Abstract
There is ample evidence that biodegradable polyelectrolyte nanocapsules are multifunctional vehicles which can smuggle drugs into cells, and release them upon endogenous activation. A large number of endogenous stimuli have already been tested in vitro, and in vivo research is escalating. Thus, the interest in the design of intelligent polyelectrolyte multilayer (PEM) drug delivery systems is clear. The need of the hour is a systematic translation of PEM-based drug delivery systems from the lab to clinical studies. Reviews on multifarious stimuli that can trigger the release of drugs from such systems already exist. This review summarizes the available literature, with emphasis on the recent progress in PEM-based drug delivery systems that are receptive in the presence of endogenous stimuli, including enzymes, glucose, glutathione, pH, and temperature, and addresses different active and passive drug targeting strategies. Insights into the current knowledge on the diversified endogenous approaches and methodological challenges may bring inspiration to resolve issues that currently bottleneck the successful implementation of polyelectrolytes into the catalog of third-generation drug delivery systems.
Introduction
Over the last decade, the layer-by-layer (LbL) method (CitationDecher and Hong 1991, CitationDecher 1997) has been employed to assemble charged polymers, termed polyelectrolytes, to form polyelectrolyte multilayers (PEMs). This programmed deposition method has opened up exciting new avenues to accomplish multiple key milestones in drug delivery, including, but not limited to, functionalized bioresorbable PEM capsules, with innovative modes of action for treatment against fatal diseases like HIV (CitationDe Haes et al. 2010) and cancer (CitationCho et al. 2014, CitationKim et al. 2007, CitationJohnston et al. 2012). The LbL method is based on the sequential electrostatic adsorption of charged polyelectrolytes on top of oppositely charged porous or non-porous templates like melamine–formaldehyde (CitationSukhorukov et al. 2004, CitationKhopade and Caruso 2004), silica nanoparticles (CitationItoh et al. 2004), gold nanoparticles (AuNPs) (CitationSchneider and Decher 2004, CitationPereira et al. 2014), calcium carbonate (Ca CO3) (CitationYashchenok et al. 2013), carbon and nickel nanotubes (CitationMayya et al. 2001, CitationArtyukhin et al. 2004), toluene (CitationTettey et al. 2010), cyclohexane (CitationKhapli et al. 2009), liquid crystal emulsions (CitationSivakumar et al. 2008), microbes like bacteria (CitationNeu et al. 2001), viruses (CitationFischlechner et al. 2005), biomolecule-based cells like erythrocytes (CitationDonath et al. 2002, CitationGeorgieva et al. 2002, CitationKreft et al. 2006), and liposomes (CitationCuomo et al. 2012), followed by the dissolution of the template. For the hierarchical assembly, natural macromolecules like DNA, RNA, poly(L-lysine) (PLL), poly(L-glutamic acid) (PGA), chitosan, cellulose, alginates, hyaluronan (CitationBecker et al. 2010, CitationSaurer et al. 2010, CitationRecksiedler et al. 2006, CitationLee et al. 2007, CitationYan et al. 2011a, CitationAntunes et al. 2011, CitationSzarpak et al. 2010), and chemically modified or synthetic polymers like dextran sulfate (DS), polystyrene sulfonic acid (PSS), poly(allylamine hydrochloride) (PAH), poly(acrylic acid) (PAA), and poly(N-isopropylacrylamide) (PNIPAm) (CitationShu et al. 2010a, CitationGao et al. 2004, CitationJian et al. 2013, CitationWang and Caruso 2006, CitationCavalieri et al. 2009) are usually employed to form thin films and hollow capsules. Any therapeutically valuable cargo (chemical compound or drug, macromolecules or nanoparticles) can be incorporated into these capsules through “pre-loading” (adsorption of cargo onto template prior to LbL assembly or entrapment) and “post-loading” (encapsulation of drugs into pre-formed capsules) by initiating changes in the permeability of the shell wall via suitable stimuli.
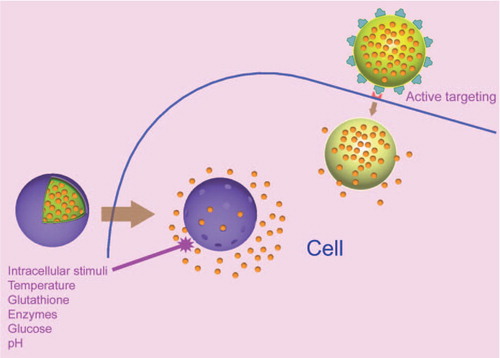
The foremost challenge in designing a smart drug delivery system is to smuggle the drug to the preferred site in the body, and then to activate the release, while protecting it from biological degradation. The method of wrapping drugs within the polyelectrolyte shells offers a wide range of inimitable properties to preserve the therapeutic potency of the molecules and to tune the drug release at the desired site in a controlled style. In contrast to non-biodegradable nanoparticles, polyelectrolytes assembled by the LbL method can be fabricated from bioresorbable materials with a low toxicity profile. The most remarkable advantage of PEMs assembled by LbL in drug delivery are their modularity, and the ability to respond to various environmental stimuli. For example, very recently, Morton et al. demonstrated LbL as a modular approach to develop targeted drug carriers via adsorption of ligand-functionalized, aqueous polyelectrolytes for tissue-specific targeting (CitationMorton et al. 2014). Currently, incorporation or in situ synthesis of metal nanoparticles, especially gold, silver, and magnetic nanoparticles in PEMs, has emerged as a promising platform in the development of light-, ultrasound-, and magnetic-responsive systems (CitationVolodkin et al. 2012, CitationRadziuk et al. 2007, CitationAngelatos et al. 2005, CitationMuñoz Javier et al. 2008, CitationAnandhakumar et al. 2012). A simple protocol for the in situ synthesis, as well as for the incorporation of biologically synthesized silver nanoparticles (AgNPs) in PAH/DS thin films that showed rupture and deformation in the presence of laser light has been demonstrated (CitationAnandhakumar and Raichur 2013, CitationSripriya et al. 2013). The AgNPs act as an energy absorption center, locally heating up the LbL film and rupturing it under the effect of laser treatment. This novel method has a significant potential in remotely activated drug delivery. Similarly, other physicochemical stimuli like electrical stimulation (CitationWood et al. 2008), ionic strength (CitationAntipov et al. 2003, CitationGao et al. 2004), solvents (CitationLvov et al. 2001), and mechanical deformation (CitationFernandes et al. 2010) can increase the permeability of the LbL films and would help in opening and closing the PEM capsules for encapsulation and release of drugs. Despite these triggers, biological properties or the presence of biomolecules specific to a disease or organ can be used to activate the release of drugs. In addition, dual or multi stimuli-responsive PEMs that can respond to more than one stimulus have also been reported (CitationNukolova et al. 2011, CitationTokuda et al. 2013, CitationShu et al. 2010b, CitationChang et al. 2011, CitationLo et al. 2005, CitationLiang et al. 2011, CitationCosta and Mano 2014).
The process of activating the drug release from PEMs by means of various stimuli can be rationally classified into: endogenous (enzymes, glucose, glutathione, receptors, pH, and temperature) and exogenous (light, ultrasonic, electric, magnetic, ionic, and mechanical) stimuli. The increase in pH and temperature is often associated with several disease conditions offering the ability for self- regulated control over drug release, thereby avoiding the need for external trigger. Hence, the thermal- and pH-responsive PEMs have been grouped under endogenous stimuli. So far, many reports on polyelectrolyte drug delivery systems have been published, in addition to the overwhelming reviews on multifarious stimuli-responsive PEMs that already exist (Citationdel Mercato et al. 2014, CitationDeshmukh et al. 2013, CitationAriga et al. 2011, CitationDe Geest et al. 2007, Citationde Villiers et al. 2011, CitationDelcea et al. 2011, CitationAntipina and Sukhorukov, 2011, CitationYan et al. 2011b, CitationWohl and Engbersen, 2012, CitationVergaro et al. 2011, CitationFleige et al. 2012, CitationDe Koker et al. 2012, CitationPavlov et al. 2013). However, a detailed review on different active and passive targeting strategies is lacking in the literature. Surface functionalization of PEM capsules with ligands offer methods to design drug delivery systems specific to diseased cells. Bioresponsive PEMs are attractive for intracellular drug delivery and have unique potential for in vivo applications, since they do not need any external triggering. The present review gives an overview of the diverse endogenous approaches available to activate the drug discharge from PEMs assembled by the LbL method, and elucidates the recent advances and challenges in this field.
Enzyme-mediated drug release
The incorporation of specific enzymatic substrates as structural components in drug delivery systems would allow a change of material property upon an enzymatic reaction. The over expressed, disease-associated enzymes or the enzyme that is found at higher concentrations at the target site can be utilized to trigger the drug release inside the cell. The wall properties of the PEM capsules can be either disintegrated in the presence of various enzymes like protease, hyaluronidase, chitosanase, and DNAse, or readily altered by varying the enzyme concentration. In the following section, the different kinds of enzyme-degradable polyelectrolyte systems for drug delivery applications are reviewed.
Proteases
Proteases or proteinases include a diverse group of enzymes like trypsin, α-chymotrypsin, pronase, etc. that could cleave or degrade the other proteins by hydrolyzing the protein–peptide bonds. The multifarious roles of proteases in diseases such as cancer, diabetes, neurological disorders, rheumatoid arthritis, cardiovascular conditions, and Alzheimer's disease make the disease-associated proteases an attractive target for drug delivery (CitationGilmore 2012). Usually, the protease-sensitive drug delivery systems are composed of a protease-sensitive substrate that releases the drug due to the cleavage at the peptide cleavage site in the presence of proteases.
CitationDe Geest et al. (2006) were the first to report the intracellular degradation of poly-L-arginine and DS in the presence of the enzyme pronase, spontaneously in VERO-1 cells, after lipid-raft-mediated uptake. Another example for pronase degrading polyelectrolytes was well-designed by Borodina and colleagues for the sustained release of encapsulated DNA. Pronase was captured by micron-sized calcium carbonate particles that were subsequently embedded into poly(L-arginine) and poly(L-aspartic acid) shells. Upon EDTA treatment to extract the calcium carbonate, pronase was released into the capsule's interior and started to digest the surrounding polyelectrolyte shell. They report that the lifetimes of such self-disintegrating capsules could be successfully adjusted to seconds, hours, or days by varying the amount of encapsulated pronase (CitationBorodina et al. 2007).
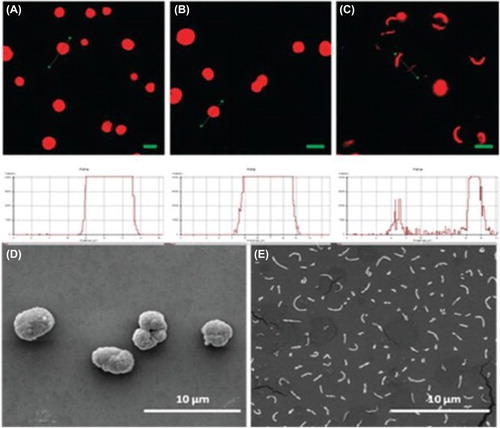
Apart from pronase, trypsin enzymes are distinctly significant since they can cleave peptides at the carboxyl side of amino acids lysine and arginine. Lee et al. successfully fabricated densely packed trypsin-responsive multilayers, by layering oppositely charged PLL and small interfering RNA (siRNA) on the surface of AuNPs. The multilayers thus prepared, termed as sRAuNPs, whose outer surface layer is PLL, could deliver siRNA into the tumor cells and silence the target gene effectively without toxicity (CitationLee et al. 2011). Similarly, Krishna et al. demonstrated the synthesis of capsules made of FDA-approved arginine-rich cationic protein, protamine (PRM), and heparin (HEP) onto a CaCO3 template doped with PSS. The trypsin treatment in about 0.04 mM phosphate-buffered saline (PBS) resulted in the immediate rupturing and sudden release of loaded dextran-tetramethylrhodamine isothiocyanate (TRITC) (CitationRadhakrishnan and Raichur 2012). shows the scanning electron microscopy (SEM) images of loaded microcapsules before and after trypsin treatment. The dual enzyme-responsive hollow microcapsule with arginine and chondroitin sulfate (CS) that disintegrates and releases the drugs upon exposure to either trypsin or in the presence of an endo-b-N-acetylhexosaminidase or hyaluronidase, has also been designed (CitationRadhakrishnan et al. 2013).
Figure 1. CLSM images of TRITC-dextran loaded (PRM/HEP)2 microcapsules before (A) and after (B) 7 weeks incubation in PBS (pH 7.4) and TRITC-dextran loaded (PRM/HEP)2 microcapsules after incubation in a 0.04 mM solution of trypsin in PBS (pH 7.4) (C) (the scale bar = 5μm). SEM images of (PRM/HEP) 2 microcapsules before (D) and after (E) incubation with 0.04mM solution of trypsin in PBS (pH 7.4). Reproduced with permission from reference (CitationRadhakrishnan and Raichur, 2012) Copyright Royal Society of Chemistry 2012.
Ochs and co-workers demonstrated a few modular approaches using PGA modified with alkyne moieties for the design of tailored drug delivery vehicles (CitationOchs et al. 2008, Citation2010a, Citation2010b). In another study, multilayer films of chitosan and pyrene-labeled poly (2-acrylamido-2- methylpropanesulfonic acid) (APy) were destroyed after the incubation in pepsin, due to enzymatic degradation of chitosan and desorption of APy (CitationWang et al. 2007). One more protease-assisted triggering phenomenon was put forth by Victor et al. by using poly(lactic acid) (PLA) nanoparticles that were enzymatically degraded by a-chymotrypsin (CitationOrozco et al. 2010). Likewise, a range of protease-responsive PEM drug delivery systems have been developed (CitationRivera-Gil et al. 2009, CitationRen et al. 2005, CitationMarchenko et al. 2012), while in contrast, protease-protective polyelectrolyte systems have been designed by CitationWoitiski et al. (2009) and CitationPechenkin et al. (2013) for the protection of encapsulated proteins from proteolytic activity.
In addition to these studies, an intriguing approach was described by Marina and co-workers. They fabricated a thin polypeptide film at a pH of 7.4 with alternating layers of two charged polypeptides, PLL/PGA, on a surface mimicking nonwoven. Complete or near complete degradation of the PLL/PGA nanofilm was obtained when the multilayer was exposed to a bacterial protease V8 glutamyl endopeptidase originating from Staphylococcus aureus, while no degradation of the nanofilm was seen in the presence of human neutrophil elastase (CitationCraig et al. 2012). The results from this study open avenues for products where the wound infection is the trigger for release of bioactive substances from a wound dressing. Thus, PEMs can be potentially tailored to design smart protease-triggered drug delivery systems that could facilitate the release of drugs for the diseases associated with deviant protease enzyme activity.
Hyaluronidase
Hyaluronic acid (HA) is an anionic mucopolysaccharide, occurring naturally in all living organisms. In mammals, the enzymatic degradation of HA results from the action of three types of enzymes—hyaluronidase (hyase), b-d-glucuronidase, and β-N-acetyl-hexosaminidase. HA is non-immunogenic and found throughout the body in various forms (CitationNecas et al. 2008). It is a major ligand for the CD44 receptor, and therefore can be used to target cancer cells that overexpress CD44 glycoproteins (CitationAuzenne et al. 2007) . The hydrophobic anticancer drug paclitaxel (PTX) was successfully loaded in PEM films designed by pre-complexing PTX with a chemically modified derivative of hyaluronic acid(alkylamino hydrazide) containing hydrophobic nanocavities, and subsequent assembly with either PLL or quaternized chitosan as polycations (CitationBoudou et al. 2012). As tumors are known to secrete high levels of hyaluronidase, the PTX-loaded microcapsules were cytotoxic to MDA-MB231 breast cancer cells due to the enzymatic degradation of the microcapsules in contact with cancer cells, leading to a transfer of the PTX from the hydrophobic nanodomains to the lipid membrane of the cells. Similarly, CitationVodouchê et al. (2006) designed a surface coating based on PLL/HA multilayers that act as a reservoir for PTX.
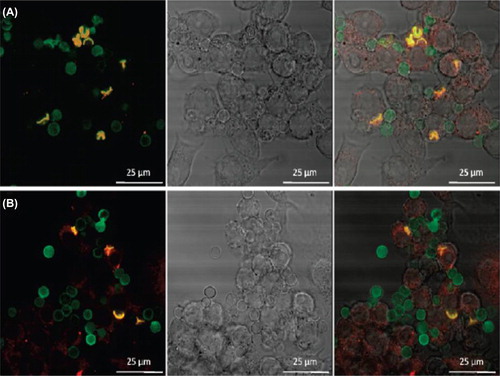
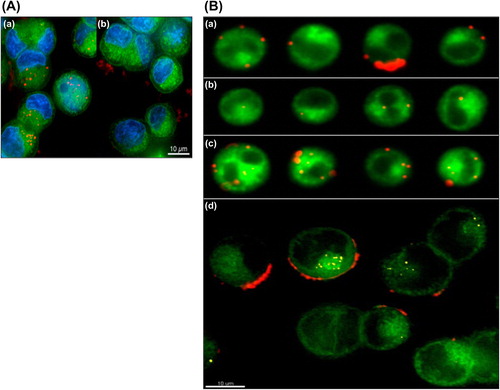
Szarpak et al. reported PEM capsules by LbL assembly of HA and PAH or PLL that are responsive to the tissue enzyme hyaluronidase (CitationSzarpak et al. 2008, Citation2010). Both chemically crosslinked HA/PLL as well as HA/PAH capsules become rapidly (i.e., within 2 h) internalized in endosomatic/lysosomatic vesicles upon incubation with in vitro cultured macrophages. Subsequently, fast intracellular opening of the capsule was observed, without the need for external triggering. shows the confocal microscopy studies of cellular uptake of capsules in which the polyelectrolyte shells remain stable outside the cells but readily break open once internalized by cells, suggesting their potential as carriers for intracellular drug delivery. Likewise, CitationLee et al. (2007) found that the bovine serum albumin (BSA) is released faster from cross linked HA/PLL as soon as hyaluronidase concentration is equal to or greater than 10 U/mL. These studies clearly indicate the perspective to use the HA-based PEM capsules for intracellular drug delivery.
Figure 2. Confocal microscopy images of (A) cross-linked HA/PAH and (B) cross-linked HA/PLL capsules after 2h coincubation with RAW mouse macrophages. Capsules are stained green fluorescent using HA-Fluorescein isothiocyanate (FITC), while the cellular lysosomes are stained using Lyso Tracker Red. The left pane gives the overlay of the green and red channel, the middle pane is the Differential interference contrast (DIC) channel and the right pane is the overlay of green, red and DIC. Colocalization between the green and red channel is observed as a yellow/orange color. Reproduced with permission from reference (CitationSzarpak et al. 2010) Copyright American Chemical Society 2010.
Chitosanase
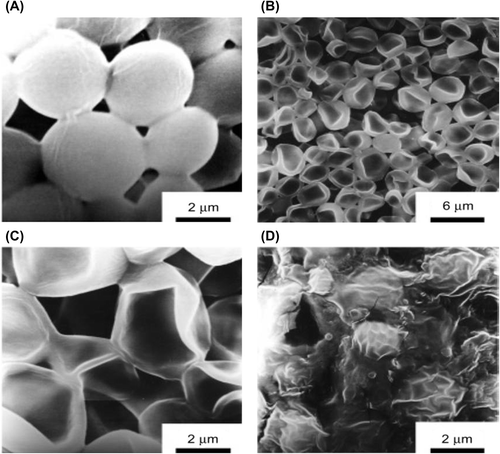
Chitosanase is an enzyme that hydrolyzes the liner polysaccharide chitosan. Designing LbL systems with chitosan will enable the drug release through multilayer disassembly in the presence of chitosanase. CitationSerizawa et al. (2002, Citation2006) were the first to demonstrate the hydrolysis of chitosan and DS by cationic chitosanase. Sustained release of the encapsulated proteins was attained via LbL assembly of chitosan and DS on protein-entrapping mesoporous silica particles (CitationItoh et al. 2006). With increasing time, the chitosan component was degraded by chitosanase, which was explored by using SEM (). Recently, CitationAlmalik et al. (2013) proved that high molecular chitosan nanoparticles are more prone to degradation by chitosanase than low molecular weight chitosan. Although chitosanase does not subsist in mammalian cells, this approach can be used to design degradable carriers for a large variety of other living organisms that produce chitosanase.
Figure 3. SEM images of the (Chitosan/DS)3 hollow capsules before (A) and after (B-D) enzymatic degradation at 37°C respectively for 5 min (B, C), and for 24 h (D). Panel C is a higher magnification of panel B. Reproduced with permission from reference (CitationItoh et al. 2006) Copyright American Chemical Society 2006.
Nucleases
The negative charge on each nucleotide unit in nucleic acids enables the possibility of assembling LbL systems. Such assembled systems can be disintegrated by means of the enzyme nucleases that slice DNA at specific sites. Johnston et al. demonstrated that films and capsules assembled solely from DNA can be engineered to contain restriction-enzyme-cut sites. The capsules degraded as the oligonucleotide in the capsule wall were cleaved specifically in the presence of EcoRI, and released the encapsulated BSA (CitationJohnston et al. 2009). But the utility of these capsules is limited since the restriction enzyme EcoRI is found only in bacteria. Earlier, CitationSerizawa et al. (2003) reported the time-controlled desorption of an LbL assembly of DNA and poly(diallyl dimethylammonium) chloride (PDDA) on the basis of the enzymatic hydrolysis by deoxyribonuclease I (DNase I), which is condensed on the PDDA surface and activated by metal ions in the media.
Redox-responsive polyelectrolyte drug delivery systems
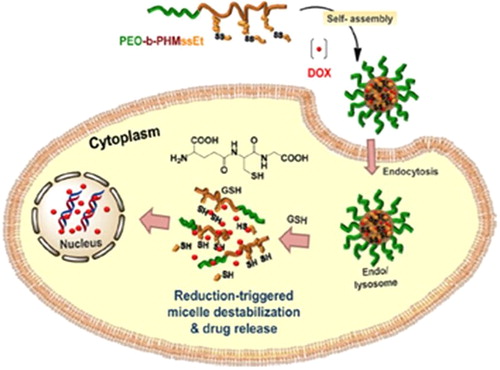
Glutathione is a tripeptide consisting of glutamate, cysteine, and glycine. The concentration of thiol- containing glutathione (GSH) is 100–1000 times higher in intracellular compartments like cytosol, lysosome, and nucleus when compared to that in the extracellular space. Besides, the level of GSH in tumor cells is about seven times higher than in the normal cells (CitationMeng et al. 2009). These facts can be utilized to design GSH-responsive drug delivery systems that can release the drug selectively inside the intracellular space, in the presence of GSH at a concentration of 2–10 mM. In order to introduce redox responsiveness, polymeric systems are functionalized at the terminus of the polymer chains with thiol groups that could enable the formation of disulfide links. When they reach the intracellular compartment in the cell, the high concentration of GSH will cleave the disulfide bond by a redox reaction to free the thiols, thereby releasing the drugs. For example, self-assembled micelles of amphiphilic block copolymers (ABPs) consisting of a pendant disulfide-labeled methacrylate polymer block (PHMssEt) and a hydrophilic poly(ethylene oxide) (PEO) block were investigated (). In response to GSH as a cellular trigger, the cleavage of pendant disulfide linkages in hydrophobic PHMssEt blocks of micellar cores caused the destabilization of self-assembled micelles due to the change in the hydrophobic/hydrophilic balance, and led to enhanced intracellular release of the anticancer drug doxorubicin (DOX) (CitationKhorsand et al. 2013).
Figure 4. Illustration of PEO-b-PHMssEt as effective intracellular drug delivery nanocarriers exhibiting enhanced release of DOX in response to GSH in cancer cells. Reproduced with permission from reference (CitationKhorsand et al. 2013) Copyright American Chemical Society 2013.
Similar redox-responsive polymeric LbL systems that contain disulfide crosslinks have been designed by many groups (CitationLi and Haynie 2004, CitationZelikin et al. 2010, CitationHaynie et al. 2004, CitationKim et al. 2010, CitationSun et al. 2013, CitationZheng et al. 2013, CitationLi et al. 2013). Caruso and colleagues reported comprehensively on redox- responsive systems (CitationZelikin et al. 2005, Citation2006, Citation2007, Citation2008, CitationDe Rose et al. 2008, CitationSivakumar et al. 2009, CitationChong et al. 2009a, Citation2009b, CitationSexton et al. 2009, CitationYan et al. 2010, CitationNg et al. 2011, CitationBecker et al. 2009). They described capsules comprised of a synthetic polymer, poly(methacrylic acid) (PMA), stabilized via biodegradable disulfide linkages (CitationZelikin et al. 2008). The capsules are obtained via the sequential deposition of thiolated PMA (PMASH) and poly(vinylpyrrolidone) (PVPON) by means of hydrogen bonding onto the template silica particles. The capsules degrade in the presence of intracellular concentrations of a natural reducing agent, GSH. These capsules have been used as carriers for globular proteins (CitationZelikin et al. 2006), single- and double-stranded DNA of varied lengths (CitationZelikin et al. 2005, Citation2007, CitationNg et al. 2011), drug-loaded oil droplets (CitationSivakumar et al. 2009), oligopeptides (CitationChong et al. 2009b), antigens (CitationDe Rose et al. 2008), and DOX, to target colorectal cancer cells (CRC) (CitationYan et al. 2010). Apart from GSH, polycationic and polyanionic poly(ferrocenyl silane)s featuring redox-active ferrocene (CitationMa et al. 2006, CitationSong et al. 2013a, Citation2013b) and sulfhydryl-containing reducing agent 1, 4-dithiothreitol (DTT) (CitationBlacklock et al. 2007) have also been used for the preparation of redox-controlled drug delivery systems. The results from these reports advocate the proficiency of the redox-responsive polyelectrolyte drug delivery systems for intracellular drug release, particularly for the rapid release of anticancer drugs.
Temperature-responsive polyelectrolyte systems
Certain polyelectrolytes like PNIPAm respond to the change in their environmental temperature. They can be engaged in drug delivery systems to discharge the drugs at physiological temperature or specifically to the diseased cell associated with the increased temperature. Polymers which become insoluble upon heating have a lower critical solution temperature (LCST), and those that become soluble upon heating have an upper critical solution temperature (UCST) (CitationSchmaljohann 2006). If the polymer solution is heated above the LCST, the polymer chain will dehydrate, become more hydrophobic, and will collapse. This change in the hydrophilic–hydrophobic balance causes the micelles to disassemble and therefore release the encapsulated drug (CitationFleige et al. 2012). PNIPAm shows inverse temperature-dependent solubility, meaning it is hydrophilic below its lower critical solution temperature (LCST), and it becomes hydrophobic above this temperature (CitationWohl and Engbersen 2012). Since the PNIPAm is not toxic and its LCST is around 32°C, close to the body temperature, it is an attractive candidate for drug delivery. PNIPAm has been extensively exploited by various research groups to design thermo-sensitive microcapsules and films for biomedical applications (CitationWei et al. 2006, CitationYuanpei et al. 2009, CitationQin et al. 2006, CitationHales et al. 2004, CitationChen et al. 2006, CitationLi et al. 2006, CitationZhao et al. 2011, CitationSteitz et al. 2002, CitationGlinel et al. 2003, CitationYe et al. 2005, CitationPrevot et al. 2006, CitationDähne et al. 2001).
Qin et al. prepared thermosensitive thin films of PNIPAm and PAA by the LbL method. The resulting multilayer films ([PAA/PNIPAm]10) were capable of loading and unloading the dye Rhodamine B when the solutions were exposed to elevated temperature (CitationQin et al. 2006). An interesting approach to treat hyperthermia was put forth by Li et al. (CitationYuanpei et al. 2009) involving the use of poly (N, N-diethylacrylamide-co-acrylamide)-block-poly (gamma-benzyl L-glutamate). The antitumor drug PTX was encapsulated and it showed thermo-sensitive controlled behavior. The cytotoxicity of PTX-loaded nanoparticles increased obviously under conditions of hyperthermia. Sukhishvili and colleagues constructed a new type of hydrogen-bonded and non-toxic LbL films for repeated on-demand release of drug from an assembly of tannic acid (TA) with the temperature-responsive block copolymer micelles (BCMs), which were pre-formed by heating solutions of a neutral diblock copolymer, poly(N-vinylpyrrolidone)-b-poly(N-isopropylacrylamide) (PVPON-b-PNIPAM), to a temperature above the LCST of PNIPAm (). The model drug, DOX, was incorporated into these films and was retained within the hydrophobic BCM pores at 37°C, whereas exposure to a lower temperature (20°C) triggered fast DOX release, demonstrating a cytotoxic effect on breast cancer cells with more than 90% killing efficacy (). Further, the films maintained their structural integrity in PBS, and each film could be repeatedly loaded with drug and used for more than 15 times with only ∼7% loss in film thickness and no obvious changes in reloading capacity or release profiles (CitationZhu et al. 2013). Earlier, the same group reported similar hydrogen-bonded temperature-sensitive systems (CitationZhu and Sukhishvili 2009, CitationZhuk et al. 2009, CitationXu et al. 2010, CitationTan et al. 2011). These studies suggest that the polymeric micelles display highly sensitive swelling/deswelling responses to temperature variations. Moreover, they are highly proficient in entrapping the drugs, and hence can be devoted to design thermosensitive delivery vehicles for pulsated and controlled drug delivery.
Figure 5. (A) Schematic illustration of the layer-by-layer assembly of PVPON-b-PNIPAM BCMs with TA into stable films at a temperature above PNIPAm's LCST. Right: hydrogen bonding formed between TA and BCMs within the LbL films. Reproduced with permission from reference (CitationZhu et al. 2013) Copyright Elsevier 2013. (B) (a) Cytotoxicity of [BCM/TA]40.5 films against MCF-7 cells after multiple temperature cycles between 37°C and 20°C. Films were immersed in culture medium at a constant temperature of 37°C, and then cooled down to 20°C for 30 min at cumulative time of 1, 2 and 3 days. After each 37°C/20°C cycling, the medium was separately collected and replaced with fresh medium at 37°C to start a new release cycle. MCF-7 cells cultured overnight were treated with collected medium samples for 24 h and analyzed by MTT assay to determine cell viability. (b) Immunofluorescence images of MCF-7 cells cultured for 24 h. F-actin was stained red with phalloidin-TRITC and cell nuclei stained blue with DAPI. (c) LIVE/DEAD staining of MCF-7 cultured for 24 h. Red = live; green = dead. Reproduced with permission from reference (CitationZhu et al. 2013) Copyright Elsevier 2013.
![Figure 5. (A) Schematic illustration of the layer-by-layer assembly of PVPON-b-PNIPAM BCMs with TA into stable films at a temperature above PNIPAm's LCST. Right: hydrogen bonding formed between TA and BCMs within the LbL films. Reproduced with permission from reference (CitationZhu et al. 2013) Copyright Elsevier 2013. (B) (a) Cytotoxicity of [BCM/TA]40.5 films against MCF-7 cells after multiple temperature cycles between 37°C and 20°C. Films were immersed in culture medium at a constant temperature of 37°C, and then cooled down to 20°C for 30 min at cumulative time of 1, 2 and 3 days. After each 37°C/20°C cycling, the medium was separately collected and replaced with fresh medium at 37°C to start a new release cycle. MCF-7 cells cultured overnight were treated with collected medium samples for 24 h and analyzed by MTT assay to determine cell viability. (b) Immunofluorescence images of MCF-7 cells cultured for 24 h. F-actin was stained red with phalloidin-TRITC and cell nuclei stained blue with DAPI. (c) LIVE/DEAD staining of MCF-7 cultured for 24 h. Red = live; green = dead. Reproduced with permission from reference (CitationZhu et al. 2013) Copyright Elsevier 2013.](/cms/asset/2dcda593-4cab-4a3b-9c71-377ce04c7c6c/ianb_a_1011801_f0005_oc.jpg)
Glucose-sensitive polyelectrolytes
Polyelectrolyte multilayer films and microcapsules have been developed to release insulin in response to change in the glucose level in blood. The presence of sugar molecules can either disrupt the linkage between polymeric multilayers or induce change in the permeability, resulting in the formation of small pores (CitationSato et al. 2011). Sato et al. reported the concanavalin/glucogen LbL systems that are sensitive to sugars (CitationSato et al. 2004), and later, drug delivery systems that can be fully disintegrated in the presence of D-glucose, D-mannose, and derivatives were also demonstrated (CitationSato et al. 2004, Citation2005, Citation2009). Novel glucose-sensitive polyelectrolyte capsules bearing phenylboronic acid moieties that are able to respond to a stimulus provided by the human body (increased glucose concentration) for the controlled delivery of insulin were reported by CitationDe Geest et al. (2006). In this study, the capsules disassembled when exposed to glucose at a concentration of 2.5 mg/L or above, in 5 min, due to the intermolecular repulsion between the sulfonate groups of PSS and the glucose/phenylboronic acid complex. Since the normal glucose concentration does not exceed 1.5 mg/L in healthy human beings, these capsules will remain intact at normal (healthy) glucose levels. Qi et al. successfully constructed glucose-responsive glucose oxidase/catalase multilayer shells by the LbL method onto insulin particles by glutaraldehyde crosslinking, for the controlled release of hypoglycemic drugs. It was observed that the coupled enzymatic reactions of glucose to gluconic acid in the assembled capsules reduced the pH value and led to the enhancement of the shell's permeability, simultaneously increasing the solubility of insulin (CitationQi et al. 2009b).
In recent years, star polymers have been utilized in a range of drug delivery applications, and their unique solution properties and high functionality make them attractive building blocks for the preparation of nanostructured thin films (CitationConnal et al. 2008). Chen et al. studied self-assembled positively charged poly [2-(dimethylamino) ethyl methacrylate] (PDMAEMA) star polymers and negatively charged insulin and glucose oxidase (GOD). The multilayer films showed faster release of insulin in response to 3 g/L glucose solution than that without glucose, and were found to be non- toxic to mouse fibroblast cells (CitationChen et al. 2011a). In addition, the same research group successfully fabricated the glucose-sensitive multilayer film by the LbL method with 21-arm poly[2-(dimethylamino)ethyl methacrylate] (star PDMAEMA) and negatively charged insulin and GOD in the form of {(Star PDMAEMA/Insulin)(4) + (Star PDMAEMA/GOD)(4) + Star PDMAEMA} (CitationChen et al. 2011b). The multilayer film shows an on–off regulation of insulin release in response to stepwise glucose challenge in vitro. Furthermore, the multilayer film could continuously release enough insulin in vivo after being subcutaneously implanted in streptocozotin-induced diabetic rats, and reduced the blood glucose level for at least two weeks. Similarly, a series of insulin-incorporated LbL films with different arrangement sequences of insulin/GOD/star-PDMAEMA were successfully fabricated, and a facile strategy to prolong the drug release time from LbL films was also demonstrated by this group (CitationChen et al. 2012). The in vivo experiments indicate that the sustained release time of insulin, as well as the hypoglycemic effect, could be prolonged from 17 to 36 days, just by rearranging the assembly sequence of LbL building blocks. Similarly, many research groups have developed LbL shells and films that deliver insulin in response to sugars (CitationMiura et al. 2006, CitationLevy et al. 2008, CitationDing et al. 2009a, Citation2009b, CitationQi et al. 2009a). In addition to these studies, glucose-responsive systems have also been designed to target cancer cells, since the tumor cells accumulate glucose faster than the normal cells. CitationManna and Patil (2010) fabricated multilayer thin films of poly(vinyl alcohol), borate, and chitosan on colloidal particles (MF particles) to deliver the anticancer drug DOX. After 10 h, 80% of encapsulated DOX was released in the presence of 25 mM glucose solution, at the physiological pH.
pH-responsive polyelectrolyte drug delivery systems
The pH gradient varies inside the human body, especially in a diseased state like cancer in which the pH of the tumor microenvironment is between 6.0 and 7.0. The pH value will drop further, from the extracellular microenvironment of a tumor to intracellular organelles, such as endosomes (pH = 5.5) and lysosomes (pH < 5.5) (CitationYang et al. 2014). Therefore, these abnormal changes in pH can help in designing pH-sensitive controlled drug delivery systems for cancer treatment. By tuning the pH values, drugs can be either encapsulated or released from PEM capsules via decomposition or enhanced permeability. PEM capsules with weak polyelectrolytes undergo protonation and deprotonation when the pH is altered, relative to the pKa value of the polyelectrolyte in the outer shell. Addition of H + ions or protonation leads to increased permeability because of stronger repulsion, resulting in the swelling of the capsule, whereas deprotonation lowers the shell permeability and results in shrinkage of the polyelectrolyte capsules. Many studies on PEM-based pH-responsive drug delivery systems have been reported earlier, for the delivery of proteins (CitationLi et al. 2012b, CitationYoshida et al. 2010, CitationYe et al. 2006), anticancer drugs (CitationLuo et al. 2012, CitationShen et al. 2013, CitationMinati et al. 2013), genes (CitationTseng et al. 2013, CitationLi et al. 2012a), and acid-susceptible drugs (CitationGujarathi et al. 2012). In addition, reversible capsules like PAH/PMA that could open at pH levels < 4 and close at pH levels > 5 (CitationAnandhakumar et al. 2010) have been designed; and carboxymethyl cellulose (CMC)/PAH that open at pH ≤ 6 and close at pH ≥ 7 (CitationTripathy and Raichur 2013) for protein encapsulation and delivery have also been designed. A detailed literature on PEM-based pH-responsive LbL films and capsules was documented by CitationSato et al. (2011). These studies evidently indicate the significance of pH-responsive PEMs in drug delivery.
Drug release to target cells by active targeting
As several classes of drug delivery systems lack specificity for diseased cells, the drugs get diffused to all other parts of the body, causing toxicity to the normal cells. In addition, the drug gets partially degraded, thereby reducing its potency. The development of drug delivery systems in response to antibody binding or receptor–ligand interaction can hinder the side-effects of drugs by targeting the diseased cell, and would help in increasing the drugs’ efficacy. In many pathological states, cells express new surface molecules that are absent in healthy cells, or strongly overexpress some of these molecules when compared with cells in a healthy state (CitationGrazú et al. 2012). These molecules encompass antibodies or their fragments, lectins like asialoglycoproteins, lipoproteins, blood plasma glycoproteins like transferrin, peptides, hormones, polysaccharides, and some low molecular weight ligands such as folate. PEM capsules can be surface-functionalized with these molecules and can be directed to an antigen or receptor overexpressed in the diseased cell.
Antibody-mediated drug delivery
One way to improve the immune response is to target the drug carriers specifically to dendritic cells (DC) that present the antigens on the surface to the T cells. Therefore, LbL capsules have been designed such that they can deliver the antigenic cargo directly to the DCs, to evoke potent immune responses (CitationDe Geest et al. 2012a, Citation2012b, CitationDe Temmerman et al. 2012, CitationDe Koker et al. 2009, CitationDe Rose et al. 2008). In vitro and in vivo studies have shown that ovalbumin (OVA)-loaded capsules elicit presentation of capsule antigen by both the major histocompatibility (MHC) complex pathways (MHC I and MHC II) (CitationSexton et al. 2009, CitationDe Geest et al. 2012a). Masereel et al. developed AuNPs with LbL coating of PAA and PSS, followed by surface coating with immunoglobulin (anti-BSA IgG) linking, through an amide bound. This coupling of AuNPs to antibodies targeting surface proteins specifically expressed in cancer cell would be an attractive challenge for the treatment of cancer (CitationMasereel et al. 2011).
The human A33 antigen is expressed by 95% of human colorectal tumor cells. Binding of humanized A33 monoclonal antibody (HuA33 mAb) to the A33 antigen is known to activate cellular internalization mechanisms, which provides a mechanism for delivering the particles inside the cells. Cortez and colleagues successfully biofunctionalized the core/shell particles and capsules made of PAH/PSS by the LbL method, with a HuA33 mAb that can bind to the A33 antigen present on the colorectal cancer cell (CRC) lines (CitationCortez et al. 2006). They also investigated the influence of size, surface properties, cell line, and kinetic parameters such as dosage (particle concentration) and incubation time on the specific binding of HuA33 mAb-coated LbL particles and capsules to CRC. In these studies, it was observed that HuA33 mAb binds to the A33 antigen present on almost all CRC, and the particles have demonstrated great promise in clinical trials as an immunotherapeutic agent for cancer therapy (CitationCortez et al. 2007). Later, the same group analyzed the interaction of alkyne-modified poly (N-vinylpyrrolidone) (PVPONAlk) capsules surface-coated with HuA33 mAb with human CRC ( and B) (CitationJohnston et al. 2012). Recently, the targeting of mouse splenic DCs using polymer capsules functionalized with DC-specific antibodies was investigated. The results from this study emphasize that simply selecting a molecule that an antibody can specifically bind to and be internalized by the target cell is not sufficient for effective internalization of a material. Consideration must be made as to whether the process by which the antigenic cargo will be internalized can accommodate the material attached to the targeting antibody (CitationMintern et al. 2013).
Figure 6. (A) Deconvolution microscopy images of (a) HuA33 or (b) IgG-functionalized PVPONAlk capsules incubated with LIM1899 CRC cells at 37°C for 24 h. Capsules are labeled with AF647 (red), cells are labeled with Lava Cell (fake colored green), and the nucleus is labeled with Hoechst 33342 (blue). Reproduced with permission from reference (CitationDe Rose et al. 2008) Copyright American Chemical Society 2012. (B) Representative imaging flow cytometry (a-c) and deconvolution microscopy (d) images of HuA33 mAb functionalized PVPONAlk capsules (red) incubated with LIM2405 + CRC cells (green) at 37°C for 4 h. (a) Surface bound capsules. (b) Cells with 1–2 internalized capsules. (c) Cells with multiple internalized capsules. (d) Deconvolution microscopy image to distinguish surface bound capsules (red) from internalized capsules (yellow). Reproduced with permission from reference (CitationDe Rose et al. 2008) Copyright American Chemical Society 2012.
Biotin-responsive LbL systems
Avidin is a protein known to strongly bind biotin and analogs (CitationWilchek and Bayer 1988). Multilayer thin films have been prepared by the LbL method by using avidin as the polycation, and -poly(vinyl sulfate) (PVS) (CitationAnzai and Nakamura 1999), concanavalin A (Con A) (CitationHoshi et al. 2002), 2-iminobiotin-labeled poly(ethyleneimine) (ib-PEI) (CitationInoue et al., 2004), PSS (CitationEndo et al. 2011), PSS, PVS, and DS (CitationAnzai et al. 2000), as polyanions on the surface of quartz slides in which avidin retained its binding activity to biotin and its analogs. In the studies demonstrated by Caruso et al. the monoclonal antibodies, HuA33 mAb and epidermal growth factor receptor monoclonal antibody (EGFR mAb) were biotinylated, complexed with neutravidin, and individually coupled to biotin-containing disulfide- stabilized poly (methacrylic acid) capsules. They demonstrated significantly enhanced cellular binding and internalization of the antibody-functionalized capsules, compared with control human immunoglobulin (IgG) - functionalized capsules, suggesting that these capsules can specifically interact with cells through antibody/antigen recognition (CitationShimoni et al. 2012).
Folate
The folate receptor has been broadly utilized in targeted drug delivery, especially to target cancer cells, as expression of the folate receptor is selectively upregulated in tumor sites of various organs such as colon, lung, prostate, ovaries, mammary glands, and brain (CitationMorris and Sharma 2011). Folic acid (FA), or folate, specifically binds to the folate receptor, and therefore folate has been conjugated to many polyelectrolytes (CitationJeong et al. 2005, CitationKim et al. 2007, CitationChiu et al. 2011, CitationNukolova et al. 2011, CitationZhu et al. 2011, CitationChiang et al. 2013, CitationMandal et al. 2011) for active targeted drug delivery to cancer cells. For example, Zhou et al. fabricated PEMs composed of chitosan and alginate assembled by the LbL method on the surface of the BSA- stabilized biocompatible poly(lactide-co-glycolide) nanoparticles. FA-grafted poly(ethylene glycol) (PEG–FA) was covalently bounded to the PEMs via carbodiimide chemistry. The combination of the chitosan/alginate LbL coating with carbodiimide chemistry provides a simple but effective way of tailoring nanoparticle surfaces in a sequential way, to first reduce unspecific interactions and then to attach other molecules to achieve specific targeting properties (CitationZhou et al. 2010). CitationHou et al. (2011) used FA as a tumor-targeting agent and designed FA− and methoxypoly(ethylene glycol) (mPEG)-conjugated chitosan nanoparticles for targeted and prolonged drug delivery systems for tumor cell-selective targeting treatments. Biodegradable polymeric micelles, self-assembled from a di-block copolymer of poly (D, L-lactic-co-glycolic acid) and PEG, were prepared to achieve delivery of DOX targeted to the folate receptor (CitationYoo and Park 2004). Besides cancer, Agarwal et al. developed a formulation to combat diabetes by LbL coating of PAH/PAA over the liposomes to which FA was appended as targeting ligand, by synthesizing an FA− poly(allylamine) hydrochloride conjugate. The insulin entrapped within the freeze-dried formulation was found stable, exhibited stability in simulated biological fluid, and showed prolonged hypoglycemic response (for up to 18 h) in diabetic rats (CitationAgrawal et al. 2013).
Transferrins
The transferrins (Tf) are a family of iron-binding proteins, typically monomeric glycoproteins with a single polypeptide chain of 670–700 amino acids. Tf have been widely applied as ligands for active targeting of anticancer agents, proteins, and genes to primary proliferating malignant cells overexpressing transferrin receptors (Tfr). Antitransferrin receptor–drug conjugates could deliver drugs and genes across the blood–brain barrier for treatment of a broad spectrum of central nervous system diseases (CitationLi and Qian 2002). Earlier, polymeric drug delivery systems targeting Tfr were designed for delivery of the anticancer drug PTX (CitationShah et al. 2009, CitationXu et al. 2005), DOX (CitationDufes et al. 2004, CitationSivakumar et al. 2009), and for the systemic delivery of oligonucleotides (ODN) to the brain (CitationVinogradov et al. 2003). PLGA nanoparticles were loaded with PTX, and the surface was epoxy- activated and modified with Tf. When Tf-modified poly(D,L-lactide-co-glycolide) was administered to Sprague-Dawley rats, the distribution of PTX to tumor tissue was highest when compared to that achieved using poly(D,L-lactide-co-glycolide) nanoparticles without Tf (CitationShah et al. 2009). In another study, Tf was conjugated to the polyelectrolyte-coated gold nanorods for targeted in vitro delivery to cancer cells. The intracellular uptake of the nanorods predominantly occurred in HeLa cells via the Tf− TfR interaction, and the nanorods were localized in vesicular structures such as endosomes (CitationDing et al. 2007). Fisher et al. showed that Tf, vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF), can each be linked to poly N-(2-hydroxypropyl) methacrylamide (HPMA)- coated poly (L-lysine)/DNA complexes. All ligand-targeted complexes demonstrated increased uptake into receptor-positive cells, thus permitting versatile targeting of genes to selected cells (CitationFisher et al. 2000).
The use of pathogenic microorganisms producing an oncolytic agent may be an approach for apoptotic therapy in cancer treatment. Granicka et al. investigated the targeting efficiency of Bacillus subtilis bacterial cells coated with polyelectrolyte shells modified with Tf to increase affinity toward the target tumor cells. It was observed that the bacterial cells coated with modified shells with incorporated Tf exhibited stronger lethal impact on leukemia cells as compared to bacterial cells with no modification of the shell coating (CitationGranicka et al. 2014).
Peptides
Peptides derived from the sequence of cell surface proteins, such as intercellular adhesion molecule-1 (ICAM-1), LHRH, Bombesin, and LFA-1, have shown potent binding affinity to the target cell surface receptors (CitationMajumdar and Siahaan 2012). Peptides chemically bound to polyelectrolytes and adsorbed or embedded in the PEM films were found to retain their biological activities. PEM films made of PLL and PGA have been functionalized by covalent binding of a synthetic analog of the anti-inflammatory peptide α-melanocyte-stimulating hormone (α-MSH) to PGA, to create biologically active coatings for tracheal prostheses (CitationSchultz et al. 2005). An elegant strategy for bi-functionalization of PEM films was developed by Meyer et al. These authors showed that it is possible to functionalize the PEM film, both for cell transfection and for activation via a peptide signaling pathway. They prepared films containing plasmid DNA pre-complexed with poly(ethyleneimine) (PEI) and a peptide molecule NBPMSH. This peptide, grafted to PGA, was used as a signal molecule for melanoma cells B16–F1, and for its ability to enhance gene delivery in a receptor-independent manner (CitationMeyer et al. 2008).
Integrins are among the most widely studied receptors for tumor-targeted drug delivery, since they play a key role in cancer metastasis and tumorigenesis. There are over 25 known integrin receptors, and most of these recognize the small tripeptide turn sequence arginine–glycine–aspartic acid (RGD), which is a part of extracellular proteins such as fibronectin and vitronectin. Toublan et al. used the electrostatic adhesion approach to adhere RGD-containing peptides to the surface of serum albumin microspheres to target tumor cells. These microspheres are selectively taken up by HT29 human colon cancer cells in vitro (CitationToublan et al. 2006). The RGD peptide has been conjugated with chitosan using a thiolation reaction. RGD enhances selective intratumoral delivery by delivering plexin domain-containing protein 1 (PLXDC1)-targeted siRNA into the alphanubeta3 integrin-positive tumor endothelial cells in A2780 tumor-bearing mice. This approach resulted in significant inhibition of tumor growth, compared with controls (CitationHan et al. 2010).
RGD peptides coupled to PEM films augment osteoblast adhesion and can be used to spatially control cell adhesion. Chua et al. functionalized chitosan–HA polyelectrolyte multilayers and immobilized RGD-containing peptide on PEM substrates, to achieve enhanced osteoblast functions while retaining antibacterial efficacy (CitationChua et al. 2008). CitationTsai et al. (2009) immobilized dual peptides containing RGD (a cell-binding domain) and LHRRVKI (a heparin-binding domain) onto polystyrene by LbL, and the effects on osteoblast cell culture were investigated. In another study, the cell proliferation in osteoblasts was enhanced in the presence of PEM films of PLL/PGA5–PLL/PGA-RGD (CitationPicart et al. 2005). Similarly, Gribova et al. investigated PEM films made of PLL and PGA as substrates of tunable stiffness that can be functionalized by an RGD-adhesive peptide, to investigate important events in myogenesis, including adhesion, migration, proliferation, and differentiation. (CitationGribova et al. 2013).
Asialoglycoprotein
The asialoglycoprotein receptor is expressed on the surface of liver hepatocytes, and specifically recognizes the terminal b-D-galactose and N-acetylgalactosamine residues (CitationRuiz and Drickamer 1996). Galactose-bound catonized polymers such as galactosylated chitosan enable direct delivery and internalization of DNA into liver through the asialoglycoprotein receptors to which galactose binds (CitationGao et al. 2005). D-galactose has been effectively incorporated into the multilayers by LbL assembly of galactosylated polyelectrolyte, by CitationZhang et al. (2006, Citation2008a, Citation2008b). They described the construction of hepatic- targeting microcapsules by self-assembly of chemo-enzymatic synthesized poly(vinyl galactose ester-co-methacryloxyethyl trimethylammonium chloride) (PGEDMC) containing galactose branches, which can be specifically recognized by membrane-bound galactose receptors (ASGPR) for the acyclovir (ACV) controlled release system (CitationZhang et al. 2008b). Earlier, the cationic D-galactose-branched copolymer PGEDMC alternated with PSS to form thin multifilms by LbL on different solid surfaces such as quartz slides, poly(ethylene terephthalate) (PET) films, silicon wafers, and polystyrene microparticles was reported. PGEDMC/PSS planar films and capsules carrying β-galactose as recognition signals have specific recognition abilities with peanut agglutinin (PNA) lectin rather than concanavalin A (Con A) lectin, observed by fluorescence spectroscopy (CitationZhang et al. 2008a). Another significant study about multilayers of galactosylated chitosan and plasmid DNA was reported by CitationCai et al. (2008) in which the films had a specific higher transfection rate on hepatoma G2 cells.
Growth factors
The growth factors and their receptors play an important role in cell growth and proliferation, and are overexpressed in a variety of tumors. Muller et al. demonstrated that vascular endothelial growth factor (VEGF) adsorbed on (PAH/PSS)4 maintained its bioactivity in vitro and stimulated endothelial cell proliferation (CitationMüller et al. 2008). In another study, the polyelectrolyte films were functionalized with brain-derived neurotrophic factor (BDNF) and semaphorin 3A (Sema3A). The viability of motoneurons on polyelectrolyte multilayers was higher compared to that on polyelectrolyte monolayers (CitationVodouhê et al. 2005). When basic fibroblast growth factor (bFGF) was immobilized onto quartz slides and collagen films by assembly with chondroitin sulfate (CS), it was found that the immobilized growth factors retained maximal bioactivity (CitationMa et al. 2007). These kinds of polyelectrolyte architectures may find diverse application in tissue engineering.
Passive targeting
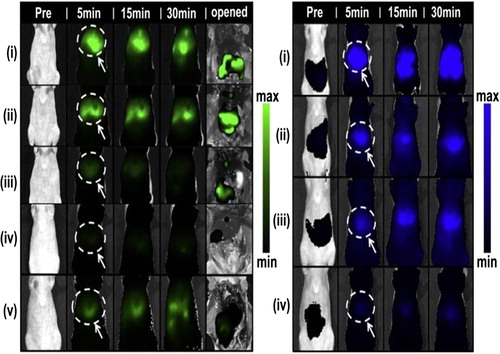
Passive targeting is based on the accumulation of drugs in the areas around the tumors with leaky vasculature, by an enhanced permeation and retention (EPR) effect. Schneider and his colleagues employed the smallest (25–100 nm) LbL nanoparticles [AuNPs coated with five primer layers of PAH and PSS and functionalized with N-(2-hydroxypropyl) methacrylamide (HPMA) copolymers] to enhance the accumulation of active drug in the tumor tissue (CitationSchneider et al. 2009). In the studies by CitationPoon et al. (2011b) carboxylated quantum dots (QDs) were sequentially coated with PLL and DS, then terminally capped with an outer layer of either PLL, DXS, or HA. After systemic intravenous injection in mice, the blood concentrations of the nanoparticles decreased in a two-phase manner (). All particles were observed to undergo a rapid distribution phase within 1 h of injection. The longer persistence of HA-terminated nanoparticles in the blood stream corroborates their superior stability and biodistribution profile. They also monitored the accumulation of the HA-terminated nanoparticles in subcutaneously induced KB tumors with intravital fluorescence imaging over a period of 72 h. It was observed that 24 h post injection, the HA-terminated particles were detectable in the tumors. Nevertheless, the nanoparticle accumulation and clearance profile in the tumor is typical of EPR-based targeting, which is short-lived, with a maximum accumulation of 24 h post injection, as there are no active mechanisms in place to promote cell uptake or extend their residence time in the tumor interstitials. Previously, trilayer architecture of PLL modified with iminobiotin, followed by the linker protein neutravidin and biotin end-functionalized PEG that enabled the layered nanoparticles to avoid rapid reticuloendothelial system (RES) clearance, allowing their accumulation in tumor interstitials due to EPR effect, was reported (CitationPoon et al. 2011a).
Figure 7. Blood circulation and tumor targeting of optimized LbL nanoparticles. (A) Blood circulation profiles of QD705, QD705/PLL/[DXS/PLL]3/HA and D705/PLL/[DXS/PLL]3/DXS. The longer persistence of QD705/PLL/[DXS/PLL]3/HA in the blood stream corroborates their superior stability and biodistribution profile. (B) Enhanced permeation and retention (EPR) based targeting of solid KB tumors induced subcutaneously on both hind flanks using QD705/PLL/[DXS/PLL]3/HA. Image is taken at the 24 h time point. (C) Time dependant accumulation of QD705/PLL/[DXS/PLL]3/HA in KB tumors. Accumulation of the nanoparticles in tumors is transient and typical of EPR dominated targeting. Data is given in mean ± SEM, n = 6. Reproduced with permission from reference (CitationPoon et al. 2011b) Copyright American Chemical society 2011.
![Figure 7. Blood circulation and tumor targeting of optimized LbL nanoparticles. (A) Blood circulation profiles of QD705, QD705/PLL/[DXS/PLL]3/HA and D705/PLL/[DXS/PLL]3/DXS. The longer persistence of QD705/PLL/[DXS/PLL]3/HA in the blood stream corroborates their superior stability and biodistribution profile. (B) Enhanced permeation and retention (EPR) based targeting of solid KB tumors induced subcutaneously on both hind flanks using QD705/PLL/[DXS/PLL]3/HA. Image is taken at the 24 h time point. (C) Time dependant accumulation of QD705/PLL/[DXS/PLL]3/HA in KB tumors. Accumulation of the nanoparticles in tumors is transient and typical of EPR dominated targeting. Data is given in mean ± SEM, n = 6. Reproduced with permission from reference (CitationPoon et al. 2011b) Copyright American Chemical society 2011.](/cms/asset/9bd90dbc-0080-4dd6-88f1-f55ba663303c/ianb_a_1011801_f0007_oc.jpg)
Conclusion and outlook
In this review, the recent progress and the various research studies carried out in the field of endogenous stimuli- responsive polyelectrolyte drug delivery systems has been summarized. As put forth by Park, there are at least four modulated delivery systems that have to be developed in the future third generation drug delivery systems. They are the glucose-sensitive transient insulin delivery with on–off switching capability, targeted delivery of anticancer agents or siRNA to tumors, long-term drug delivery ranging from 6 months to 1 year, and in vitro testing methods that can predict in vivo pharmacokinetic profiles (CitationPark 2014). Of these, the most challenging is a mode of delivery which mimics the pulsatile levels of insulin in blood. At the moment, it is not clear which endogenous stimuli could be utilized for such a delivery system. Inspiration might come from exogenous principles. One potential route, which holds promise in modulating blood insulin levels in vivo, is based on the principle of the light-cell-pump (CitationSommer et al. 2010), which has been recently exploited to force permeable nanovesicles to release their cargo (CitationCarter et al. 2014).
PEMs are particularly attractive for third generation applications because of their responsiveness to a variety of environmental stimuli, and because their properties can be easily tailored to specific needs. Moreover, PEMs hold great promise for designing theranostic platforms. For instance, recently CitationZhang et al. (2014) prepared multifunctional magnetic PAH/PSS microcapsules that can be used in drug delivery, hyperthermia, and also simultaneously as MRI contrast agents for remote cancer cell therapy and imaging. However, there are a few bottleneck challenges to the successful implementation of PEMs into the catalog of third generation drug delivery systems. One major challenge would be designing PEM-based delivery systems with stability against physiological barriers. Furthermore, detailed knowledge about the internalization behaviour of PEM within the cells, the biocompatibility, and biodistribution in vivo is only partially available. Recent progress regarding these aspects was achieved by Morton et al. in a model study which employed a poly (lactic-co-glycolic acid) drug-containing core as a substrate for LbL deposition. The authors fabricated a range of coated systems to investigate the potential of these polyelectrolytes to stabilize the drug for delivery, as well as to improve the pharmacokinetics of both the drug and carrier (CitationMorton et al. 2013). shows the stability assessments of different nanoparticle architectures using the IVIS system, a highly versatile in vivo imaging technology, for simultaneous tracking of both drug and nanoparticle. In another study, the multiple internalization pathways of PEMs into the mammalian cells were explored by CitationKastl et al. (2013).
Figure 8. Biodistribution channels for both drug, cardiogreen (CG820) and nanoparticle (PLL700) with significant differences in the accumulated signal intensities for different formulations of the layered architectures in the liver, relative to the controls, within the first 30 min post-administration on both channels. Reproduced with permission from reference (CitationMorton et al. 2013) Copyright Elsevier 2013.
Thus, there is ample evidence that biodegradable polyelectrolyte nanocapsules are multifunctional vehicles which can smuggle drugs into cells, and release them upon endogenous activation. Therefore, the interest in the design of intelligent PEM drug delivery systems is clear, and the need of the hour is a systematic translation of PEM-based drug delivery systems from the lab to clinical studies. In addition to applications in drug delivery, polyelectrolytes find increasing applications in other different fields like biosensors (CitationCampos et al. 2014), biomaterial coatings (CitationPanayotov et al. 2014), encapsulation of living cells (CitationStange et al. 1993, CitationGranicka et al. 2009), bioelectrochemistry (CitationWiemann et al. 2008), photoelectrochemistry (CitationYuan et al. 2014) etc. and consequently, any progress in LbL-based PEM could lead to significant advancement in these fields as well.
Declaration of interest
The author reports no declaration of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agrawal AK, Harde H, Thanki K, Jain S. 2013. Improved stability and antidiabetic potential of insulin containing folic acid functionalized polymer stabilized multilayered liposomes following oral administration. Biomacromolecules. 15:350–360.
- Almalik A, Donno R, Cadman CJ, Cellesi F, Day PJ, Tirelli N. 2013. Hyaluronic acid-coated chitosan nanoparticles: Molecular weight-dependent effects on morphology and hyaluronic acid presentation. J Control Release. 172:1142–1150.
- Anandhakumar S, Raichur AM. 2013. Polyelectrolyte/silver nanocomposite multilayer films as multifunctional thin film platforms for remote activated protein and drug delivery. Acta Biomater. 9:8864–8874.
- Anandhakumar S, Mahalakshmi V, Raichur AM. 2012. Silver nanoparticles modified nanocapsules for ultrasonically activated drug delivery. Mater Sci Eng C. 32:2349–2355.
- Anandhakumar S, Nagaraja V, Raichur AM. 2010. Reversible polyelectrolyte capsules as carriers for protein delivery. Colloids Surf B Biointerfaces. 78:266–274.
- Angelatos AS, Radt B, Caruso F. 2005. Light-responsive polyelectrolyte/gold nanoparticle microcapsules. J Phys Chem B. 109:3071–3076.
- Antipina MN, Sukhorukov GB. 2011. Remote control over guidance and release properties of composite polyelectrolyte based capsules. Adv Drug Deliv Rev 63:716–729.
- Antipov AA, Sukhorukov GB, Möhwald H. 2003. Influence of the ionic strength on the polyelectrolyte multilayers‘permeability. Langmuir. 19:2444–2448.
- Antunes JC, Pereira CL, Molinos M, Ferreira-da- ilva F, Dess M, Gloria A, et al. 2011. Layer-by-layer self-assembly of chitosan and poly(γ-glutamic acid) into polyelectrolyte complexes. Biomacromolecules. 12:4183–4195.
- Anzai JI, Hoshi T, Nakamura N. 2000. Construction of multilayer thin films containing avidin by a layer-by-layer deposition of avidin and poly(anion)s. Langmuir. 16:6306–6311.
- Anzai JI, Nakamura N. 1999. Preparation of active avidin films by a layer-by-layer deposition of poly(vinyl sulfate) and avidin on a solid surface. J Chem Soc Perkin Trans. 2:2413–2414.
- Ariga K, Lvov YM, Kawakami K, Ji Q, Hill JP. 2011. Layer-by-layer self-assembled shells for drug delivery. Adv Drug Delivery Rev. 63:762–771.
- Artyukhin AB, Bakajin O, Stroeve P, Noy A. 2004. Layer-by-layer electrostatic self- assembly of polyelectrolyte nanoshells on individual carbon nanotube templates. Langmuir. 20:1442–1448.
- Auzenne E, Ghosh SC, Khodadadian M, Rivera B, Farquhar D, Price RE, et al. 2007. Hyaluronic acid- paclitaxel: antitumor efficacy against cd44(+) human ovarian carcinoma xenografts. Neoplasia. 9:479–486.
- Becker AL, Johnston APR, Caruso F. 2010. Peptide nucleic acid films and capsules: assembly and enzymatic degradation. Macromol Biosci. 10:488–495.
- Becker AL, Zelikin AN, Johnston APR, Caruso F. 2009. Tuning the formation and degradation of layer-by-layer assembled polymer hydrogel microcapsules. Langmuir. 25:14079–14085.
- Blacklock J, Handa H, Soundara Manickam D, Mao G, Mukhopadhyay A, Oupický D. 2007. Disassembly of layer-by-layer films of plasmid DNA and reducible TAT polypeptide. Biomaterials. 28:117–124.
- Borodina T, Markvicheva E, Kunizhev S, Möhwald H, Sukhorukov GB, Kreft O. 2007. Controlled release of DNA from self-degrading microcapsules. Macromol Rapid Commun. 28:1894–1899.
- Boudou T, Kharkar P, Jing J, Guillot R, Pignot-Paintrand I, Auzely-Velty R, Picart C. 2012. Polyelectrolyte multilayer nanoshells with hydrophobic nanodomains for delivery of paclitaxel. J Control Release. 159:403–412.
- Cai K, Hu Y, Luo Z, Kong T, Lai M, Sui X, et al. 2008. Cell-specific gene transfection from a gene-functionalized poly(d,l-lactic acid) substrate fabricated by the layer-by-layer assembly technique. Angew Chem Int Ed Engl. 47:7479–7481.
- Campos PP, Moraes ML, Volpati D, Miranda PB, Oliveira ON, Ferreira M. 2014. Amperometric detection of lactose using β-galactosidase immobilized in layer-by- layer films. ACS Appl Mater Interfaces. 6:11657–11664.
- Carter KA, Shao S, Hoopes MI, Luo D, Ahsan B, Grigoryants VM, et al. 2014. Porphyrin–phospholipid liposomes permeabilized by near-infrared light. Nat Commun. 5:3546.
- Cavalieri F, Postma A, Lee L, Caruso F. 2009. Assembly and functionalization of DNA- polymer microcapsules. ACS Nano. 3:234–240.
- Chang B, Sha X, Guo J, Jiao Y, Wang C, Yang W. 2011. Thermo and pH dual responsive, polymer shell coated, magnetic mesoporous silica nanoparticles for controlled drug release. J Mater Chem. 21:9239–9247.
- Chen X, Ding X, Zheng Z, Peng Y. 2006. Thermosensitive cross-linked polymer vesicles for controlled release system. New J Chem. 30:577–582.
- Chen X, Guo Z, Xin J, Li J, Li J. 2011a. Modulated insulin release from glucose-sensitive multilayer films. J Control Release. 152:e152–e154.
- Chen X, Luo J, Wu W, Tan H, Xu F, Li J. 2012. The influence of arrangement sequence on the glucose-responsive controlled release profiles of insulin-incorporated LbL films. Acta Biomater. 8:4380–4388.
- Chen X, Wu W, Guo Z, Xin J, Li J. 2011b. Controlled insulin release from glucose-sensitive self-assembled multilayer films based on 21-arm star polymer. Biomaterials. 32:1759–1766.
- Chiang WH, Huang WC, Chang CW, Shen MY, Shih ZF, Huang YF, et al. 2013. Functionalized polymersomes with outlayered polyelectrolyte gels for potential tumor-targeted delivery of multimodal therapies and MR imaging. J Control Release. 168:280–288.
- Chiu CC, Lin YT, Sun SL, Sung KH, Wang LF. 2011. Anticancer activity of released doxorubicin from a folate-mediated polyelectrolyte complex. J Biomater Sci Polym Ed. 22:1487–1507.
- Cho Y, Lee JB, Hong J. 2014. Controlled release of an anti-cancer drug from DNA structured nano-films. Sci Rep. 4:4078.
- Chong SF, Chandrawati R, Städler B, Park J, Cho J, Wang Y, et al. 2009a. Stabilization of polymer-hydrogel capsules via thiol–disulfide exchange. Small. 5:2601–2610.
- Chong S-F, Sexton A, De Rose R, Kent SJ, Zelikin AN, Caruso F. 2009b. A paradigm for peptide vaccine delivery using viral epitopes encapsulated in degradable polymer hydrogel capsules. Biomaterials. 30:5178–5186.
- Chua PH, Neoh KG, Kang ET, Wang W. 2008. Surface functionalization of titanium with hyaluronic acid/chitosan polyelectrolyte multilayers and RGD for promoting osteoblast functions and inhibiting bacterial adhesion. Biomaterials. 29:1412–1421.
- Connal LA, Li Q, Quinn JF, Tjipto E, Caruso F, Qiao GG. 2008. pH-responsive poly(acrylic acid) core cross-linked star polymers: morphology transitions in solution and multilayer thin films. Macromolecules. 41:2620–2626.
- Cortez C, Tomaskovic-Crook E, Johnston APR, Radt B, Cody SH, Scott AM, et al. 2006. Targeting and uptake of multilayered particles to colorectal cancer cells. Adv Mater. 18:1998–2003.
- Cortez C, Tomaskovic-Crook E, Johnston APR, Scott AM, Nice EC, Heath JK, Caruso F. 2007. Influence of size, surface, cell line, and kinetic properties on the specific binding of a33 antigen-targeted multilayered particles and capsules to colorectal cancer cells. ACS Nano. 1:93–102.
- Costa RR, Mano JF. 2014. Polyelectrolyte multilayered assemblies in biomedical technologies. Chem Soc Rev. 43:3453–3479.
- Craig M, Bordes R, Holmberg K. 2012. Polypeptide multilayer self-assembly and enzymatic degradation on tailored gold surfaces studied by QCM-D. Soft Matter. 8:4788–4794.
- Cuomo F, Lopez F, Ceglie A, Maiuro L, Miguel MG, Lindman B. 2012. PH-responsive liposome-templated polyelectrolyte nanocapsules. Soft Matter. 8:4415–4420.
- Dähne L, Leporatti S, Donath E, Möhwald H. 2001. Fabrication of micro reaction cages with tailored properties. J Am Chem Soc. 123:5431–5436.
- De Geest BG, Vandenbroucke RE, Guenther AM, Sukhorukov GB, Hennink WE, Sanders NN, et al. 2006. Intracellularly degradable polyelectrolyte microcapsules. Adv Mater. 18:1005–1009.
- De Geest BG, Jonas AM, Demeester J, De Smedt SC. 2006. Glucose-responsive polyelectrolyte capsules. Langmuir. 22:5070–5074.
- De Geest BG, Sanders NN, Sukhorukov GB, Demeester J, De Smedt SC. 2007. Release mechanisms for polyelectrolyte capsules. Chem Soc Rev. 36:636–649.
- De Geest BG, Willart MA, Hammad H, Lambrecht BN, Pollard C, Bogaert P, et al. 2012a. Polymeric multilayer capsule-mediated vaccination induces protective immunity against cancer and viral infection. ACS Nano. 6:2136–2149.
- De Geest BG, Willart MA, Lambrecht BN, Pollard C, Vervaet C, Remon JP, et al. 2012b. Surface-engineered polyelectrolyte multilayer capsules: synthetic vaccines mimicking microbial structure and function. Angew Chem Int Ed Engl. 51:3862–3866.
- De Haes W, De Koker S, Pollard C, Atkinson D, Vlieghe E, Hoste J, et al. 2010. Polyelectrolyte capsules-containing hiv-1 p24 and poly i:c modulate dendritic cells to stimulate hiv-1-specific immune responses. Mol Ther. 18:1408–1416.
- De Koker S, De Geest BG, Singh SK, De Rycke R, Naessens T, Van Kooyk Y, et al. 2009. Polyelectrolyte microcapsules as antigen delivery vehicles to dendritic cells: uptake, processing, and cross-presentation of encapsulated antigens. Angew Chem Int Ed Engl. 48:8485–8489.
- De Koker S, Hoogenboom R, De Geest BG. 2012. Polymeric multilayer capsules for drug delivery. Chem Soc Rev. 41:2867–2884.
- De Rose R, Zelikin AN, Johnston APR, Sexton A, Chong SF, Cortez C, et al. 2008. Binding, internalization, and antigen presentation of vaccine- loaded nanoengineered capsules in blood. Adv Mater. 20:4698–4703.
- De Temmerman ML, Rejman J, Vandenbroucke RE, De Koker S, Libert C, Grooten J, et al. 2012. Polyelectrolyte LbL microcapsules versus PLGA microparticles for immunization with a protein antigen. J Control Release. 158:233–239.
- de Villiers MM, Otto DP, Strydom SJ, Lvov YM. 2011. Introduction to nanocoatings produced by layer-by-layer (LbL) self-assembly. Adv Drug Deliv Rev. 63:701–715.
- Decher G, Hong JD. 1991. Buildup of ultrathin multilayer films by a self-assembly process: II. consecutive adsorption of anionic and cationic bipolar amphiphiles and polyelectrolytes on charged surfaces . Ber Bunsen-Ges Phys Chem. 95:1430–1434.
- Decher G. 1997. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science. 277:1232–1237.
- del Mercato LL, Ferraro MM, Baldassarre F, Mancarella S, Greco V, Rinaldi R, Leporatti S. 2014. Biological applications of LbL multilayer capsules: from drug delivery to sensing. Adv Colloid Interface Sci. 207:139–154.
- Delcea M, Möhwald H, Skirtach AG. 2011. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv Drug Delivery Rev. 63:730–747.
- Deshmukh PK, Ramani KP, Singh SS, Tekade AR, Chatap VK, Patil GB, Bari SB. 2013. Stimuli-sensitive layer-by-layer (LbL) self-assembly systems: targeting and biosensory applications. J. Controlled Release. 166:294–306.
- Ding H, Yong KT, Roy I, Pudavar HE, Law WC, Bergey EJ, Prasad PN. 2007. Gold nanorods coated with multilayer polyelectrolyte as contrast agents for multimodal imaging. J Phys Chem C. 111:12552–12557.
- Ding Z, Guan Y, Zhang Y, Zhu XX. 2009a. Layer-by-layer multilayer films linked with reversible boronate ester bonds with glucose-sensitivity under physiological conditions. Soft Matter. 5:2302–2309.
- Ding Z, Guan Y, Zhang Y, Zhu XX. 2009b. Synthesis of glucose-sensitive self-assembled films and their application in controlled drug delivery. Polymer. 50:4205–4211.
- Donath E, Moya S, Neu B, Sukhorukov GB, Georgieva R, Voigt A, et al. 2002. Hollow polymer shells from biological templates: fabrication and potential applications. Chem Eur J 8:5481–5485.
- Dufes C, Muller JM, Couet W, Olivier JC, Uchegbu I, Schätzlein A. 2004. Anticancer drug delivery with transferrin targeted polymeric chitosan vesicles. Pharm Res. 21:101–107.
- Endo Y, Sato K, Anzai JI. 2011. Preparation of avidin-containing polyelectrolyte microcapsules and their uptake and release properties. Polym Bull. 66:711–720.
- Fernandes PAL, Delcea M, Skirtach AG, Mohwald H, Fery A. 2010. Quantification of release from microcapsules upon mechanical deformation with AFM. Soft Matter. 6:1879–1883.
- Fischlechner M, Zschönig O, Hofmann J, Donath E. 2005. Engineering virus functionalities on colloidal polyelectrolyte lipid composites. Angew Chem Int Ed Engl. 117:2952–2955.
- Fisher KD, Ulbrich K, Subr V, Ward CM, Mautner V, Blakey D, Seymour LW. 2000. A versatile system for receptor-mediated gene delivery permits increased entry of DNA into target cells, enhanced delivery to the nucleus and elevated rates of transgene expression. Gene Ther. 7:1337–1343.
- Fleige E, Quadir MA, Haag R. 2012. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: concepts and applications. Adv Drug Deliv Rev. 64:866–884.
- Gao C, Möhwald H, Shen JC. 2004. Enhanced biomacromolecule encapsulation by swelling and shrinking procedures. Chem Phys Chem. 5:116–120.
- Gao S, Chen J, Dong L, Ding Z, Yang YH, Zhang J. 2005. Targeting delivery of oligonucleotide and plasmid DNA to hepatocyte via galactosylated chitosan vector. Eur J Pharm Biopharm. 60: 327–334.
- Georgieva R, Moya S, Hin M, Mitlöhner R, Donath E, Kiesewetter H, et al. 2002. Permeation of macromolecules into polyelectrolyte microcapsules. Biomacromolecules. 3:517–524.
- Gilmore BF. 2012. Proteases as selective activators of triggered drug release: a potential answer to the problem of biomaterial-associated infections? J Biotechnol Biomater. 2:e111. doi:10.4172/2155-952X.1000e111.
- Glinel K, Sukhorukov GB, Möhwald H, Khrenov V, Tauer K. 2003. Thermosensitive hollow capsules based on thermoresponsive polyelectrolytes. Macromol Chem Phys. 204:1784–1790.
- Granicka LH, Antosiak-Iwáńska M, Godlewska E, Hoser G, Strawski M, Szklarczyk M, Dudziński K. 2009. The Experimental tudy of polyelectrolyte coatings uitability for encapsulation of cells. Artif Cells Blood Substit Immobil Biotechnol. 37:187–194.
- Granicka LH, Borkowska M, Grzeczkowicz A, Stachowiak R, Szklarczyk M, Bielecki J, Strawski M. 2014. The targeting nanothin polyelectrolyte shells in system with immobilized bacterial cells for antitumor factor production. J Biomed Mater Res A. 102:2662–2668.
- Grazú V, Moros M, Sánchez-Espinel C. 2012. Chapter 14 - Nanocarriers as nanomedicines: design concepts and recent advances. In: Jesus MD, de la Fuente, Grazu V, Eds. Frontiers of Nanoscience. New Jersey: Elsevier.
- Gribova V, Gauthier-Rouvière C, Albigès-Rizo C, Auzely-Velty R, Picart C. 2013. Effect of RGD functionalization and stiffness modulation of polyelectrolyte multilayer films on muscle cell differentiation. Acta Biomater. 9:6468–6480.
- Gujarathi NA, Rane BR, Patel JK. 2012. pH sensitive polyelectrolyte complex of O− carboxymethyl chitosan and poly (acrylic acid) cross-linked with calcium for sustained delivery of acid susceptible drugs. Int J Pharm. 436:418–425.
- Hales M, Barner-Kowollik C, Davis TP, Stenzel MH. 2004. Shell-cross-linked vesicles synthesized from block copolymers of poly(d,l-lactide) and poly(N-isopropyl acrylamide) as thermoresponsive nanocontainers. Langmuir. 20:10809–10817.
- Han HD, Mangala LS, Lee JW, Shahzad MMK, Kim HS, Shen D, et al. 2010. Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clin Cancer Res. 16:3910–3922.
- Haynie DT, Palath N, Liu Y, Li B, Pargaonkar N. 2004. Biomimetic nanostructured materials: inherent reversible tabilization of polypeptide microcapsules. Langmuir. 21:1136–1138.
- Hoshi T, Akase S, Anzai JI. 2002. Preparation of multilayer thin films containing avidin through ugar− lectin interactions and their binding properties. Langmuir. 18:7024–7028.
- Hou Z, Zhan C, Jiang Q, Hu Q, Li L, Chang D, et al. 2011. Both FA− and mPEG-conjugated chitosan nanoparticles for targeted cellular uptake and enhanced tumor tissue distribution. Nanoscale Res Lett. 6:1–11.
- Inoue H, Sato K, Anzai JI. 2004. Disintegration of layer-by-layer assemblies composed of 2-iminobiotin-labeled poly(ethyleneimine) and avidin. Biomacromolecules. 6:27–29.
- Itoh Y, Matsusaki M, Kida T, Akashi M. 2004. Preparation of biodegradable hollow nanocapsules by silica template method. Chem Lett. 33:1552–1553.
- Itoh Y, Matsusaki M, Kida T, Akashi M. 2006. Enzyme-responsive release of encapsulated proteins from biodegradable hollow capsules. Biomacromolecules. 7:2715–2718.
- Jeong JH, Kim SH, Kim SW, Park TG. 2005. In vivo tumor targeting of ODN-PEG-folic acid/PEI polyelectrolyte complex micelles. J Biomater Sci Polym Ed. 16:1409–1419.
- Jian W, Xu S, Wang J, Feng S. 2013. Layer-by-layer assembly of poly(allylamine hydrochloride)/polyurethane and its loading and release behavior for methylene orange. J Appl Polym Sci. 129:2070–2075.
- Johnston APR, Kamphuis MMJ, Such GK, Scott AM, Nice EC, Heath JK, Caruso F. 2012. Targeting cancer cells: controlling the binding and internalization of antibody- functionalized capsules. ACS Nano. 6:6667–6674.
- Johnston APR, Lee L, Wang Y, Caruso F. 2009. Controlled degradation of DNA capsules with engineered restriction-enzyme cut sites. Small. 5:1418–1421.
- Kastl L, Sasse D, Wulf V, Hartmann R, Mircheski J, Ranke C, et al. 2013. Multiple internalization pathways of polyelectrolyte multilayer capsules into mammalian cells. ACS Nano. 7:6605–6618.
- Khapli S, Kim JR, Montclare JK, Levicky R, Porfiri M, Sofou S. 2009. Frozen cyclohexane-in-water emulsion as a sacrificial template for the synthesis of multilayered polyelectrolyte microcapsules. Langmuir. 25:9728–9733.
- Khopade AJ, Caruso F. 2004. Two-component, ultrathin microcapsules prepared by a core- mediated layer-by-layer approach. Chem Mater. 16:2107–2112.
- Khorsand B, Lapointe G, Brett C, Oh JK. 2013. Intracellular drug delivery nanocarriers of glutathione-responsive degradable block copolymers having pendant disulfide linkages. Biomacromolecules. 14:2103–2111.
- Kim E, Kim D, Jung H, Lee J, Paul S, Selvapalam N, et al. 2010. Facile, template-free synthesis of stimuli-responsive polymer nanocapsules for targeted drug delivery. Angew Chem Int Ed Engl. 49:4405–4408.
- Kim SH, Jeong JH, Mok H, Lee SH, Kim SW, Park TG. 2007. Folate receptor targeted delivery of polyelectrolyte complex micelles prepared from ODN-PEG-folate conjugate and cationic lipids. Biotechnol Prog. 23:232–237.
- Kreft O, Georgieva R, Bäumler H, Steup M, Müller-Roher B, Sukhorukov GB, Mähwald H. 2006. Red blood cell templated polyelectrolyte capsules: a novel vehicle for the stable encapsulation of DNA and proteins. Macromol Rapid Commun. 27:435–440.
- Lee H, Jeong Y, Park TG. 2007. Shell cross-linked hyaluronic acid/polylysine layer-by- layer polyelectrolyte microcapsules prepared by removal of reducible hyaluronic acid microgel cores. Biomacromolecules. 8:3705–3711.
- Lee SK, Han MS, Asokan S, Tung C-H. 2011. Effective gene silencing by multilayered sirna-coated gold nanoparticles. Small. 7:364–370.
- Levy T, Déjugnat C, Sukhorukov GB. 2008. Polymer microcapsules with carbohydrate- sensitive properties. Adv Funct Mater. 18:1586–1594.
- Li B, Haynie DT. 2004. Multilayer biomimetics: reversible covalent tabilization of a nanostructured biofilm. Biomacromolecules. 5:1667–1670.
- Li H, Zhang JZ, Tang Q, Du M, Hu J, Yang D. 2013. Reduction-responsive drug delivery based on mesoporous silica nanoparticle core with crosslinked poly(acrylic acid) shell. Mater Sci Eng C. 33:3426–3431.
- Li H, Qian ZM. 2002. Transferrin/transferrin receptor-mediated drug delivery. Med Res Rev. 22:225–250.
- Li P, Liu D, Miao L, Liu C, Sun X, Liu Y, Zhang N. 2012a. A pH-sensitive multifunctional gene carrier assembled via layer-by-layer technique for efficient gene delivery. Int J Nanomed. 7:925–939.
- Li Y, Lokitz BS, McCormick CL. 2006. Thermally responsive vesicles and their structural “locking” through polyelectrolyte complex formation. Angew Chem. 118:5924–5927.
- Li Y, Lu L, Zhang H, Wang J. 2012b. The pH regulated phycobiliproteins loading and releasing of polyelectrolytes multilayer microcapsules. Colloids Surf B. 93:121–126.
- Liang K, Such GK, Zhu Z, Yan Y, Lomas H, Caruso F. 2011. Charge-shifting click capsules with dual-responsive cargo release mechanisms. Adv Mater. 23:H273–H277.
- Lo CL, Lin KM, Hsiue GH. 2005. Preparation and characterization of intelligent core-shell nanoparticles based on poly(D,l-lactide)-g-poly(N-isopropyl acrylamide-co-methacrylic acid). J Controlled Release. 104:477–488.
- Luo GF, Xu XD, Zhang J, Yang J, Gong YH, Lei Q, et al. 2012. Encapsulation of an adamantane-doxorubicin prodrug in pH-responsive polysaccharide capsules for controlled release. ACS Appl Mater Interfaces. 4:5317–5324.
- Lvov Y, Antipov AA, Mamedov A, Möhwald H, Sukhorukov GB. 2001. Urease encapsulation in nanoorganized microshells. Nano Lett. 1:125–128.
- Ma L, Zhou J, Gao C, Shen J. 2007. Incorporation of basic fibroblast growth factor by a layer-by-layer assembly technique to produce bioactive substrates. J Biomed Mater Res B Appl Biomater. 83:285–292.
- Ma Y, Dong WF, Hempenius MA, Möhwald H, Julius Vancso G. 2006. Redox-controlled molecular permeability of composite-wall microcapsules. Nat Mater. 5:724–729.
- Majumdar S, Siahaan TJ. 2012. Peptide-mediated targeted drug delivery. Med Res Rev. 32:637–658.
- Mandal S, Bonifacio A, Zanuttin F, Sergo V, Krol S. 2011. Synthesis and multidisciplinary characterization of polyelectrolyte multilayer-coated nanogold with improved stability toward aggregation. Colloid Polym Sci. 289:269–280.
- Manna U, Patil S. 2010. Glucose-triggered drug delivery from borate mediated layer-by- layer self-assembly. ACS Appl Mater Interfaces. 2:1521–1527.
- Marchenko I, Yashchenok A, Borodina T, Bukreeva T, Konrad M, Möhwald H, Skirtach A. 2012. Controlled enzyme-catalyzed degradation of polymeric capsules templated on CaCO3: influence of the number of LbL layers, conditions of degradation, and disassembly of multicompartments. J Control Release. 162:599–605.
- Masereel B, Dinguizli M, Bouzin C, Moniotte N, Feron O, Gallez B, et al. 2011. Antibody immobilization on gold nanoparticles coated layer-by- layer with polyelectrolytes. J Nanopart Res. 13:1573–1580.
- Mayya KS, Gittins DI, Dibaj AM, Caruso F. 2001. Nanotubes prepared by templating sacrificial Nickel Nanorods. Nano Lett. 1:727–730.
- Meng F, Hennink WE, Zhong Z. 2009. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials. 30:2180–2198.
- Meyer F, Dimitrova M, Jedrzejenska J, Arntz Y, Schaaf P, Frisch B, et al. 2008. Relevance of bi-functionalized polyelectrolyte multilayers for cell transfection. Biomaterials. 29:618–624.
- Minati L, Antonini V, Dalla Serra M, Speranza G, Enrichi F, Riello P. 2013. pH-activated doxorubicin release from polyelectrolyte complex layer coated mesoporous silica nanoparticles. Microporous Mesoporous Mater. 180:86–91.
- Mintern JD, Percival C, Kamphuis MMJ, Chin WJ, Caruso F, Johnston APR. 2013. Targeting dendritic cells: the role of specific receptors in the internalization of polymer capsules. Adv Healthcare Mater. 2:940–944.
- Miura S, Teramura Y, Iwata H. 2006. Encapsulation of islets with ultra-thin polyion complex membrane through poly(ethylene glycol)-phospholipids anchored to cell membrane. Biomaterials. 27:5828–5835.
- Morris VB, Sharma CP. 2011. Folate mediated l-arginine modified oligo (alkylaminosiloxane) graft poly (ethyleneimine) for tumor targeted gene delivery. Biomaterials. 32:3030–3041.
- Morton SW, Poon Z, Hammond PT. 2013. The architecture and biological performance of drug-loaded LbL nanoparticles. Biomaterials. 34:5328–5335.
- Morton SW, Shah NJ, Quadir MA, Deng ZJ, Poon Z, Hammond PT. 2014. Osteotropic therapy via targeted layer-by-layer nanoparticles. Adv. Healthcare Mater. 3:867–875.
- Müller S, Koenig G, Charpiot A, Debry C, Voegel JC, Lavalle P, Vautier D. 2008. VEGF- functionalized polyelectrolyte multilayers as proangiogenic prosthetic coatings. Adv Funct Mater. 18:1767–1775.
- Muñoz Javier A, del Pino P, Bedard MF, Ho D, Skirtach AG, Sukhorukov GB, et al. 2008. Photoactivated release of cargo from the cavity of polyelectrolyte capsules to the cytosol of cells. Langmuir. 24:12517–12520.
- Necas J, Bartosikova L, Brauner P, Kolar J. 2008. Hyaluronic acid (hyaluronan): a review. Vet Med Czech. 53:397–411.
- Neu B, Voigt A, Mitlöhner R, Leporatti S, Gao CY, Donath E, et al. 2001. Biological cells as templates for hollow microcapsules. J Microencapsul. 18:385–395.
- Ng SL, Such GK, Johnston APR, Antequera-García G, Caruso F. 2011. Controlled release of DNA from poly(vinylpyrrolidone) capsules using cleavable linkers. Biomaterials. 32:6277–6284.
- Nukolova NV, Oberoi HS, Cohen SM, Kabanov AV, Bronich TK. 2011. Folate-decorated nanogels for targeted therapy of ovarian cancer. Biomaterials. 32:5417–5426.
- Ochs CJ, Such GK, Städler B, Caruso F. 2008. Low-fouling, biofunctionalized, and biodegradable click capsules. Biomacromolecules. 9:3389–3396.
- Ochs CJ, Such GK, Yan Y, van Koeverden MP, Caruso F. 2010b. Biodegradable click capsules with engineered drug-loaded multilayers. ACS Nano. 4:1653–1663.
- Ochs CJ, Such GK, Caruso F. 2010a. Modular assembly of layer-by-layer capsules with tailored degradation profiles. Langmuir. 27:1275–1280.
- Orozco VH, Kozlovskaya V, Kharlampieva E, López BL, Tsukruk VV. 2010. Biodegradable self-reporting nanocomposite films of poly(lactic acid) nanoparticles engineered by layer-by-layer assembly. Polymer. 51:4127–4139.
- Panayotov IV, Collart-Dutilleul PY, Salehi H, Martin M, Végh A, Yachouh J, et al. 2014. Sprayed cells and polyelectrolyte films for biomaterial functionalization: the influence of physical PLL– PGA film treatments on dental pulp cell behavior. Macromol Biosci. 14:1771–1782.
- Park K. 2014.Controlled drug delivery systems: past forward and future back. J Control Release. 198:3–8.
- Pavlov AM, De Geest BG, Louage B, Lybaert L, De Koker S, Koudelka Z, et al. 2013. Magnetically engineered microcapsules as intracellular anchors for remote control over cellular mobility. Adv Mater. 25:6945–6950.
- Pechenkin MA, Balabushevich NG, Zorov IN, Izumrudov VA, Klyachko NL, Kabanov AV, Larionova NI. 2013. Use of protease inhibitors in composite polyelectrolyte microparticles in order to increase the bioavailability of perorally administered encapsulated proteins. Pharm Chem J. 47:62–69.
- Pereira SO, Barros-Timmons A, Trindade T. 2014. Biofunctionalisation of colloidal gold nanoparticles via polyelectrolytes assemblies. Colloid Polym Sci. 292:33–50.
- Picart C, Elkaim R, Richert L, Audoin F, Arntz Y, Da Silva Cardoso M, et al. 2005. Primary cell adhesion on RGD-functionalized and covalently crosslinked thin polyelectrolyte multilayer films. Adv Funct Mater. 15:83–94.
- Poon Z, Chang D, Zhao X, Hammond PT. 2011a. Layer-by-Layer nanoparticles with a pH- sheddable layer for in vivo targeting of tumor hypoxia. ACS Nano. 5:4284–4292.
- Poon Z, Lee JB, Morton SW, Hammond PT. 2011b. Controlling in vivo stability and biodistribution in electrostatically assembled nanoparticles for systemic delivery. Nano Lett. 11:2096–2103.
- Prevot M, Déjugnat C, Möhwald H, Sukhorukov GB. 2006. Behavior of temperature-sensitive PNIPAM confined in polyelectrolyte capsules. Chem Phys Chem. 7:2497–2502.
- Qi W, Yan X, Duan L, Cui Y, Yang Y, Li J. 2009a. Glucose-sensitive microcapsules from glutaraldehyde cross-linked hemoglobin and glucose oxidase. Biomacromolecules . 10:1212–1216.
- Qi W, Yan X, Fei J, Wang A, Cui Y, Li J. 2009b. Triggered release of insulin from glucose-sensitive enzyme multilayer shells. Biomaterials. 30:2799–2806.
- Qin S, Geng Y, Discher DE, Yang S. 2006. Temperature-controlled assembly and release from polymer vesicles of poly(ethylene oxide)-block- poly(N-isopropylacrylamide). Adv Mater. 18:2905–2909.
- Radhakrishnan K, Tripathy J, Raichur AM. 2013. Dual enzyme responsive microcapsules simulating an “OR” logic gate for biologically triggered drug delivery applications. Chem Commun. 49:5390–5392.
- Radhakrishnan K, Raichur AM. 2012. Biologically triggered exploding protein based microcapsules for drug delivery. Chem Commun. 48:2307–2309.
- Radziuk D, Shchukin DG, Skirtach A, Möhwald H, Sukhorukov G. 2007. Synthesis of silver nanoparticles for remote opening of polyelectrolyte microcapsules. Langmuir. 23:4612–4617.
- Recksiedler CL, Deore BA, Freund MS. 2006. A novel layer-by-layer approach for the fabrication of conducting polymer/RNA multilayer films for controlled release. Langmuir. 22:2811–2815.
- Ren K, Ji J, Shen J. 2005. Tunable DNA release from cross-linked ultrathin DNA/PLL multilayered films. Bioconjug Chem. 17:77–83.
- Rivera-Gil P, De Koker S, De Geest BG, Parak WJ. 2009. Intracellular processing of proteins mediated by biodegradable polyelectrolyte capsules. Nano Lett. 9:4398–4402.
- Ruiz NI, Drickamer K. 1996. Differential ligand binding by two subunits of the rat liver asialoglycoprotein receptor. Glycobiology. 6:551–559.
- Sato K, Imoto Y, Sugama J, Seki S, Inoue H, Odagiri T, Anzai JI. 2004. Sugar-sensitive thin films composed of concanavalin A and glycogen. Anal Sci. 20:1247–1248.
- Sato K, Kodama D, Endo Y, Anzai JI. 2009. Preparation of insulin-containing microcapsules by a layer-by-layer deposition of concanavalin A and glycogen. J Nanosci Nanotechnol. 9:386–390.
- Sato K, Kodama D, Anzai JI. 2005. Sugar-sensitive thin films composed of concanavalin a and sugar-bearing polymers. Anal Sci. 21:1375–1378.
- Sato K, Yoshida K, Takahashi S, Anzai JI. 2011. pH- and sugar-sensitive layer-by-layer films and microcapsules for drug delivery. Adv Drug Deliv Rev. 63:809–821.
- Saurer EM, Flessner RM, Sullivan SP, Prausnitz MR, Lynn DM. 2010. Layer-by-layer assembly of DNA- and protein-containing films on microneedles for drug delivery to the skin. Biomacromolecules. 11:3136–3143.
- Schmaljohann D. 2006. Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 58:1655–1670.
- Schneider G, Decher G. 2004. From functional core/shell nanoparticles prepared via layer-by- layer deposition to empty nanospheres. Nano Lett. 4:1833–1839.
- Schneider GF, Subr V, Ulbrich K, Decher G. 2009. Multifunctional cytotoxic stealth nanoparticles. A model approach with potential for cancer therapy . Nano Lett. 9:636–642.
- Schultz P, Vautier D, Richert L, Jessel N, Haikel Y, Schaaf P, et al. 2005. Polyelectrolyte multilayers functionalized by a synthetic analogue of an anti- inflammatory peptide, α-MSH, for coating a tracheal prosthesis. Biomaterials. 26:2621–2630.
- Serizawa T, Iida K, Matsuno H, Kurita K. 2006. Prolonged degradation of end-capped polyelectrolyte multilayer films. Polym Bull. 57:407–413.
- Serizawa T, Yamaguchi M, Akashi M. 2002. Alternating bioactivity of polymeric layer-by- layer assemblies: anticoagulation vs procoagulation of human blood. Biomacromolecules. 3:724–731.
- Serizawa T, Yamaguchi M, Akashi M. 2003. Time-controlled desorption of ultrathin polymer films triggered by enzymatic degradation. Angew Chem Int Ed Engl. 42:1115–1118.
- Sexton A, Whitney PG, Chong SF, Zelikin AN, Johnston APR, De Rose R, et al. 2009. A protective vaccine delivery system for in vivo T cell stimulation using nanoengineered polymer hydrogel capsules. ACS Nano. 3:3391–3400.
- Shah N, Chaudhari K, Dantuluri P, Murthy RSR, Das S. 2009. Paclitaxel-loaded PLGA nanoparticles surface modified with transferrin and Pluronic®P85, an in vitro cell line and in vivo biodistribution studies on rat model. J Drug Target. 17:533–542.
- Shen HJ, Shi H, Ma K, Xie M, Tang LL, Shen S, et al. 2013. Polyelectrolyte capsules packaging BSA gels for pH-controlled drug loading and release and their antitumor activity. Acta Biomater. 9:6123–6133.
- Shimoni O, Postma A, Yan Y, Scott AM, Heath JK, Nice EC, Zelikin AN, Caruso F. 2012. Macromolecule Functionalization of Disulfide-Bonded Polymer Hydrogel Capsules and Cancer Cell Targeting. ACS Nano. 6:1463–1472.
- Shu S, Sun C, Zhang X, Wu Z, Wang Z, Li C. 2010a. Hollow and degradable polyelectrolyte nanocapsules for protein drug delivery. Acta Biomater. 6:210–217.
- Shu S, Zhang X, Wu Z, Wang Z, Li C. 2010b. Gradient cross-linked biodegradable polyelectrolyte nanocapsules for intracellular protein drug delivery. Biomaterials. 31:6039–6049.
- Sivakumar S, Bansal V, Cortez C, Chong S-F, Zelikin AN, Caruso F. 2009. Degradable, surfactant-free, monodisperse polymer-encapsulated emulsions as anticancer drug carriers. Adv Mater. 21:1820–1824.
- Sivakumar S, Gupta JK, Abbott NL, Caruso F. 2008. Monodisperse emulsions through templating polyelectrolyte multilayer capsules. Chem Mater. 20:2063–2065.
- Sommer AP, Zhu D, Scharnweber T. 2010. Laser modulated transmembrane convection: Implementation in cancer chemotherapy. J Control Release. 148:131–134.
- Song J, Jańczewski D, Ma Y, Hempenius M, Xu J, Vancso GJ. 2013a. Disassembly of redox responsive poly(ferrocenylsilane) multilayers: the effect of blocking layers, supporting electrolyte and polyion molar mass. J. Colloid Interface Sci. 405:256–261.
- Song J, Jańczewski D, Ma Y, Hempenius M, Xu J, Vancso GJ. 2013b. Redox-controlled release of molecular payloads from multilayered organometallic polyelectrolyte films. J Mater Chem B. 1:828–834.
- Sripriya J, Anandhakumar S, Achiraman S, Antony JJ, Siva D, Raichur AM. 2013. Laser receptive polyelectrolyte thin films doped with biosynthesized silver nanoparticles for antibacterial coatings and drug delivery applications. Int J Pharm. 457:206–213.
- Stange J, Mitzner S, Dautzenberg H, Ramlow W, Knippel M, et al. 1993. Prolonged biochemical and morphological stability of encapsulated liver cells—a new method. Biomater Artif Cells Immobiliz Biotechnol. 21:343–352.
- Steitz R, Leiner V, Tauer K, Khrenov V, v. Klitzing R. 2002. Temperature-induced changesin polyelectrolyte films at the solid–liquid interface. Appl Phys A Mater Sci Process. 74:s519–s521.
- Sukhorukov GB, Shchukin DG, Dong WF, Möhwald H, Lulevich VV, Vinogradova OI. 2004. Comparative analysis of hollow and filled polyelectrolyte microcapsules templated on melamine formaldehyde and carbonate cores. Macromol Chem Phys. 205:530–535.
- Sun JT, Piao JG, Wang LH, Javed M, Hong CY, Pan CY. 2013. One-pot synthesis of redox-responsive polymers-coated mesoporous silica nanoparticles and their controlled drug release. Macromol Rapid Commun. 34:1387–1394.
- Szarpak A, Cui D, Dubreuil F, De Geest BG, De Cock LJ, Picart C, et al. 2010. Designing hyaluronic acid-based layer-by-layer capsules as a carrier for intracellular drug delivery. Biomacromolecules. 11:713–720.
- Szarpak A, Pignot-Paintrand I, Nicolas C, Picart C, Auzély-Velty R. 2008. Multilayer assembly of hyaluronic acid/poly(allylamine): control of the buildup for the production of hollow capsules. Langmuir. 24:9767–9774.
- Tan WS, Zhu Z, Sukhishvili SA, Rubner MF, Cohen RE. 2011. Effect of block copolymer architecture on the thermally induced swelling of micelle-containing multilayer thin films. Macromolecules. 44:7767–7774.
- Tettey KE, Yee MQ, Lee D. 2010. Layer-by-layer assembly of charged particles in nonpolar media. Langmuir. 26:9974–9980.
- Tokuda Y, Miyagishima T, Tomida K, Wang B, Takahashi S, Sato K, Anzai JI. 2013. Dual pH-sensitive layer-by-layer films containing amphoteric poly(diallylamine-co-maleic acid). J Colloid Interface Sci. 399:26–32.
- Toublan FJJ, Boppart S, Suslick KS. 2006. Tumor targeting by surface-modified protein microspheres. J Am Chem Soc 128:3472–3473.
- Tripathy J, Raichur AM. 2013. Designing carboxymethyl cellulose based layer-by-layer capsules as a carrier for protein delivery. Colloids Surf B Biointerfaces. 101:487–492.
- Tsai WB, Chen RPY, Wei KL, Chen YR, Liao TY, Liu HL, Lai JY. 2009. Polyelectrolyte multilayer films functionalized with peptides for promoting osteoblast functions. Acta Biomater. 5:3467–3477.
- Tseng SJ, Zeng YF, Deng YF, Yang PC, Liu JR, Kempson IM. 2013. Switchable delivery of small interfering RNA using a negatively charged pH-responsive polyethylenimine- based polyelectrolyte complex. Chem Commun. 49:2670–2672.
- Vergaro V, Scarlino F, Bellomo C, Rinaldi R, Vergara D, Maffia M, et al. 2011. Drug-loaded polyelectrolyte microcapsules for sustained targeting of cancer cells. Adv Drug Deliv Rev. 63: 847–864.
- Vinogradov SV, Batrakova EV, Kabanov AV. 2003. Nanogels for oligonucleotide delivery to the brain. Bioconjugate Chem. 15:50–60.
- Vodouhê C, Guen EL, Garza JM, Francius G, Déjugnat C, Ogier J, et al. 2006. Control of drug accessibility on functional polyelectrolyte multilayer films. Biomaterials. 27:4149–4156.
- Vodouhê C, Schmittbuhl M, Boulmedais F, Bagnard D, Vautier D, Schaaf P, et al. 2005. Effect of functionalization of multilayered polyelectrolyte films on motoneuron growth. Biomaterials. 26:545–554.
- Volodkin D, Skirtach A, Möhwald H. 2012. Bioapplications of light-sensitive polymer films and capsules assembled using the layer-by-layer technique. Polym Int. 61:673–679.
- Wang C, Ye S, Dai L, Liu X, Tong Z. 2007. Enzymatic desorption of layer-by-layer assembled multilayer films and effects on the release of encapsulated indomethacin microcrystals. Carbohydr Res. 342:2237–2243.
- Wang Y, Caruso F. 2006. Template synthesis of stimuli-responsive nanoporous polymer- based spheres via sequential assembly. Chem Mater. 18:4089–4100.
- Wei H, Zhang XZ, Zhou Y, Cheng SX, Zhuo RX. 2006. Self-assembled thermoresponsive micelles of poly(N-isopropylacrylamide-b-methyl methacrylate). Biomaterials. 27:2028–2034.
- Wiemann LO, Buthe A, Klein M, van den Wittenboer A, Dähne L, Ansorge-Schumacher MB. 2008. Encapsulation of synthetically valuable biocatalysts into polyelectrolyte multilayer systems. Langmuir. 25:618–623.
- Wilchek M, Bayer EA. 1988. The avidin-biotin complex in bioanalytical applications. Anal Biochem. 171:1–32.
- Wohl BM, Engbersen JFJ. 2012. Responsive layer-by-layer materials for drug delivery. J Control Release. 158:2–14.
- Woitiski CB, Neufeld RJ, Ribeiro AJ, Veiga F. 2009. Colloidal carrier integrating biomaterials for oral insulin delivery: Influence of component formulation on physicochemical and biological parameters. Acta Biomater. 5:2475–2484.
- Wood KC, Zacharia NS, Schmidt DJ, Wrightman SN, Andaya BJ, Hammond PT. 2008. Electroactive controlled release thin films . Proc Natl Acad Sci . 105:2280–2285.
- Xu L, Zhu Z, Sukhishvili SA. 2010. Polyelectrolyte multilayers of diblock copolymer micelles with temperature-responsive cores. Langmuir. 27:409–415.
- Xu Z, Gu W, Huang J, Sui H, Zhou Z, Yang Y, et al. 2005. In vitro and in vivo evaluation of actively targetable nanoparticles for paclitaxel delivery. Int J Pharm. 288:361–368.
- Yang KN, Zhang CQ, Wang W, Wang PC, Zhou JP, Liang XJ. 2014. pH- responsive mesoporous silica nanoparticles employed in controlled drug delivery systems for cancer treatment. Cancer Biol Med. 11:34–43.
- Yan S, Zhu J, Wang Z, Yin J, Zheng Y, Chen X. 2011a. Layer-by-layer assembly of poly(l- glutamic acid)/chitosan microcapsules for high loading and sustained release of 5- fluorouracil. Eur J Pharm Biopharm. 78:336–345.
- Yan Y, Johnston APR, Dodds SJ, Kamphuis MMJ, Ferguson C, Parton RG, et al. 2010. Uptake and intracellular fate of disulfide-bonded polymer hydrogel capsules for doxorubicin delivery to colorectal cancer cells. ACS Nano. 4:2928–2936.
- Yan Y, Such GK, Johnston APR, Lomas H, Caruso F. 2011b. Toward therapeutic delivery with layer-by-layer engineered particles. ACS Nano. 5:4252–4257.
- Yashchenok A, Parakhonskiy B, Donatan S, Kohler D, Skirtach A, Mohwald H. 2013. Polyelectrolyte multilayer microcapsules templated on spherical, elliptical and square calcium carbonate particles. J Mater Chem B. 1:1223–1228.
- Ye S, Wang C, Liu X, Tong Z, Ren B, Zeng F. 2006. New loading process and release properties of insulin from polysaccharide microcapsules fabricated through layer-by-layer assembly. J Control Release. 112:79–87.
- Ye S, Wang C, Liu X, Tong Z. 2005. Deposition temperature effect on release rate of indomethacin microcrystals from microcapsules of layer-by-layer assembled chitosan and alginate multilayer films. J Control Release. 106:319–328.
- Yoo HS, Park TG. 2004. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J Control Release. 96:273–283.
- Yoshida K, Sato K, Anzai JI. 2010. Layer-by-layer polyelectrolyte films containing insulin for pH-triggered release. J Mater Chem. 20:1546–1552.
- Yuan S, Mu J, Mao R, Li Y, Zhang Q, Wang H. 2014. All-nanoparticle self-assembly zno/tio2 heterojunction thin films with remarkably enhanced photoelectrochemical activity. ACS Appl Mater Interfaces. 6:5719–5725.
- Yuanpei L, Shirong P, Wei Z, Zhuo D. 2009. Novel thermo-sensitive core–shell nanoparticles for targeted paclitaxel delivery. Nanotechnology. 20:065104.
- Zelikin AN, Becker AL, Johnston APR, Wark KL, Turatti F, Caruso F. 2007. A general approach for DNA encapsulation in degradable polymer microcapsules. ACS Nano. 1:63–69.
- Zelikin AN, Li Q, Caruso F. 2006. Degradable polyelectrolyte capsules filled with oligonucleotide sequences. Angew Chem. 118:7907–7909.
- Zelikin AN, Li Q, Caruso F. 2008. Disulfide-stabilized poly(methacrylic acid) capsules: formation, cross-linking, and degradation behavior. Chem Mater. 20:2655–2661.
- Zelikin AN, Price AD, Städler B. 2010. Poly(methacrylic acid) polymer hydrogel capsules: drug carriers, sub-compartmentalized microreactors, artificial organelles. Small. 6:2201–2207.
- Zelikin AN, Quinn JF, Caruso F. 2005. Disulfide cross-linked polymer capsules: en route to biodeconstructible systems. Biomacromolecules. 7:27–30.
- Zhang F, Wu Q, Chen Z-C, Li X, Jiang XM, Lin XF. 2006. Bioactive galactose-branched polyelectrolyte multilayers and microcapsules: Self-assembly, characterization, and biospecific lectin adsorption. Langmuir. 22:8458–8464.
- Zhang F, Wu Q, Chen ZC, Zhang M, Lin XF. 2008a. Hepatic-targeting microcapsules construction by self-assembly of bioactive galactose-branched polyelectrolyte for controlled drug release system. J Colloid Interface Sci. 317:477–484.
- Zhang F, Wu Q, Liu LJ, Chen ZC, Lin XF. 2008b. Thermal treatment of galactose-branched polyelectrolyte microcapsules to improve drug delivery with reserved targetability. Int J Pharm. 357:22–31.
- Zhang W, Deng L, Wang G, Guo X, Li Q, Zhang J, Khashab NM. 2014. Low-magnetization magnetic microcapsules: a synergistic theranostic platform for remote cancer cells therapy and imaging. Part Part Syst Charact. 31:911.
- Zhao Y, Fan X, Liu D, Wang Z. 2011. PEGylated thermo-sensitive poly(amidoamine) dendritic drug delivery systems. Int J Pharm. 409:229–236.
- Zheng C, Zhang XG, Sun L, Zhang ZP, Li CX. 2013. Biodegradable and redox-responsive chitosan/poly(l-aspartic acid) submicron capsules for transmucosal delivery of proteins and peptides. J Mater Sci Mater Med. 24:931–939.
- Zhou J, Romero G, Rojas E, Ma L, Moya S, Gao C. 2010. Layer by layer chitosan/alginate coatings on poly(lactide-co-glycolide) nanoparticles for antifouling protection and Folic acid binding to achieve selective cell targeting. J Colloid Interface Sci. 345:241–247.
- Zhu H, Liu F, Guo J, Xue J, Qian Z, Gu Y. 2011. Folate-modified chitosan micelles with enhanced tumor targeting evaluated by near infrared imaging system. Carbohydr Polym. 86:1118–1129.
- Zhu Z, Gao N, Wang H, Sukhishvili SA. 2013. Temperature-triggered on-demand drug release enabled by hydrogen-bonded multilayers of block copolymer micelles. J Control Release. 171:73–80.
- Zhu Z, Sukhishvili SA. 2009. Temperature-induced swelling and small molecule release with hydrogen-bonded multilayers of block copolymer micelles. ACS Nano. 3:3595–3605.
- Zhuk A, Pavlukhina S, Sukhishvili SA. 2009. Hydrogen-bonded layer-by-layer temperature-triggered release films. Langmuir. 25:14025–14029.
