Abstract
Biological containers such as virus-like particles (VLPs) have gained increasing interest in the fields of gene therapy and vaccine development. Several virus-based materials have been studied, but the toxicity, biodistribution, and immunology of these systems still require extensive investigation. The specific goal of this review is to provide information about nodaviruses, which are causative infectious agents of insects and aquatic animals, but not humans. By understanding the structure and biophysical properties of such viruses, further chemical or genetic modification for novel nanocarriers could be developed. Therefore, their application for therapeutic purposes, particularly in humans, is of great interest.
Introduction
In recent years, interest has increased in the use of biological cages such as small heat shock proteins, ferritins, and viral capsids for drug delivery and other biomedical applications (CitationMa et al. 2012). One of the characteristic features of these protein cages is their morphological uniformity, which can be further modified, either genetically or chemically, to allow site-specific recognition and presentation of various molecules proven to be beneficial for protein cage targeting (CitationUchida et al. 2007). Among available biological containers, virus-like particles (VLPs), or recombinant protein cages mimicking the viral capsid structure, offer the huge advantages of biocompatibility and easy functionalization, for example, host cell recognition, fusion, and entry (CitationLudwig and Wagner 2007). With such versatile properties, many attempts have been made to manipulate VLPs to carry and display foreign epitopes or to serve as building blocks for carrying heterogeneous genetic materials to target other diseases. Based on their self-assembly and disassembly properties, VLPs with the ability to encapsulate and deliver plasmid DNA into cells in vitro and in vivo include the mouse polyoma virus (CitationKrauzewicz et al. 2000), human papillomavirus (CitationCombita et al. 2001), hepatitis E virus (CitationTakamura et al. 2004), and simian virus 40 (CitationDamodaran et al. 2002). Since these VLPs are derivatives of human infectious viruses that cause harmful diseases, their application as biological containers is thus still questionable and requires intensive investigation. This review therefore investigates the plausibility of using nodavirus, a causative agent of fish, insect, and crustacean diseases, as a nanocontainer. An overview of its genome organization and the structural and chemical properties of the viral capsid protein, development of nodavirus VLPs as an epitope-presenting system and vector for gene therapy as well as its advantage and disadvantage and the other synthetic-based nanocontainers are also included.
Nodavirus genome organization and virus-like particle formation
Nodavirus, a member of the nodaviridae family, is a non-enveloped icosahedral virus with a diameter between 29 and 35 nm. The viral genome consists of bipartite linear positive-sense RNA including RNA1 and RNA2. RNA1 (3.0–3.2 kb in length) encodes RNA-dependent RNA polymerase (RdRp) and subgenomic RNA3, which translates one or two proteins, while RNA 2 (1.3–1.4 kb) encodes a capsid protein precursor (CitationJohnson et al. 2001). Each genome segment is capped at the 5′ end but has no poly (A) at the 3′ end. Based on known amino acid sequences of their capsid proteins (CitationNikolaev et al. 1997, CitationBonami and Sri Widada 2011), phylogenetic trees of nodaviridae contain three distinguished groups: alphanodaviruses which primarily infect insects, betanodaviruses which cause infections in fish, and prawn and shrimp nodaviruses which infect crustaceans. The members of each group are shown in .
Table I. Member of each genera of nodavirus.
The structure, assembly, and RNA packaging of nodaviruses have been studied extensively. Capsid protein of insect and fish nodaviruses can be produced in Sf 21 cells using a recombinant baculovirus vector and E.coli system (CitationShetty et al. 2012, CitationSahul Hameed and Bonami 2012, CitationTang et al. 2001). Malabaricus grouper nervous necrosis virus (MGNNV), FHV, PaV, and BBV spontaneously form icosahedral VLPs with T = 3 quasi-symmetry (CitationTang et al. 2002, Citation2001, CitationTihova et al. 2004b). Recently, expression of Macrobrachium rosenbergii nodavirus capsid protein in E.coli and its self-assembly into VLPs with diameter of approximately 30 ± 3 nm has been reported (CitationGoh et al. 2011). The three-dimensional and crystal structures of the MrNv-VLPs are not yet available and require further study.
Three-dimensional structure of nodavirus-like particles
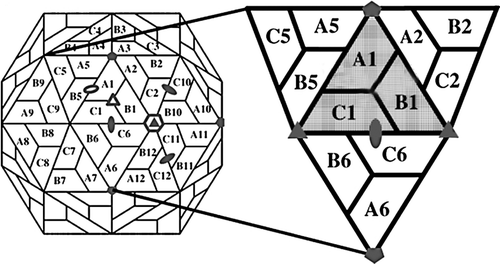
Knowledge of non-enveloped virus replication remains poorly established. Generally, capsids are solely responsible for attachment of a virus to the host cell prior to subsequent release of the genome for replication. For the simplest viruses, the completed icosaheral shell comprises 60 copies of single-typed protein forming an identical triangulation subunit of T = 1. When there are > 60 protein units (180, 240, or 360), these interact to form a complex closed shell and chemically identical structural subunits with slight changes in the bonding pattern (CitationBaker et al. 1999). Structural comparison through cryo-EM and three-dimensional analysis indicate that MGNNV, PaV, and FHV are assembled as T = 3 icosaheral particles; however, details of their radial density distribution and morphology differed remarkably.
The MGNNV capsid is formed by 180 copies of a 38 kDa protein unit with a maximal diameter of 380 Ǻ and is composed of two shells: an inner shell with a radius between 112 and 154 Ǻ and an outer shell with a radius between 154 and 192 Ǻ. The inner density at the lower radius (< 112 Ǻ) corresponds to RNA packaging within the capsid cavity (CitationTang et al. 2002). Sequence comparison of capsid protein of several fish nodaviruses has indicated a highly conserved region with a similarity of 92.5% and a variable region at the C-terminus (CitationNishizawa et al. 1995). For MGNNV, the N-terminal residues (1–82) share more than 79% identity with the three fish nodaviruses including Dicentrarchus labrax encephalitis virus, striped jack nervous necrosis virus, and atlantic halibut virus. They are also enriched in the positively charged amino acid residues, 9 arginine and 6 lysine, which allow interaction with negatively charged RNA during virus assembly and RNA packaging (CitationMarshall and Schneemann 2001). Residues 83 to 216 form a conserved β-sandwich domain for the shell of the capsid and the variable region at the C-terminus (residues 217–338) are responsible for formation of the protruding domain. At the quasi-three-fold axis, the protrusion of MGNNV is significantly greater than that of FHV and PaV and requires insertion of polypeptides to form a structure similar to that of insect nodaviruses (CitationLin et al. 2001).
Unlike the beta-nodavirus, alpha one requires self-catalyzed cleavage for capsid maturation. After particle assembly, the precursor protein alpha (subunit A) is cleaved to produce protein beta (subunit B) and gamma (subunit C) (CitationVenter and Schneemann 2008b). X-ray crystallography has shown that PaV and FHV are significantly smaller than MGNNV with an approximate diameter of 360 Ǻ and 350 Ǻ, respectively (CitationTihova et al. 2004a, CitationTang et al. 2002). For PaV, the A-subunits forming the 12 pentamers of the capsid are related to five-fold symmetry while the B- and C-subunits alternate around three-fold symmetry to form 20 hexamers (). The density map of PaV can be divided into three layers at different radii: an outermost layer which is a protein shell, a thin middle layer, and a central core for RNA. Similar to other viruses, residues 83–321-fold as an eight-stranded antiparallel β-sandwich to form the viral capsid, and residues 196–209 of three subunits at the C-terminus form the protruding domain with a size of about 15 Ǻ (CitationTang et al. 2001).
Figure 1. Diagram of the subunit packing in a T = 3 icosahedral lattice. The rhombic icosahedron is composed of 12 pentameric and 20 hexameric capsomeres for a total of 180 capsid proteins. The reference asymmetric unit composed of A1, B1, and C1 subunits is shown as a shaded triangle. Filled ovals, triangles, and pentagons define the locations of the two-, three-, and five-fold symmetry elements, respectively. Open ovals, triangles, and hexagons define the locations of quasi two-, three-, and six-fold symmetry elements, respectively. Modified from CitationDamodaran et al. (2002).
For FHV, a 43 kD subunit A is cleaved at conserved site Asn 363/Ala 364 to produce subunits B and C with molecular masses of 38 kD and 5 kD, respectively (CitationGallagher and Rueckert 1988). Protein beta forms a central anti-parallel β barrel, and the peptide at the N terminus is located inside the particle in contact with encapsulated RNA (CitationVenter and Schneemann 2008a). Sequence comparison of BBV reveals 87% similarity to FHV capsid proteins. Overall amino acid organization is also similar to FHV; namely, the N-terminus predominantly contains basic amino acid residues which are believed to interact with RNA and the β-barrel domain forms the protrusion. Calculated by X-ray scattering, the BBV shell is 312 Ǻ in diameter. Its perpendicular diameter to icosahedral two-fold axes can be divided into three zones including the innermost (extending 95–120 Ǻ), central (extending 120–145 Ǻ), and outermost regions (extending 145–170 Ǻ) (CitationKaesberg et al. 1990).
Although MrNv was first discovered in 1997 (CitationMa et al. 2012), information about its viral capsid protein is not available due to deficiency of the crustacean cell lines that can be used for virus replication and propagation (CitationSudhakaran et al. 2007). Transmission electron microscopy (TEM) and dynamic light scattering (DLS) confirmed that the MrNv capsid protein expressed in E.coli self-assembles to form VLPs with a diameter of 30 ± 3 nm (CitationGoh et al. 2011). Nevertheless, its high-resolution structure, determining viral capsid angulation, and its significant functional domains remain a topic for further investigation.
Essential calcium ions and disulfide bonds for nodavirus-like particle formation
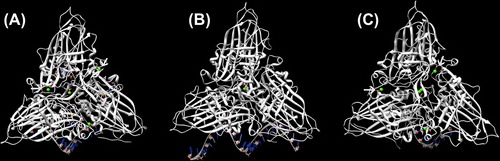
Disassembly and reassembly of viral capsids are two major events of viral replication. Several reports have revealed that calcium ion-mediated interactions between capsid protein subunits play an important role in VLP formation and that disulfide bonds are necessary for particle stability (CitationChen et al. 2001, CitationIshizu et al. 2001, CitationSatheshkumar et al. 2004, CitationSherman et al. 2006). Sequence alignment has revealed that insect and fish nodaviruses contain a calcium-binding motif in the highly conserved region of capsid protein. This result agrees well with available atomic structures of FHV, PaV, and BBV capsid proteins which incorporate four, one, and five calcium ions, respectively (), with negatively charged aspartate and glutamate residues in each icosahedral asymmetric unit (iASU) (CitationTang et al. 2001, CitationBanerjee et al. 2010). The importance of metal ions in particle formation has been tested by both metal chelators and mutation at the metal-binding sites. When calcium-coordinating residues in FHV capsid protein alpha are mutated, not only the particle is less stable than wild-type, but also virus entry is affected and infectivity is compromised (CitationBanerjee et al. 2010). Like other beta-nodaviruses, dragon grouper nervous necrosis (DGNNV) shares an identical calcium-binding motif, DxxDxD, at the 130–135 residues of capsid protein. Mutations in Asp130 and Asp135 produce intact and broken particles, while mutation of Asp133 disrupts particle formation (CitationWu et al. 2008). We have recently tested the effects of reducing and chelating agents on MrNv-VLP stability (CitationJariyapong et al. 2014) and found that this shrimp nodavirus requires both Ca2+ ions and disulfide linkages for its assembly process as well as stability of VLP capsid integrity. Interestingly, the assembly of MrNv-VLP selectively depends on calcium ions—only EGTA can disrupt VLP particles in the presence of DTT and not EDTA, which instead causes swelling of particles. This finding firmly pinpoints the importance of Ca2+ and disulfide bridging for nodavirus VLP formation.
Figure 2. Calcium-binding site in FHV, PaV, and BBV. (A) The capsid of FHV contains one calcium ion at the quasi three-fold axis, which is coordinated by aspartate 249 and glutamate 251. The three other calcium ions at the interface between pairs of subunits are coordinated by aspartate-221 from one subunit and aspartate 161 and glutamate 257 from the other subunit (PDB: 4FSJ). (B) One calcium ion is coordinated by aspartate 249 and glutamate 251 at the three-fold axis of aV (PDB: 1F8V). (C) Conserved acidic residues at an identical position as BBV incorporates five calcium ions in each iASU (PDB: 2BBV).
Stability of nodavirus-like particles under harsh hydrolytic conditions
Since many pathogenic viruses and bacteria establish their initial infection through the mucosal surface, development of mucosal vaccines has been widely studied (CitationZhai et al. 2013, CitationRivera-Hernandez et al. 2013). Induction of mucosal immunity through oral vaccine administration is beneficial due to its convenience; however, there are several difficulties in oral immunization with non-replicating molecules such as proteolytic enzymes in the gastrointestinal tract and an acidic environment. VLPs derived from harmful human viruses such as the hepatitis-E virus-like particle (HEV-VLP) have previously been found to be tolerant and still intact after exposure to digestive juices (CitationJariyapong et al. 2013). This is because the quaternary arrangement of VLP protein protects it from the cleavage of protease enzymes. Recently, MrNv-VLP has been reported to be stable under harsh hydrolytic conditions. Linear sequence analysis has revealed abundant potential sites for cleavage by pepsin, trypsin, and chymotrypsin throughout the capsid protein sequence. However, testing the stability of MrNv-VLPs under strong hydrolytic conditions to measure their suitability for oral delivery systems revealed that treatment with these digestive enzymes changed the band intensity of MrNv-VLPs but not their band mobility (CitationJariyapong et al. 2014), although the mechanism of tolerance is still unknown. Like HEV-VLP, it is possible that the quaternary structure of the MrNv capsid protein also protects these potential cleavage sites from enzyme accessibility. Further work on the atomic structure of the MrNv protein is required to confirm this hypothesis.
Application of nodaviruses in antigen presenting system and delivery system
Many researchers have already launched attempts to either re-engineer exterior capsid surfaces or load plasmid DNA into VLP of nodaviruses with variable degrees of success. FHV capsid protein has been reported as a foreign epitope presenting system, particularly in vaccine development. The first study employing FHV capsid protein as a carrier focused on the antigenicity of a short peptide derived from the V3 loop of HIV-1 gp 120, which was inserted into four sites on the outer surface of FHV capsid protein at amino acid positions 303, 264, 205, and 131 (CitationTisminetzky et al. 1994). The results showed that the peptide-insertion position on the capsid protein most efficiently recognized by HIV antisera is at position 264 (CitationVenter and Schneemann 2008a). Additional studies using peptides derived from hepatitis C virus core protein (CitationBuratti et al. 1997) and hepatitis B virus S-antigen (CitationChen et al. 2006) have demonstrated the potency of the FHV epitope presenting system for characterization of patient antisera; however, these studies employed chimeric FHV in the monomeric form. The display of many foreign peptides have also utilized the FHV-VLP system (CitationScodeller et al. 1995). As mentioned above, a seven amino acid peptide from the V3 loop of HIV-1 gp 120 has either replaced FHV amino acids 131–134 or been inserted between residues 305 and 306. These particles induced a high titer of HIV antibodies after immunization of guinea pigs. In addition, it has also been shown that FHV-VLP can carry the extracellular domain of anthrax toxin receptor 2 (ANTXR2), which was inserted into positions 206 and 264 on the FHV capsid protein. Successful insertion of this peptide into VLP particles was confirmed by comparing EM density maps of non-inserted FHV-VLP. Re-engineered FHV-VLP inserted with the ANTXR2 peptide revealed notable differences in the pattern of the ANTXR2 domain on the VLP surface. For insertion at position 206, the domains were clustered in group of six at the two-fold axes whereas insertions into other sites resulted in variable distributions of heterologous proteins. The capability of chimeric 264 FHV-VLP to protect rats against anthrax lethal toxin (LeTx) was subsequently tested in vivo. After intravenous administration with a lethal dose of LeTx either with or without chimera 264, this complex VLP elicited a toxin-neutralizing antibody response that protected rats from LeTx after a single immunization with no signs of infection (CitationManayani et al. 2007).
As described earlier in Section 2, the N-terminus of nodaviridae peptides contains basic amino acids which are folded inward in the capsid quaternary structure and responsible for binding with negatively charged RNA. This property could be a key success factor in allowing researchers to pack therapeutic agents, for instance, double strand RNA, antibodies, and vector-based gene therapy, into the central cavity of VLPs. We have recently encapsulated Pie1-EGFP plasmid DNA by VLP of MrNv for delivery to insect cell lines (CitationJariyapong et al. 2014). An intense green fluorescence of the GFP signal was detected as a punctuate staining pattern throughout the cytoplasm of live Sf9 cells after 48 h incubation with loaded VLPs. These results could be useful for applying MrNv-VLP for dsRNA encapsulation and further delivering it in vivo for treating shrimp virus infections. Additionally, targeting surface-modified VLPs to human affected cells through capsid re-engineering technology with small peptides or even chemical modification could be an area of future investigation.
Advantages and disadvantages of VLP and other synthetic-based nanocontainers
Development of an effective delivery system is one of the most challenging steps in gene therapy and vaccination. Ideally, the delivery system should be able to target the necessary antigen-production region, allow interaction with the cells of interest, and induce minimal immune responses. In general, delivery vehicles are divided into non-viral and viral delivery systems. Non-viral synthetic vectors possess many advantages over their viral counterparts as they are safe and easy to manufacture on a large scale. Several non-viral nanoparticles derived from biopolymers such as poly-lactide polyglycolide (PLGA or PLG) (CitationFredriksen and Grip 2012) and chitosan (CitationVimal et al. 2013, CitationRampino et al. 2013) are now being investigated and developed as vaccine delivery systems for aquatic animals. Chitosan is safer than PLGA as no organic solvent is required during antigen entrapment since, because it is positively charged, it has the ability to bind with negatively charged immunogenic DNA/RNA (CitationSaroja et al. 2011). It can also open up tight junctions and increase the paracellular transport of antigens (Citationvan der Lubben et al. 2001). Calcium phosphate is another inorganic material that has been reportedly used as an antigen delivery system in fish. Similar to chitosan, calcium phosphate is biocompatible and biodegradable. When S-layer proteins (of Aeromonas hydrophila) are absorbed on a nano-sized calcium phosphate particle, this elicits both innate and adaptive immune systems which persist up to 63 days post-immunization (CitationBehera and Swain 2011).
Several publications have reported the application of virus-based nanocarriers for drug delivery in humans (CitationMa et al. 2012, CitationBolhassani et al. 2011, CitationDestito et al. 2009). However, there is only limited information available about antigen/vaccine carriers constructed from the capsid protein of fish or crustacean viruses. VLPs offer great advantages over other non-viral nanoparticles because of their excellent bio-compatibity, morphological uniformity, and ready functionalization, for example, host cell recognition, fusion, and entry (CitationLudwig and Wagner 2007). Moreover, they can be found in a variety of distinctive shapes including icosahedrons, spheres, and tubes that range from ˜10 nm to over a micron (CitationPorta et al. 2003, CitationTang et al. 2006, CitationUchida et al. 2007, CitationLee et al. 2009, CitationDestito et al. 2009), a size that is readily taken into antigen presenting cells (APC). A comparison of diameter and length of each VLP with that of other synthetic nanocontainers is shown in . As mentioned in Section 3, nodavirus-like particles are icosahedrous particles with diameter < 5 μm, and are thus favorable for uptake by APC via macropinocytosis and endocytosis, followed by directing antigen processing in late endosomes and presentation of epitopes by the MHC class system (CitationLudwig and Wagner 2007, CitationDestito et al. 2009). Based on this pathway, nodavirus-like particles have proven to be not only potent immunogens (CitationThiéry et al. 2006) but also nanocontainers in their host species.
Table II. Comparison between virus-like particle and synthetic-based nanocontainer in size and shape.
Recent application of VLPs has been directed toward nanocontainers for drug ferries to convey substances directly from the bloodstream to their specific destination in the brain through the blood–brain barrier (BBB). Findings from these applications would therefore open new treatment aspects of human brain disorders, including neurodegenerative diseases, viral infections, and bacterial infections. VLPs of West Nile virus (WNV) have shown the ability to cross human endothelial cells via a transcellular pathway without disrupting the integrity of the BBB (CitationHasebe et al. 2010). Mice immunized with WNV-VLP (containing WNV structural protein, prME) showed no morbidity or mortality after being challenged with WNV. This chimeric VLP could also induce sterilizing immunity without producing any evidence of viremia or viral RNA in brain (CitationQiao et al. 2004).
Conclusions
Nodaviruses are causative infectious agents of crustaceans and some aquatic animals. Their capsid protein structure and physicochemical properties have been studied extensively to gain fundamental knowledge of RNA viruses and development of antiviral immunity. Many reports have indicated success in utilizing non-enveloped-virus-derived VLPs, including MrNv-VLPs, as a biological container for many therapeutic materials. Attempts should be launched to study specific targeting properties of these non-enveloped VLPs in vivo and in animal models using available bioengineering technology, for instance, GFP tagged plasmids and advanced microscopy. Once this information is available, delivery of therapeutic materials encapsulated in VLPs into specific organs in animals will be highly feasible. The next challenging task is the encapsulation of dsRNA against shrimp viruses or hormones involved in reproductive controls into VLPs and their delivery to known sites of infection for viral deterioration or even enhancement of reproductive performance. Along with non-causative infection of mammals, including humans, one of the ultimate applications of nodavirus-derived capsid proteins may be as a powerful biological container for human therapeutic purposes.
Acknowledgments
The author would like to thank Assoc. Prof. Wattana Weerachatyanukul for his support and encouragement during preparation of this article.
Declaration of interest
The author report no declarations of interest. The author alone is responsible for the content and writing of the paper.
This research was supported by Institute of Research and Development, Walailak University, Nakhon Si Thammarat, Thailand.
References
- Baker TS, Olson NH, Fuller SD. 1999. Adding the third dimension to virus life cycles: three-dimensional reconstruction of icosahedral viruses from cryo-electron micrographs. Microbiol Mol Biol Rev. 63:862–922.
- Banerjee M, Speir JA, Kwan MH, Huang R, Aryanpur PP, Bothner B, Johnson JE. 2010. Structure and function of a genetically engineered mimic of a nonenveloped virus entry intermediate. J Virol. 84:4737–4746.
- Behera T, Swain P. 2011. Antigen adsorbed calcium phosphate nanoparticles stimulate both innate and adaptive immune response in fish, Labeo rohita H. Cell Immunol. 271:350–359.
- Bolhassani A, Safaiyan S, Rafati S. 2011. Improvement of different vaccine delivery systems for cancer therapy. Mol Cancer. 10:3.
- Bonami J-R, Sri Widada J. 2011. Viral diseases of the giant fresh water prawn Macrobrachium rosenbergii: a review. J Invertebr Pathol. 106:131–142.
- Buratti E, Di Michele M, Song P, Monti-Bragadin C, Scodeller EA, Baralle FE, Tisminetzky SG. 1997. Improved reactivity of hepatitis C virus core protein epitopes in a conformational antigen-presenting system. Clin Diagn Lab Immunol. 4:117–121.
- Chen P-L, Wang M, Ou W-C, Lii C-K, Chen L-S, Chang D. 2001. Disulfide bonds stabilize JC virus capsid-like structure by protecting calcium ions from chelation. FEBS Lett. 500:109–113.
- Chen Y, Xiong X, Liu X, Li J, Wen Y, Chen Y, et al. 2006. Immunoreactivity of HCV/HBV epitopes displayed in an epitope-presenting system. Mol Immunol. 43:436–442.
- Combita AL, Touzé A, Bousarghin L, Sizaret PY, Muoz N, Coursaget P. 2001. Gene transfer using human papillomavirus pseudovirions varies according to virus genotype and requires cell surface heparan sulfate. FEMS Microbiol Lett. 204:183–188.
- Damodaran KV, Reddy VS, Johnson JE, Brooks CL. III. 2002. A General method to quantify quasi-equivalence in icosahedral viruses. J Mol Biol. 324:723–737.
- Destito G, Schneemann A, Manchester M. 2009. Biomedical nanotechnology using virus-based nanoparticles. In: Manchester M, Steinmetz N, Eds. Viruses and Nanotechnology. Berlin, Heidelberg: Springer. 95–122.
- Fredriksen BN, Grip J. 2012. PLGA/PLA micro- and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of Atlantic salmon (Salmo salar L.). Vaccine. 30:656–667.
- Gallagher TM, Rueckert RR. 1988. Assembly-dependent maturation cleavage in provirions of a small icosahedral insect ribovirus. J Virol. 62:3399–3406.
- Goh ZH, Tan SG, Bhassu S, Tan WS. 2011. Virus-like particles of Macrobrachium rosenbergii nodavirus produced in bacteria. J Virol Methods. 175:74–79.
- Hasebe R, Suzuki T, Makino Y, Igarashi M, Yamanouchi S, Maeda A, et al. 2010. Transcellular transport of West Nile virus-like particles across human endothelial cells depends on residues 156 and 159 of envelope protein. BMC Microbiol. 10:165.
- Ishizu K-I, Watanabe H, Han S-I, Kanesashi S-N, Hoque M, Yajima H, et al. 2001. Roles of disulfide linkage and calcium ion-mediated interactions in assembly and disassembly of virus-like particles composed of simian virus 40 VP1 capsid protein. J Virol. 75:61–72.
- Jariyapong P, Chotwiwatthanakun C, Somrit M, Jitrapakdee S, Xing L, Cheng HR, Weerachatyanukul W. 2014. Encapsulation and delivery of plasmid DNA by virus-like nanoparticles engineered from Macrobrachium rosenbergii nodavirus. Virus Res. 179:140–146.
- Jariyapong P, Xing L, VAN Houten NE, Li T-C, Weerachatyanukul W, Hsieh B, et al. 2013. Chimeric hepatitis E virus-like particle as a carrier for oral-delivery. Vaccine. 31:417–424.
- Johnson KN, Johnson KL, Dasgupta R, Gratsch T, Ball LA. 2001. Comparisons among the larger genome segments of six nodaviruses and their encoded RNA replicases. J Gen Virol. 82:1855–1866.
- Kaesberg P, Dasgupta R, Sgro JY, Wery JP, Selling BH, Hosur MV, Johnson JE. 1990. Structural homology among four nodaviruses as deduced by sequencing and X-ray crystallography. J Mol Biol. 214:423–435.
- Krauzewicz N, Stokrová J, Jenkins C, Elliott M, Higgins CF, Griffin BE. 2000. Virus-like gene transfer into cells mediated by polyoma virus pseudocapsids. Gene Therapy. 7:2122–2131.
- Lee LA, Niu Z, Wang Q. 2009. Viruses and virus-like protein assemblies—Chemically programmable nanoscale building blocks. Nano Res. 2:349–364.
- Lin C-S, Lu M-W, Tang L, Liu W, Chao C-B, Lin CJ, et al. 2001. Characterization of virus-like particles assembled in a recombinant baculovirus system expressing the capsid protein of a fish nodavirus. Virology. 290:50–58.
- Ludwig C, Wagner R. 2007. Virus-like particles-universal molecular toolboxes. Curr Opin Biotechnol. 18:537–545.
- Ma Y, Nolte RJ, Cornelissen JJ 2012. Virus-based nanocarriers for drug delivery. Adv Drug Deliv Rev. 64:811–825.
- Manayani DJ, Thomas D, Dryden KA, Reddy V, Siladi ME, Marlett JM, et al. 2007. A viral nanoparticle with dual function as an anthrax antitoxin and vaccine. PLoS Pathog. 3:1422–1431.
- Marshall D, Schneemann A. 2001. Specific packaging of nodaviral RNA2 requires the N-terminus of the capsid protein. Virology. 285:165–175.
- Nikolaev VK, Leontovich AM, Drachev VA, Brodsky LI. 1997. Building multiple alignment using iterative dynamic improvement of the initial motif alignment. Biochemistry (Moscow). 62:578–582.
- Nishizawa T, Mori K-I, Furuhashi M, Nakai T, Furusawa I, Muroga K. 1995. Comparison of the coat protein genes of five fish nodaviruses, the causative agents of viral nervous necrosis in marine fish. J Gen Virol. 76:1563–1569.
- Porta C, Spall VE, Findlay KC, Gergerich RC, Farrance CE, Lomonossoff GP. 2003. Cowpea mosaic virus-based chimaeras: Effects of inserted peptides on the phenotype, host range, and transmissibility of the modified viruses. Virology. 310:50–63.
- Qiao M, Ashok M, Bernard KA, Palacios G, Zhou ZH, Lipkin WI, Liang TJ. 2004. Induction of sterilizing immunity against West Nile Virus (WNV), by Immunization with WNV-Like particles produced in insect cells. J Infect Dis. 190:2104–2108.
- Rampino A, Borgogna M, Blasi P, Bellich B, Cesàro A. 2013. Chitosan nanoparticles: Preparation, size evolution and stability. Int J Pharm. 455:219–228.
- Rivera-Hernandez T, Hartas J, Wu Y, Chuan YP, Lua LHL, Good M, et al. 2013. Self-adjuvanting modular virus-like particles for mucosal vaccination against group A streptococcus (GAS). Vaccine. 31: 1950–1955.
- Sahul Hameed AS, Bonami JR. 2012. White tail disease of freshwater prawn, macrobrachium rosenbergii. Indian J Virol. 23:134–140.
- Saroja C, Lakshmi P, Bhaskaran S. 2011. Recent trends in vaccine delivery systems: a review. Int J Pharm Investig. 1:64–74.
- Satheshkumar PS, Lokesh GL, Sangita V, Saravanan V, Vijay CS, Murthy MRN, Savithri HS. 2004. Role of metal ion-mediated interactions in the assembly and stability of sesbania mosaic virus T = 3 and T = 1 capsids. J MolBiol. 342:1001–1014.
- Scodeller EA, Tisminetzky SG, Porro F, Schiappacassi M, DE Rossi A, Chiecco-Bianchi L, Baralle FE. 1995. A new epitope presenting system displays a HIV-1 V3 loop sequence and induces neutralizing antibodies. Vaccine. 13:1233–1239.
- Sherman MB, Guenther RH, Tama F, Sit TL, Brooks CL, Mikhailov AM, et al. 2006. Removal of divalent cations induces structural transitions in red clover necrotic mosaic virus, revealing a potential mechanism for RNA release. J Virol. 80:10395–10406.
- Shetty M, Maiti B, Shivakumar Santhosh K, Venugopal MN, Karunasagar I. 2012. Betanodavirus of marine and freshwater fish: distribution, genomic organization, diagnosis and control measures. Indian J Virol. 23:114–123.
- Sudhakaran R, Parameswaran V, Sahul Hameed AS. 2007. In vitro replication of Macrobrachium rosenbergii nodavirus and extra small virus in C6/36 mosquito cell line. J Virol Methods. 146: 112–118.
- Takamura S, Niikura M, Li TC, Takeda N, Kusagawa S, Takebe Y, et al. 2004. DNA vaccine-encapsulated virus-like particles derived from an orally transmissible virus stimulate mucosal and systemic immune responses by oral administration. Gene Ther. 11: 628–635.
- Tang J, Johnson JM, Dryden KA, Young MJ, Zlotnick A, Johnson JE. 2006. The role of subunit hinges and molecular “switches” in the control of viral capsid polymorphism. J Struct Biol. 154:59–67.
- Tang L, Johnson KN, Ball LA, Lin T, Yeager M, Johnson JE. 2001. The structure of Pariacoto virus reveals a dodecahedral cage of duplex RNA. Nat Struct Mol Biol. 8:77–83.
- Tang L, Lin C-S, Krishna NK, Yeager M, Schneemann A, Johnson JE. 2002. Virus-like particles of a fish nodavirus display a capsid subunit domain organization different from that of insect nodaviruses. J Virol. 76:6370–6375.
- Thiéry R, Cozien J, Cabon J, Lamour F, Baud M, Schneemann A. 2006. Induction of a protective immune response against viral nervous necrosis in the European sea bass dicentrarchus labrax by using betanodavirus virus-like particles. J Virol. 80:10201–10207.
- Tihova M, Dryden KA, Le TVL, Harvey SC, Johnson JE, Yeager M, Schneemann A. 2004a. Nodavirus coat protein imposes dodecahedral RNA structure independent of nucleotide sequence and length. J Virol. 78:2897–2905.
- Tihova M, Dryden KA, Le TVL, Harvey SC, Johnson JE, Yeager M, Schneemann A. 2004b. Nodavirus coat protein imposes dodecahedral RNA structure independent of nucleotide sequence and length. J Virol. 78:2897–2905.
- Tisminetzky S, Scodeller EA, Evangelisti P, Chen Y, Schiappacassi M, Porro F, et al. 1994. Immunoreactivity of chimeric proteins carrying the HIV-1 epitope IGPGRAF. Correlation between predicted conformation and antigenicity. FEBS Lett. 353:1–4.
- Uchida M, Klem MT, Allen M, Suci P, Flenniken M, Gillitzer E, et al. 2007. Biological containers: Protein cages as multifunctional nanoplatforms. Adv Mater. 19:1025–1042.
- van der Lubben IM, Verhoef JC, Borchard G, Junginger HE. 2001. Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur J Pharm Sci. 14:201–207.
- Venter P, Schneemann A. 2008a. Recent insights into the biology and biomedical applications of Flock House virus. Cell Mol Life Sci. 65:2675–2687.
- Venter PA, Schneemann A. 2008b. Nodaviruses. In: Mahy BWJ, van Regenmortel MHV, Eds. Encyclopedia of Virology (Third Edition). Oxford: Academic Press. 430–438.
- Vimal S, Abdul Majeed S, Taju G, Nambi KSN, Sundar Raj N, Madan N, et al. 2013. Chitosan tripolyphosphate (CS/TPP) nanoparticles: Preparation, characterization and application for gene delivery in shrimp. Acta Tropica. 128:486–493.
- Wu Y-M, Hsu C-H, Wang C-H, Liu W, Chang W-H, Lin C-S. 2008. Role of the DxxDxD motif in the assembly and stability of betanodavirus particles. ArchVirol. 153:1633–1642.
- Zhai Y, Zhong Z, Zariffard M, Spear GT, Qiao L. 2013. Bovine papillomavirus-like particles presenting conserved epitopes from membrane-proximal external region of HIV-1 gp41 induced mucosal and systemic antibodies. Vaccine. 31:5422–5429.
