–498.
- Saka Ö, Karakoç V, Andaç M, Türkmen D, Denizli A. 2013. Dye- attached magnetic poly(hydroxyethyl methacrylate) nanospheres for albumin depletion from human plasma. Artificial Cells, Nanomedicine and Biotechnology. [Epub ahead of print].
- Sattarzadeh S, Golipour H. 2012. Chemical modification of glycidyl methacrylate copolymers with oximes containing pyridine groups. Der Pharma Chemica. 4:2340–2346.
- Steel LF, Trotter MG, Nakajima PB, Mattu TS, Gonye G, Block T. 2003. Efficient and specific removal of albumin from human serum samples. Mol Cell Proteomics. 2:262–270.
- Tan CJ, Wangrangsimakul S, Bai R, Tong YW. 2008. Defining the interactions between proteins and surfactants for nanoparticle surface imprinting through miniemulsion polymerization. Chem Mater. 20:118–127.
- Travis J, Pannell R. 1973. Selective removal of albumin from plasma by affinity chromatography. Clin Chim Acta. 49:49–52.
- Young CL, Woolfit AR, McWilliam LG, Mour H, Boye AE, Barr JR. 2005. Survey of albumin purification methods for the analysis of albumin-organic toxicant adducts by liquid chromatography–electrospray ionization–tandem mass spectrometry. Proteomics. 5:4973–4979.